Are Mixed Tropical Tree Plantations More Resistant to Drought than Monocultures?
Abstract
:1. Tropical Plantation Forestry and Biodiversity
2. Climate Change Effects on Tropical Tree Plantations
3. A Case Study from Panama
3.1. Seasonal Drought a Natural Phenomenon for Panama
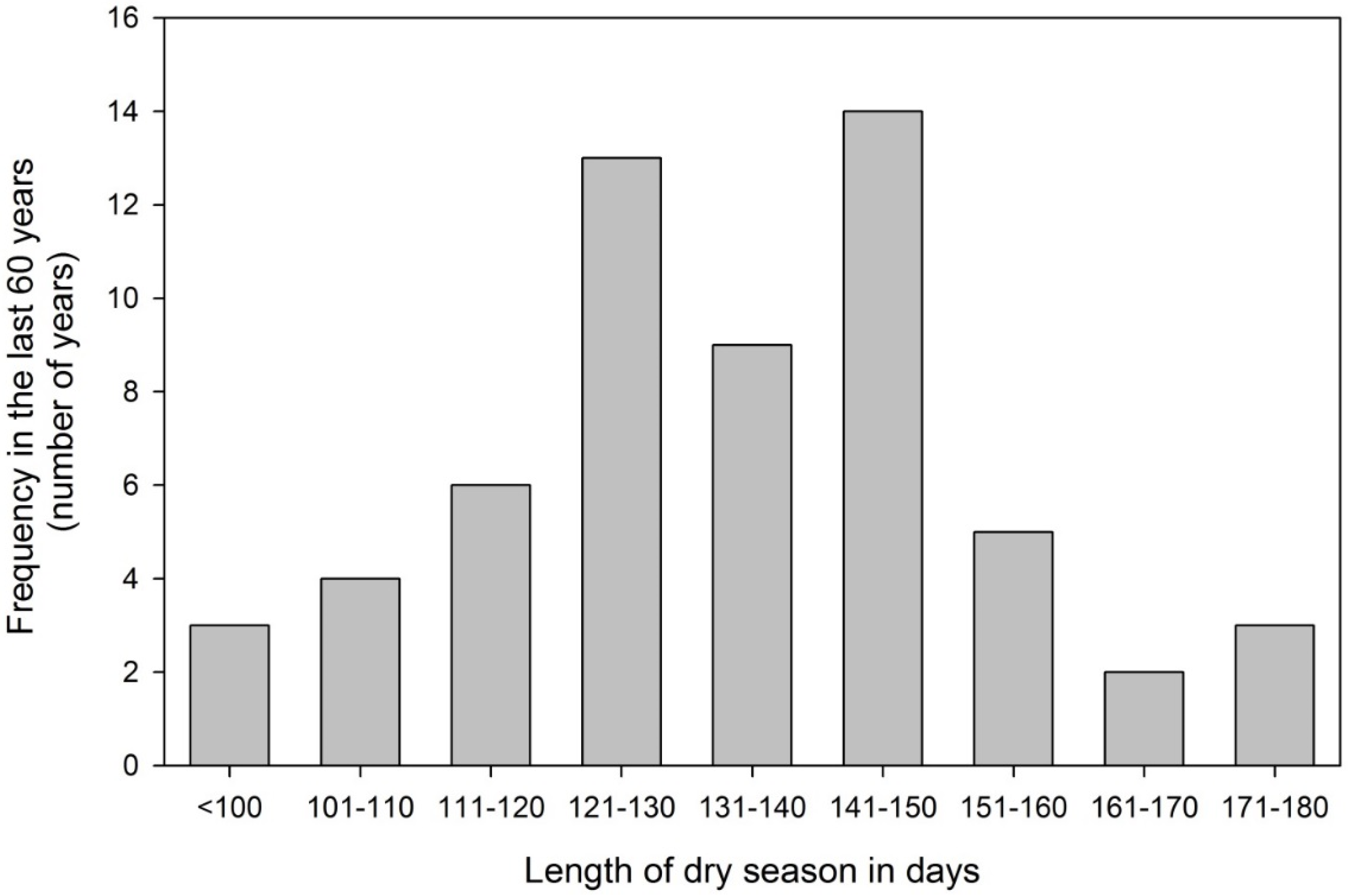
3.2. The Experimental Site in Panama
| Mixture type | Number of plots | Replication of species |
|---|---|---|
| Monoculture | 12 | Each species replicated in two plots |
| 3-species mixture | 6 | Three species, chosen randomly, for each successional group |
| 6-species mixture | 6 | Adding three species, each from a different successional group, to the existing combination in a three species mixture |
3.3. Results on Tree Water Hydraulics and Underlying Mechanisms of Tree Functionality
3.3.1. Different Traits in the Water Uptake Pattern of Species Lead to Diversity Effects
| Leaf phenology | Rooting depth | Water acquisition (soil depth) | Species |
|---|---|---|---|
| Dry season deciduous | shallow | 0‒30 cm | Cedrela odorata |
| Dry season leaf senescence | intermediate | 30‒60 cm | Luehea seemannii, Anacardium excelsum, Hura crepitans |
| Dry season green | deep | >60 cm | Tabebuia rosea |
3.3.2. Mixed Species Stands Show Higher Dry Season Stand Transpiration Rates
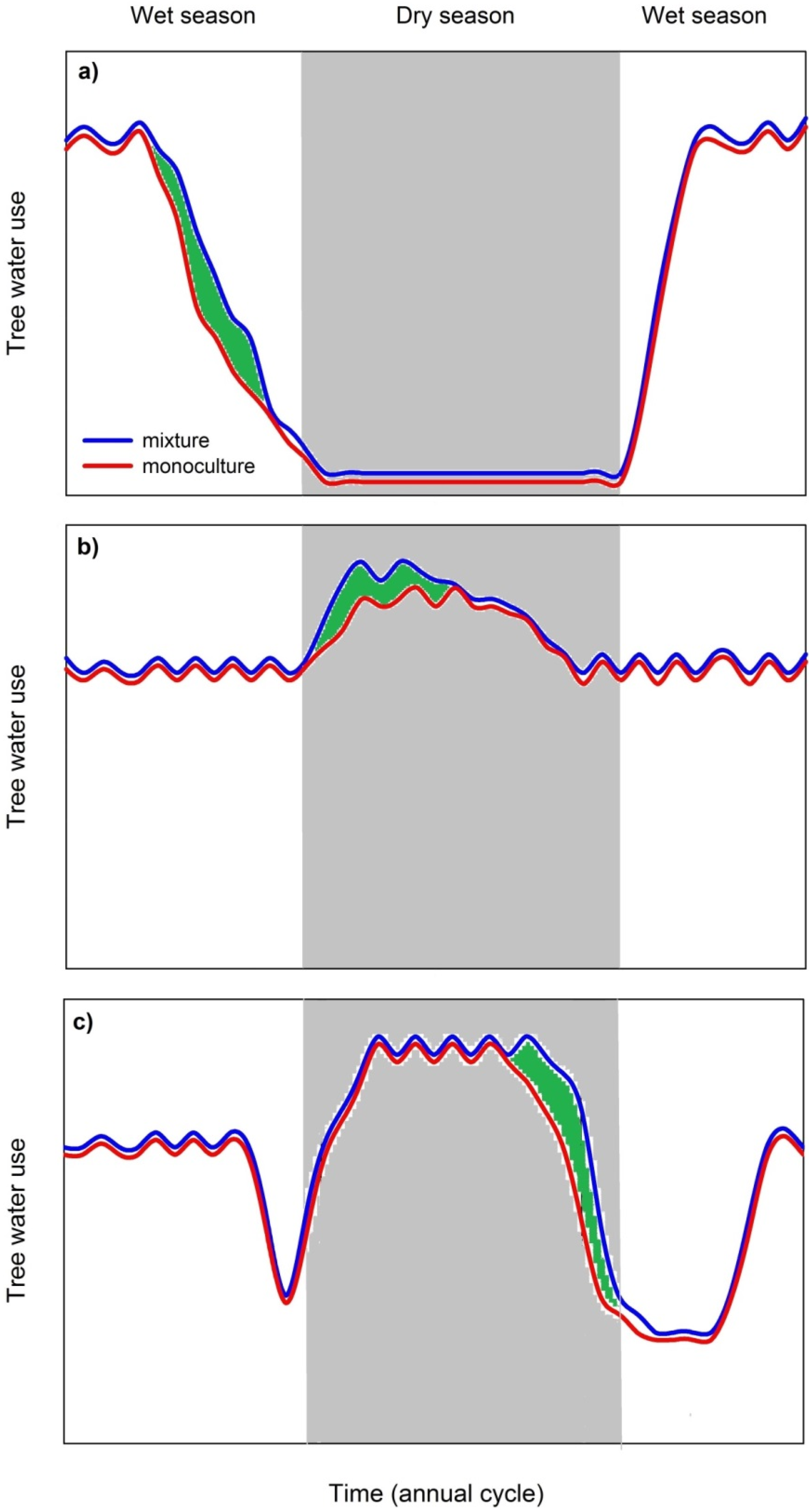
3.3.3. Possible Limitations of Species Facilitation and Urgent Research Needs
4. Conclusions and Suggestions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Saatchi, S.S.; Harris, N.L.; Brown, S.; Lefsky, M.; Mitchard, E.T.A.; Salas, W.; Zutta, B.R.; Buermann, W.; Lewis, S.L.; Hagen, S.; et al. Benchmark map of forest carbon stocks in tropical regions across three continents. Proc. Natl. Acad. Sci. USA 2011, 108, 9899–9904. [Google Scholar] [CrossRef] [PubMed]
- Bawa, K.S.; Kress, W.J.; Nadkarni, N.M.; Lele, S.; Raven, P.H.; Janzen, D.H.; Lugo, A.E.; Ashton, P.S.; Lovejoy, T.E. Tropical ecosystems into the 21st century. Science 2004, 306, 227–228. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.J.; Muller-Landau, H.C. The future of tropical forest species1. Biotropica 2006, 38, 287–301. [Google Scholar] [CrossRef]
- FAO. Global Forest Land-use Change 1990–2005; Food and Agriculture Organization of the United Nations: Rome, Italy, 2012. [Google Scholar]
- Bauhus, J.; van der Meer, P.; Kanninen, M. Ecosystem Goods and Services from Plantation Forests; Earthscan: London, UK, 2010. [Google Scholar]
- Canadell, J.G.; Raupach, M.R. Managing forests for climate change mitigation. Science 2008, 320, 1456–1457. [Google Scholar] [CrossRef] [PubMed]
- ITTO. Encouraging Industrial Forest Plantations in the Tropics; International tropical timber organization: Yokokama, Japan, 2009. [Google Scholar]
- Lamb, D.; Erskine, P.D.; Parrotta, J.A. Restoration of degraded tropical forest landscapes. Science 2005, 310, 1628–1632. [Google Scholar] [CrossRef] [PubMed]
- Montagnini, F. Accumulation in above-ground biomass and soil storage of mineral nutrients in pure and mixed plantations in a humid tropical lowland. For. Ecol. Manag. 2000, 134, 257–270. [Google Scholar] [CrossRef]
- Healy, C.; Gotelli, N.J.; Potvin, C. Partitioning the effects of biodiversity and environmental heterogeneity for productivity and mortality in a tropical tree plantation. J. Ecol. 2008, 96, 903–913. [Google Scholar] [CrossRef]
- Ruiz-Jaen, M.C.; Potvin, C. Can we predict carbon stocks in tropical ecosystems from tree diversity? Comparing species and functional diversity in a plantation and a natural forest. New Phytol. 2011, 189, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Oelmann, Y.; Potvin, C.; Mark, T.; Werther, L.; Tapernon, S.; Wilcke, W. Tree mixture effects on aboveground nutrient pools of trees in an experimental plantation in panama. Plant Soil 2010, 326, 199–212. [Google Scholar] [CrossRef]
- Potvin, C.; Mancilla, L.; Buchmann, N.; Monteza, J.; Moore, T.; Murphy, M.; Oelmann, Y.; Scherer-Lorenzen, M.; Turner, B.L.; Wilcke, W.; et al. An ecosystem approach to biodiversity effects: Carbon pools in a tropical tree plantation. For. Ecol. Manag. 2011, 261, 1614–1624. [Google Scholar] [CrossRef]
- Forrester, D.I.; Theiveyanathan, S.; Collopy, J.J.; Marcar, N.E. Enhanced water use efficiency in a mixed eucalyptus globulus and acacia mearnsii plantation. For. Ecol. Manag. 2010, 259, 1761–1770. [Google Scholar] [CrossRef]
- Zeugin, F.; Potvin, C.; Jansa, J.; Scherer-Lorenzen, M. Is tree diversity an important driver for phosphorus and nitrogen acquisition of a young tropical plantation? For. Ecol. Manag. 2010, 260, 1424–1433. [Google Scholar] [CrossRef]
- Richards, A.E.; Forrester, D.I.; Bauhus, J.; Scherer-Lorenzen, M. The influence of mixed tree plantations on the nutrition of individual species: A review. Tree Physiol. 2010, 30, 1192–1208. [Google Scholar] [CrossRef] [PubMed]
- Loreau, M.; Hector, A. Partitioning selection and complementarity in biodiversity experiments. Nature 2001, 412, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Menalled, F.D.; Kelty, M.J.; Ewel, J.J. Canopy development in tropical tree plantations: A comparison of species mixtures and monocultures. For. Ecol. Manag. 1998, 104, 249–263. [Google Scholar] [CrossRef]
- Petit, B.; Montagnini, F. Growth equations and rotation ages of ten native tree species in mixed and pure plantations in the humid neotropics. For. Ecol. Manag. 2004, 199, 243–257. [Google Scholar] [CrossRef]
- Piotto, D.; Vı́quez, E.; Montagnini, F.; Kanninen, M. Pure and mixed forest plantations with native species of the dry tropics of costa rica: A comparison of growth and productivity. For. Ecol. Manag. 2004, 190, 359–372. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bauhus, J.; Khanna, P.K. Growth dynamics in a mixed-species plantation of eucalyptus globulus and acacia mearnsii. For. Ecol. Manag. 2004, 193, 81–95. [Google Scholar] [CrossRef]
- Forrester, D.I. Transpiration and water-use efficiency in mixed-species forests versus monocultures: Effects of tree size, stand density and season. Tree Physiol. 2015, 35, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Parrotta, J.A. Productivity, nutrient cycling, and succession in single- and mixed-species plantations of casuarina equisetifolia, eucalyptus robusta, and leucaena leucocephala in puerto rico. For. Ecol. Manag. 1999, 124, 45–77. [Google Scholar] [CrossRef]
- Kunert, N.; Mercado Cardenas, A. Effects of xylem water transport on CO2 efflux of woody tissue in a tropical tree, amazonas state, brazil. Hoehnea 2012, 39, 139–144. [Google Scholar] [CrossRef]
- Grossiord, C.; Granier, A.; Gessler, A.; Pollastrini, M.; Bonal, D. The influence of tree species mixture on ecosystem-level carbon accumulation and water use in a mixed boreal plantation. For. Ecol. Manag. 2013, 298, 82–92. [Google Scholar] [CrossRef]
- Kunert, N.; Schwendenmann, L.; Potvin, C.; Hölscher, D. Tree diversity enhances tree transpiration in a panamanian forest plantation. J. Appl. Ecol. 2012, 49, 135–144. [Google Scholar] [CrossRef]
- Schwendenmann, L.; Pendall, E.; Sanchez-Bragado, R.; Kunert, N.; Hölscher, D. Tree water uptake in a tropical plantation varying in tree diversity: Interspecific differences, seasonal shifts and complementarity. Ecohydrology 2015, 8, 1–12. [Google Scholar] [CrossRef]
- Sapijanskas, J.; Potvin, C.; Loreau, M. Beyond shading: Litter production by neighbors contributes to overyielding in tropical trees. Ecology 2012, 94, 941–952. [Google Scholar] [CrossRef]
- Sapijanskas, J.; Paquette, A.; Potvin, C.; Kunert, N.; Loreau, M. Tropical tree diversity enhances light capture through crown plasticity and spatial and temporal niche differences. Ecology 2014, 95, 2479–2492. [Google Scholar] [CrossRef]
- IPCC. Annex i: Atlas of Global and Regional Climate Projections. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group i to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Intergovernmental Panel on Climate Change: Cambridge, UK; New York, NY, USA, 2013; p. 1311. [Google Scholar]
- Wu, Z.; Dijkstra, P.; Koch, G.W.; Penuelas, J.; Hungate, B.A. Responses of terrestrial ecosystems to temperature and precipitation change: A meta-analysis of experimental manipulation. Glob. Chang. Biol. 2011, 17, 927–942. [Google Scholar] [CrossRef]
- Condit, R.; Hubbell, S.P.; Foster, R.B. Mortality rates of 205 neotropical tree and shrub species and the impact of a severe drought. Ecol. Monogr. 1995, 65, 419–439. [Google Scholar] [CrossRef]
- Potts, M.D. Drought in a bornean everwet rain forest. J. Ecol. 2003, 91, 467–474. [Google Scholar] [CrossRef]
- Phillips, O.L.; van der Heijden, G.; Lewis, S.L.; López-González, G.; Aragão, L.E.O.C.; Lloyd, J.; Malhi, Y.; Monteagudo, A.; Almeida, S.; Dávila, E.A.; et al. Drought-mortality relationships for tropical forests. New Phytol. 2010, 187, 631–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunert, N.; Barros, P.; Higuchi, N. Do palm water use characteristics explain the spatial distribution of palms in the central amazon? Acta Hortic. 2013, 991, 197–204. [Google Scholar]
- Westman, W.E. Measuring the inertia and resilience of ecosystems. BioScience 1978, 28, 705–710. [Google Scholar] [CrossRef]
- Scherer-Lorenzen, M. The functional role of biodiversity in the context of global change. In Forest and Global Change; Coomes, A.D., Burslem, D.F.R.P., Simonson, W.D., Eds.; Cambrigde University Press: Cambridge, UK, 2014; p. 195. [Google Scholar]
- Spiecker, H. Overview of recent growth trends in european forests. Water Air Soil Pollut. 1999, 116, 33–46. [Google Scholar] [CrossRef]
- Bruijnzeel, L.A. Predicting the hydrological impacts of land cover transformation in the humid tropics: The need for integrated research. In Amazonian Deforestation and Climate; Gash, J.H.C., Nobre, C.A., Roberts, J.M., Victoria, R.L., Eds.; John Wiley & Sons: New York, NY, USA, 1996; pp. 15–55. [Google Scholar]
- Zhang, L.; Dawes, W.R.; Walker, G.R. Predicting the Effect of Vegetation Changes on Catchment Average Water Balance; ACT: Canberra Australia, 1999. [Google Scholar]
- Köhler, M.; Schwendenmann, L.; Hölscher, D. Throughfall reduction in a cacao agroforest: Tree water use and soil water budgeting. Agric. For. Meteorol. 2010, 150, 1079–1089. [Google Scholar] [CrossRef]
- Van Ruijven, J.; Berendse, F. Diversity enhances community recovery, but not resistance, after drought. J. Ecol. 2010, 98, 81–86. [Google Scholar] [CrossRef]
- Paton, S. 2013 Meteorological and Hydrological Summary for Barro Colorado Island; Smithsonian Tropical Research Institute: Panama City, Panama, 2013. [Google Scholar]
- Engelbrecht, B.J.; Kursar, T.; Tyree, M. Drought effects on seedling survival in a tropical moist forest. Trees 2005, 19, 312–321. [Google Scholar] [CrossRef]
- Holdridge, L.R.; Budowski, G. Report on an ecological survey of the republic of panama. Caribb. For. 1956, 17, 92–109. [Google Scholar]
- Kunert, N.; Schwendenmann, L.; Hölscher, D. Seasonal dynamics of tree sap flux and water use in nine species in panamanian forest plantations. Agric. For. Meteorol. 2010, 150, 411–419. [Google Scholar] [CrossRef]
- Tyree, M.T.; Engelbrecht, B.M.J.; Vargas, G.; Kursar, T.A. Desiccation tolerance of five tropical seedlings in panama. Relationship to a field assessment of drought performance. Plant Physiol. 2003, 132, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Condit, R. Ecological implications of changes in drought patterns: Shifts in forest composition in panama. Clim. Chang. 1998, 39, 413–427. [Google Scholar] [CrossRef]
- Condit, R.; Aguilar, S.; Hernandez, A.; Perez, R.; Lao, S.; Angehr, G.; Hubbell, S.P.; Foster, R.B. Tropical forest dynamics across a rainfall gradient and the impact of an el niño dry season. J. Trop. Ecol. 2004, 20, 51–72. [Google Scholar] [CrossRef]
- Condit, R.; Ashton, P.S.; Manokaran, N.; LaFrankie, J.V.; Hubbell, S.P.; Foster, R.B. Dynamics of the forest communities at pasoh and barro colorado: Comparing two 50-ha plots. Philos. Trans. R. Soc. B: Biol. Sci. 1999, 354, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Kane, J.M.; Anderegg, L.D.L. Consequences of widespread tree mortality triggered by drought and temperature stress. Nat. Clim. Chang. 2013, 3, 30–36. [Google Scholar] [CrossRef]
- FAO. World Reference Base for Soil Resources; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998; p. 88. [Google Scholar]
- Leigh, E.G.J.; Rand, A.S.; Windsor, D.W. The Ecology of a Tropical Forest, 2nd ed.; Smithsonian Press: Washington, DC, USA, 1996. [Google Scholar]
- Abraham, M. Spatial Variation in Soil Organic Carbon and Stable Carbon Isotope Signature in a Pasture and a Primary Forest in Central Panamá; McGill University: Montreal, Canada, 2004. [Google Scholar]
- Scherer-Lorenzen, M.; Luis Bonilla, J.; Potvin, C. Tree species richness affects litter production and decomposition rates in a tropical biodiversity experiment. Oikos 2007, 116, 2108–2124. [Google Scholar] [CrossRef]
- Potvin, C.; Gotelli, N.J. Biodiversity enhances individual performance but does not affect survivorship in tropical trees. Ecol. Lett. 2008, 11, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Meinzer, F.; Andrade, J.; Goldstein, G.; Holbrook, N.; Cavelier, J.; Wright, S. Partitioning of soil water among canopy trees in a seasonally dry tropical forest. Oecologia 1999, 21, 293–301. [Google Scholar] [CrossRef]
- Schneebeli, M.; Wolf, S.; Kunert, N.; Eugster, W.; Mätzler, C. Relating the x-band opacity of a tropical tree canopy to sapflow, rain interception and dew formation. Remote Sens. Environ. 2011, 115, 2116–2125. [Google Scholar] [CrossRef]
- Erwin, T.L. Tropical forests: Their richness in coleoptera and other arthropod species. Coleopt. Bull. 1982, 36, 74–75. [Google Scholar]
- Kunert, N. Tree Transpiration in Forest Plantations: Effects of Species, Seasonality and Diversity (Panama); Georg-August-Universität Göttingen: Goettingen, Germany, 2010. [Google Scholar]
- Dierick, D.; Kunert, N.; Köhler, M.; Schwendenmann, L.; Hölscher, D. Comparison of tree water use characteristics in reforestation and agroforestry stands across the tropics. In Tropical Rainforests and Agroforests under Global Change; Tscharntke, T., Leuschner, C., Veldkamp, E., Faust, H., Guhardja, E., Bidin, A., Eds.; Spinger: Berlin, Germany, 2010; pp. 293–308. [Google Scholar]
- Neumann, R.B.; Cardon, Z.G. The magnitude of hydraulic redistribution by plant roots: A review and synthesis of empirical and modeling studies. New Phytol. 2012, 194, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Prieto, I.; Armas, C.; Pugnaire, F.I. Water release through plant roots: New insights into its consequences at the plant and ecosystem level. New Phytol. 2012, 193, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Haggar, J.P.; Ewel, J.J. Primary productivity and resource partitioning in model tropical ecosystems. Ecology 1997, 78, 1211–1221. [Google Scholar] [CrossRef]
- Meinzer, F.C.; Goldstein, G.; Andrade, J.L. Regulation of water flux through tropical forest canopy trees: Do universal rules apply? Tree Physiol. 2001, 21, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Bond-Lamberty, B.; Wang, C.; Gower, S.T. Aboveground and belowground biomass and sapwood area allometric equations for six boreal tree species of northern manitoba. Can. J. For. Res. 2002, 32, 1441–1450. [Google Scholar] [CrossRef]
- McDowell, N.G. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties, 2nd ed.; John Wiley & Sons: West Sussex, UK, 2002; p. 456. [Google Scholar]
- Beier, C.; Beierkuhnlein, C.; Wohlgemuth, T.; Penuelas, J.; Emmett, B.; Körner, C.; de Boeck, H.; Christensen, J.H.; Leuzinger, S.; Janssens, I.A.; et al. Precipitation manipulation experiments-challenges and recommendations for the future. Ecol. Lett. 2012, 15, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.; Adams, H.D.; Anderegg, W.R.L.; Jansen, S.; Zeppel, M.J.B. Research frontiers in drought-induced tree mortality: Crossing scales and disciplines. New Phytol. 2015, 205, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Nepstad, D.C.; Moutinho, P.; Dias-Filho, M.B.; Davidson, E.; Cardinot, G.; Markewitz, D.; Figueiredo, R.; Vianna, N.; Chambers, J.; Ray, D.; et al. The effects of partial throughfall exclusion on canopy processes, aboveground production, and biogeochemistry of an amazon forest. J. Geophys. Res. Atmos. 2002, 107, 1–18. [Google Scholar] [CrossRef]
- Belk, E.L.; Markewitz, D.; Rasmussen, T.C.; Carvalho, E.J.M.; Nepstad, D.C.; Davidson, E.A. Modeling the effects of throughfall reduction on soil water content in a brazilian oxisol under a moist tropical forest. Water Resour. Res. 2007, 43, 1–14. [Google Scholar] [CrossRef]
- Fisher, R.A.; Williams, M.; Da Costa, A.L.; Malhi, Y.; Da Costa, R.F.; Almeida, S.; Meir, P. The response of an eastern amazonian rain forest to drought stress: Results and modelling analyses from a throughfall exclusion experiment. Glob. Chang. Biol. 2007, 13, 2361–2378. [Google Scholar] [CrossRef]
- Moser, G.; Schuldt, B.; Hertel, D.; Horna, V.; Coners, H.; Barus, H.; Leuschner, C. Replicated throughfall exclusion experiment in an indonesian perhumid rainforest: Wood production, litter fall and fine root growth under simulated drought. Glob. Chang. Biol. 2014, 20, 1481–1497. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunert, N.; Cárdenas, A.M. Are Mixed Tropical Tree Plantations More Resistant to Drought than Monocultures? Forests 2015, 6, 2029-2046. https://doi.org/10.3390/f6062029
Kunert N, Cárdenas AM. Are Mixed Tropical Tree Plantations More Resistant to Drought than Monocultures? Forests. 2015; 6(6):2029-2046. https://doi.org/10.3390/f6062029
Chicago/Turabian StyleKunert, Norbert, and Alida Mercado Cárdenas. 2015. "Are Mixed Tropical Tree Plantations More Resistant to Drought than Monocultures?" Forests 6, no. 6: 2029-2046. https://doi.org/10.3390/f6062029





