Effect of Hydropriming and Biopriming on Seed Germination and Growth of Two Mexican Fir Tree Species in Danger of Extinction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Rhizobacterial Strains
2.3. Substrate and Fertilization
2.4. Experiment 1: Determining Germination Optimum Response Time with Hydropriming
2.5. Experiment 2: Evaluating Germination Response with Hydropriming and Biopriming
2.6. Experiment 3: Measuring Morphological Growth Response with Hydropriming and Biopriming
2.7. Data Analysis
3. Results
3.1. Experiment 1: Germination Optimum Response Time with Hydropriming
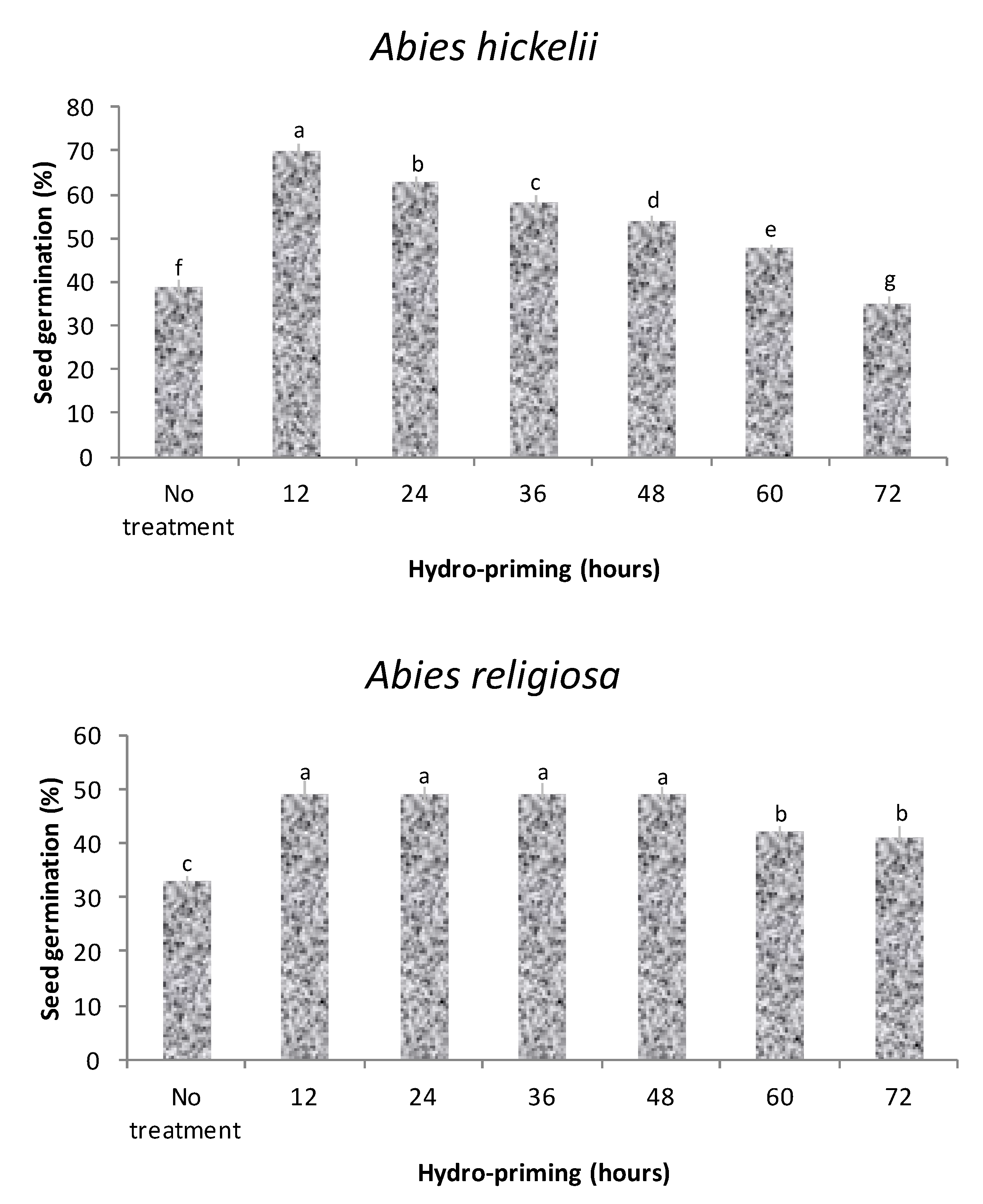
3.2. Experiment 2: Germination Response with Hydropriming and Biopriming
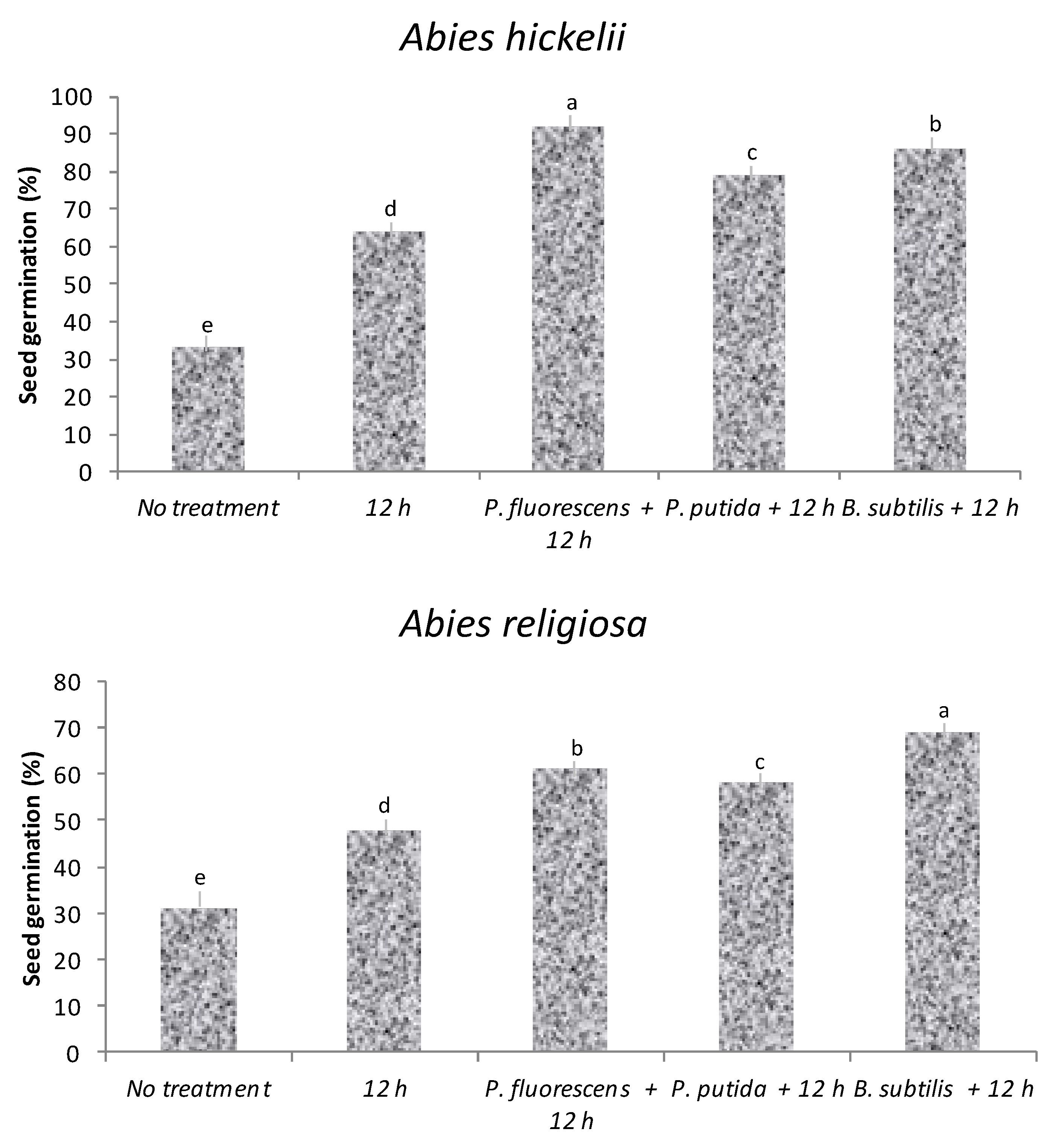
3.3. Experiment 3: Morphological Growth Response with Hydropriming and Biopriming
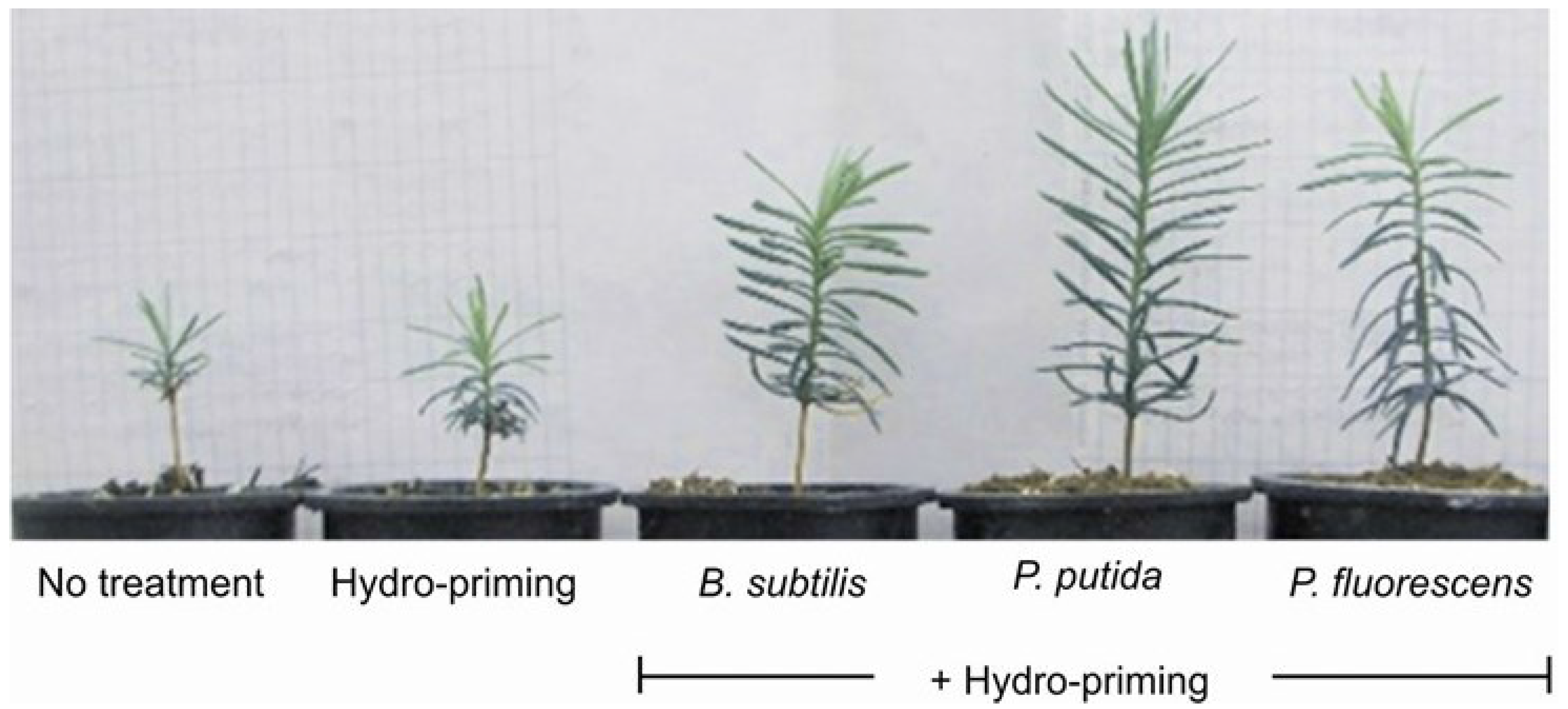
| Seed Treatment | Height (cm) | Stem diameter (mm) | Root length (cm) | Total biomass (g) | Radicle volume (cm3) |
|---|---|---|---|---|---|
| No treatment | 7.1 bc | 1.31 b | 8.1 b | 0.41 e | 0.42 c |
| Hydropriming | 7.8 bc | 1.39 b | 9.9 a | 0.58 de | 0.52 c |
| Hydropriming + P. fluorescens | 9.2 a | 1.65 a | 10.2 a | 0.80 bc | 1.12 a |
| Hydropriming + P. putida | 9.1 a | 1.63 a | 10.1 a | 1.15 a | 0.85 ab |
| Hydropriming + Bacilus. subtilis | 8.2 ab | 1.58 a | 10.1 a | 0.79 bc | 0.76 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- FAO. State of the World’s Forests; United Nations Food and Agriculture Organization: Rome, Italy, 2007; p. 157. [Google Scholar]
- FAO. Global Forest Resources Assessment 2000; United Nations Food and Agriculture Organization: Rome, Italy, 2001; p. 482. [Google Scholar]
- Shandra, J.M.; Restivo, M.; London, B. Non-Governmental Organizations and Deforestation: Reconsidering the Cross-National Evidence. Int. Rev. Mod. Soc. 2008, 34, 109–132. [Google Scholar]
- Truesdale, D.; Goldammer, J.G. Strategy for Future Development of International Cooperation in Wildland Fire Management; United Nations Food and Agriculture Organization: Rome, Italy, 2003. [Google Scholar]
- White, R.; Murray, S.; Rohweder, M. Pilot Analysis of Global Ecosystems: Grassland Ecosystems; World Resources Institute: Washington, DC, USA, 2000; p. 112. [Google Scholar]
- FAO. Livestock’s Long Shadow. Environmental Issues and Options; United Nations Food and Agriculture Organization: Rome, Italy, 2006; p. 133. [Google Scholar]
- United Nations Forum on Forests Tenth Session, Istanbul, Turkey. Collaborative Partnership on Forests’ Framework. Patent E/CN. 18/2003/INF/1, 8–19 April 2003. [Google Scholar]
- SEMARNAT. Inventario Nacional Forestal, Anexo VI.7; Diccionario del Inventario Nacional Forestal, Investigaciones Geográficas, Boletín del Instituto de Geografía, Universidad Autónoma de Mexico: Mexico, Mexico, 2001. (In Spanish) [Google Scholar]
- Liu, T. A Monograph of the Genus Abies; Department of Forestry, College of Agriculture, National Taiwan University: Taipei, Taiwan, 1971; p. 608. [Google Scholar]
- Velázquez, A.; Durán, E.; Mas, J.F.; Bray, D.B.; Bocco, G. Situación actual y prospectiva del cambio de la cubierta vegetal y usos del suelo en México. In Más allá de las Metas de Desarrollo del Milenio; CONAPO: Mexico City, Mexico, 2005; pp. 391–416. (In Spanish) [Google Scholar]
- Mas, J.; Velázquez, A.; Couturier, S. La evaluación de los cambios de cobertura/uso del suelo en la República Mexicana. Investig. Ambient. 2009, 1, 23–39. (In Spanish) [Google Scholar]
- Martínez, M. Las Pináceas Mexicanas; Universidad Nacional Autonoma de Mexico: Mexico City, Mexico, 1953; pp. 98–117. [Google Scholar]
- Ortega, J.H. Propiedades Físicas y Mecánicas de la Madera de Abies Religiosa (H.B.K.) Schl. et Cham. y Pinus Hartwegii Lindl; Escuela Nacional de Agricultura: Chapingo, México, 1962. [Google Scholar]
- Manzanilla, H. Investigaciones Epidométricas y Silvícolas en Bosques Mexicanos de Abies religiosa; Secretaría de Agricultura y Ganadería: Mexico City, Mexico, 1974. [Google Scholar]
- Sánchez-González, A.; López-Mata, L. Clasificación y ordenación de la vegetación del norte de la Sierra Nevada, a lo largo de un gradiente altitudinal. Univ. Nac. Autón. Méx. 2003, 74, 47–71. (In Spanish) [Google Scholar]
- González-Elizondo, M.; Jurado, E.; Návar, J.; González-Elizondo, M.S.; Villanueva, J.; Aguirre, O.; Jiménez, J. Tree-rings and climate relationships for Douglas-fir chronologies from the Sierra Madre Occidental, México: A 1681–2001 rain reconstruction. For. Ecol. Manag. 2005, 213, 39–53. [Google Scholar] [CrossRef]
- Sanchez-Azofeifa, G.A.; Rivard, B.; Calvo, J.; Moorthy, I. Dynamics of tropical deforestation around national parks: Remote sensing of forest change on the Osa Peninsula of Costa Rica. BioOne Mt. Res. Dev. 2002, 22, 352–358. [Google Scholar] [CrossRef]
- Narave, F.H. La vegetación del Cofre de Perote, Veracruz, México. Biotica 1985, 10, 35–151. (In Spanish) [Google Scholar]
- Dredge, D.D.; Gómez, G.O. Planeación y Manejo Sustentable. Proyecto CO24. México. Available online: http://www.conabio.gob.mx/institucion/proyectos/resultados/InfC024%20primera%20parte.pdf (accessed on 22 October 2014).
- NOM-059-ECOL-2001. Protección Ambiental-Especies Nativas de México de Flora y Fauna Silvestres-Categorías de Riesgo y Especificaciones Para su Inclusión, Exclusión o Cambio-Lista de Especies en Riesgo; National Standard: Mexico City, Mexico, 2002. [Google Scholar]
- Narave, F.H.; Taylor, K. Pinaceae. Flora de Veracruz Fascículo 98; INECOL-University of California: Xalapa, México, 1997. [Google Scholar]
- NOM-059-SEMARNAT-2010. Protección Ambiental-Especies Nativas de México de Flora y Fauna Silvestres-Categorías de Riesgo y Especificaciones Para su Inclusión, Exclusión o Cambio-Lista de Especies en Riesgo; National Standard: Mexico City, Mexico, 2010. [Google Scholar]
- Red List of Threatened Species, Ver 2012.2; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2012.
- Patiño, V.F.; Garza, P.; Villagómez, Y.A.; Talvera, I.A.; Talvera, I.; Camacho, M.F. Guia para la recolección y manejo de semillas de especies forestales. Bol. Divulg. 1983, 63, 1–181. (In Spanish) [Google Scholar]
- Hansen, E.M.; Goheen, D.J.; Jules, E.S.; Ullian, B. Managing Port-Orford cedar and the introduced pathogen Phytophthora lateralis. Plant Dis. 2000, 84, 4–13. [Google Scholar] [CrossRef]
- Hansen, E.; Sutton, W.; Parke, J.; Linderman, R. Phytophthora ramorum and Oregon forest trees—One pathogen, three diseases. In Proceedings of the Sudden Oak Death Science Symposium, USDA Forest Service and University of California, Berkeley, CA, USA, 17–18 December 2002; pp. 78–79.
- Hansen, E.M.; Sutton, W. Persistence of Phytophthora ramorum after eradication efforts in Oregon Tanoak Forests. In Proceedings of the Sudden Oak Death Second Science Symposium: The State of Our Knowledge, Monterey, CA, USA, 18–21 January 2005; Frankel, S.J., Shea, P.J., Eds.; Pacific Southwest Research Station, Forest Service, United States Department of Agriculture: Albany, CA, USA, 2006. [Google Scholar]
- Isely, D. Vigor tests. Proc. Assoc. Off. Seed Anal. 1957, 47, 176–182. [Google Scholar]
- Caballero, D.M. Efecto del tamaño de semilla y de tres tipos de sustrato en la germinación y desarrollo inicial de Pinus pseudostrobus var. oaxacana Martínez. INIF, México. Bol. Téc. 1967, 23, 1–25. (In Spanish) [Google Scholar]
- Abraham, F.; Ruiz, M. Laboratorio de semillas forestales. In Bosques y Desarrollo 14; Organización Internacional de Maderas Tropicales: Madrid, Spain, 1995; pp. 24–28. [Google Scholar]
- Kozlowski, T.T.; Kramer, P.J.; Pallardy, S.G. The Physiological Ecology of Woody Plants; Academic Press: San Diego, CA, USA, 1991; pp. 303–337. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecological, Biogeography, and Evolution of Dormancy and Germination; Academic Press: London, UK, 1998. [Google Scholar]
- Tikabu, M.; Ogden, P.C. Effect of scarification, gibberellic acid and temperature on seed germination of two multipurpose Albizia species from Ethiopia. Seed Sci. Technol. 2001, 29, 11–20. [Google Scholar]
- Luna, I.; Alcántara, O.; Ruiz, C.; Contreras-Medina, R. Composition and structure of humid montane oak forests at different sites in central and eastern Mexico. In Ecology and Conservation of Neotropical Montane oak Forests Ecological Studies 185; Kappelle, M., Ed.; Springer-Verlag: Heidelberg, Germany, 2006; pp. 101–112. [Google Scholar]
- Khan, A.A. Preplant physiological seed conditioning. In Horticultural Reviews; Janick, J., Ed.; John Wiley and Sons: New York, NY, USA, 1992; pp. 131–181. [Google Scholar]
- Sadeghian, S.Y.; Yavari, N. Effect of warer-deficit stress on germination and early seedling growth in sugar beet. J. Agron. Crop Sci. 2004, 190, 138–144. [Google Scholar] [CrossRef]
- Abdulrahmani, B.; Ghassemi-Golezani, K.; Valizadeh, M.; Feizi-Asl, V. Seed priming and seedling establishment of barley (Hordeum vulgare L.). J. Food Agric. Environ. 2007, 5, 179–184. [Google Scholar]
- Sebastian, J.; Wong, M.K.; Tang, E.; Dinneny, J.R. Methods to promote germination of dormant Setaria viridis seeds. PLoS ONE 2014, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi-Golezani, K.; Sheikhzadeh-Mosaddegh, P.; Valizadeh, M. Effects of hydro-priming duration and limited irrigation on field performance of chickpea. Res. J. Seed Sci. 2008, 1, 34–40. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Aliloo, A.A.; Valizadeh, M.; Moghaddam, M. Effects of different priming techniques on seed invigoration and seedling establishment of lentil (Lens culinaris Medik). J. Food Agric. Environ. 2008, 6, 222–226. [Google Scholar]
- McDonald, M.B. Seed deterioration: Physiology, repair and assessment. Seed Sci. Technol. 1999, 27, 177–237. [Google Scholar]
- Duffus, C.; Slaugther, C. Las Semillas y Sus Usos; AGT editor: Mexico City, Mexico, 1985; p. 188. [Google Scholar]
- Afzal, I.; Hussain, B.; Basra, S.M.A.; Rehman, H. Priming with moringa leaf extract reduces imbibitional chilling injury in spring maize. Seed Sci. Technol. 2012, 40, 271–276. [Google Scholar] [CrossRef]
- Bakhtavar, M.A.; Afzal, I.; Basra, S.M.A.; Ahmad, A.U.H.; Noor, M.A. Physiological Strategies to Improve the performance of Spring Maize (Zea mays L.) planted under early and optimum sowing conditions. PLoS ONE 2015, 10, e0124441. [Google Scholar] [CrossRef] [PubMed]
- El-Mohamedy, R.S.R.; Abd Alla, M.A.; Badiaa, R.I. Soil amendment and seed bio-priming treatments as alternative fungicides for controlling root rot diseases on cowpea plants in Nobaria Province. Res. J. Agric. Biol. Sci. 2006, 2, 391–398. [Google Scholar]
- Rao, M.S.L.; Kulkarni, S.; Lingaraju, S.; Nadaf, H.L. Biopriming of seeds: A potential tool in the integrated management of Alternaria blight of sunflower. Helia 2009, 32, 107–114. [Google Scholar] [CrossRef]
- El-Mougy, N.S.; Abdel-Kader, M.M. Long-term activity of bio-priming seed treatment for biological control of faba bean root rot pathogens. Aust. Plant Pathol. 2008, 37, 464–471. [Google Scholar] [CrossRef]
- Begum, M.M.; Sariah, M.; Puteh, A.B.; Zainal Abidin, M.A.; Rahman, M.A.; Siddiqui, Y. Field performance of bio-primed seeds to suppress Colletotrichum truncatum causing damping-off and seedling stand of soybean. Biol. Control 2010, 53, 18–23. [Google Scholar] [CrossRef]
- Jensen, B.; Knudsen, I.M.; Madsen, M.; Jensen, D.F. Biopriming of infected carrot seed with an antagonist, Clonostachys rosea, selected for control of seed-borne Alternaria spp. Phytopathology 2004, 94, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Montiel, L.G.; Escalona, M.A. Microorganismos que benefician a las plantas: Las bacterias PGPR. Cienci. Hombre 2003, 16, 19–25. [Google Scholar]
- Warton, D.I.; Hui, F.K.C. The arcsine is asinine: The analysis of proportions in ecology. Ecology 2011, 92, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Artola, A.G.; Carrillo-Castaneda, G.D.; Santos, L. Hydropriming: A strategy to increase Lotus corniculatus L. seed vigor. Seed Sci. Technol. 2003, 31, 455–463. [Google Scholar] [CrossRef]
- Murray, G.A.; Swensen, J.B.; Beaver, G. Emergence of sugar beet seedlings at low soil temperature following seed soaking and priming. Hort. Sci. 1993, 28, 31–32. [Google Scholar]
- Moreno-Chávez, L.R.; López-López, M.A.; Estañol-Botello, E.; Velázquez-Martínez, A. Diagnóstico de necesidades de fertilización de Abies religiosa (H.B.K.) Schl. et Cham. en vivero mediante el DRIS. Madera Bosques 2002, 8, 51–60. (In Spanish) [Google Scholar]
- Avila, C.H. Distribución, Ecología y usos de Abies hickelii Flous et Gaussen en Veracruz. Informe Técnico final. INIFAP—CIRGOC; Córdoba: Veracruz, México, 1987; p. 85. [Google Scholar]
- Madrigal, S.X. Clave para la identificación de las coníferas silvestres del estado de Michoacán. Instituto Nacional de Investigaciones Forestales. Mex. Bol. Divulg. 1982, 58, 7–15. (In Spanish) [Google Scholar]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Fuentes-Ramírez, L.E.; Caballero-Mellado, J. Bacterial biofertilizers. In PGPR: Biocontrol and Biofertilization; Siddiqui, Z.A., Ed.; Springer Netherlands: Berlin, Germany, 2006; pp. 143–172. [Google Scholar]
- Kloepper, J.W.; Lifshitz, R.; Novacky, A. Pseudomonas inoculation to benefit plant production. Anim. Plant Sci. 1988, 1, 60–64. [Google Scholar]
- Timmusk, S.; van West, P.; Gow, N.A.R.; Wagner, E.G.H. Antagonistic effects of Paenibacillus polymyxa towards the oomycete plant pathogens Phytophthora palmivora and Pythium aphanidermatum. In Mechanism of Action of the Plant Growth Promoting Bacterium Paenibacillus polymyxa; Uppsala University: Uppsala, Sweden, 2003; pp. 1–28. [Google Scholar]
- Chanway, C.P.; Shishido, M.; Nairn, J.; Jungwirth, S.; Markham, J.; Holl, F.B. Endophytic colonization and field responses of hybrid spruce seedlings after inoculation with plant growth-promoting rhizobacteria. For. Ecol. Manag. 2000, 133, 81–88. [Google Scholar] [CrossRef]
- Deepa, C.K.; Dastager, S.G.; Pandey, A. Plant growth-promoting activity in newly isolated Bacillus thioparus (NII-0902) from Western ghat forest, India. World J. Microbiol. Biotechnol. 2010, 26, 2277–2283. [Google Scholar] [CrossRef]
- Mafia, R.G.; Alfenas, A.C.; Ferreira, E.M.; Binoti, D.H.B.; Mafia, G.M.V.; Mounteer, A.H. Root colonization and interaction among growth promoting rhizobacteria isolates and eucalypts species. Rev. Árvore 2009, 33, 1–9. [Google Scholar] [CrossRef]
- Díaz, P.; Ferrera-Cerrato, R.; Almaraz-Suárez, J.; Alcántara, G. Inoculation of plant growth promoting bacteria in Lettuce. Terra 2001, 19, 327–333. [Google Scholar]
- Duda, B.; Orlikowski, L.B. Rhizoctonia solani on coniferous seedlings in forest nurseries. J. Plant Prot. Res. 2004, 44, 175–180. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulueta-Rodríguez, R.; Hernández-Montiel, L.G.; Murillo-Amador, B.; Rueda-Puente, E.O.; Capistrán, L.L.; Troyo-Diéguez, E.; Córdoba-Matson, M.V. Effect of Hydropriming and Biopriming on Seed Germination and Growth of Two Mexican Fir Tree Species in Danger of Extinction. Forests 2015, 6, 3109-3122. https://doi.org/10.3390/f6093109
Zulueta-Rodríguez R, Hernández-Montiel LG, Murillo-Amador B, Rueda-Puente EO, Capistrán LL, Troyo-Diéguez E, Córdoba-Matson MV. Effect of Hydropriming and Biopriming on Seed Germination and Growth of Two Mexican Fir Tree Species in Danger of Extinction. Forests. 2015; 6(9):3109-3122. https://doi.org/10.3390/f6093109
Chicago/Turabian StyleZulueta-Rodríguez, Ramón, Luis G. Hernández-Montiel, Bernardo Murillo-Amador, Edgar O. Rueda-Puente, Liliana Lara Capistrán, Enrique Troyo-Diéguez, and Miguel V. Córdoba-Matson. 2015. "Effect of Hydropriming and Biopriming on Seed Germination and Growth of Two Mexican Fir Tree Species in Danger of Extinction" Forests 6, no. 9: 3109-3122. https://doi.org/10.3390/f6093109







