1. Introduction
The American chestnut (
Castanea dentata (Marsh.) Borkh) was a dominant forest tree in the eastern United States until two nonnative pathogens virtually eliminated it as a canopy species.
Phytophthora cinnamomi Rands, a soil-borne oomycete that causes ink disease, was likely introduced into the US in the mid-19th century and is credited with eradicating chestnut in bottomland and poorly drained soils in the southern states [
1]. Chestnut blight fungus (
Cryphonectria parasitica (Murr.) Barr), which arrived in the late 19th century, killed most mature chestnuts throughout the tree’s range by the 1950s. The tree was once ecologically important as a source of mast for wildlife [
2,
3,
4], and economically valuable for its rot-resistant lumber, high tannin content and edible nuts
cf. [
5,
6]. The predominant strategy to develop a blight-resistant American chestnut uses blight resistance from Chinese (
C. mollissima Blume) or Japanese (
C. crenata Sieb. & Zucc.) chestnuts in a backcross breeding approach [
6,
7,
8]. This approach incorporates an initial cross between a Chinese or Japanese chestnut and an American chestnut (F
1), followed by a series of backcrosses to American chestnuts (BC
1F
1, BC
2F
1, and BC
3F
1) and an intercrossing generation (BC
3F
2). Each generation is screened for resistance and timber form. The final cross in the breeding scheme is among third generation backcross trees (BC
3F
2) to reach the BC
3F
3 generation. In theory, this final generation will have the growth habit of American chestnut and blight resistance of an Asian chestnut species [
6,
7,
8].
Blight-resistant American chestnut hybrids suitable for reforestation may become widely available from The American Chestnut Foundation’s (TACF) breeding program after further testing of selections is conducted [
9]. However, restoration will require more than blight resistance. Success of planting will require artificial regeneration of seedlings that can compete with natural vegetation, persevere or escape deer browse pressure, and tolerate extreme abiotic conditions, such as drought. Only two studies, however, have examined nursery production of high quality sensu [
10] American chestnut seedlings [
11,
12], and few studies have examined the effect of seedling quality on chestnut in forested settings [
12,
13,
14,
15]. While the effect of seedling quality on American chestnut establishment success and competitive ability has not been thoroughly assessed, the importance of seedling quality to oak (
Quercus spp. L.) establishment has been tested for many decades [
16]. As oak and chestnut are within the same taxonomic family, we can use the results of experiments with oak species to infer chestnut response.
Planting high quality northern red oak (
Q. rubra L.) seedlings tends to result in increased survival and growth, as compared to planting smaller seedlings [
16,
17,
18,
19,
20]. Clark
et al. [
14] found similar trends in planting high quality American, Chinese and hybrid chestnut seedlings. Rhoades
et al. [
13] found that larger American chestnut seedlings performed better in high-light harvest treatments, while seedling size did not affect performance in low light treatments. Many studies demonstrate a positive correlation between both number of first order lateral roots (FOLR) and root collar diameter (RCD) with growth and survival of northern red oak [
16,
19,
20,
21,
22,
23]. Both traits are highly correlated with root mass, an important characteristic affecting transplant shock [
16]. Height is also an important factor in seedling survival and growth [
16]. Tall seedlings have a better chance of escaping browsing by deer, a serious threat to seedling establishment throughout much of American chestnut’s range, and of competing with other vegetation for light [
16].
In order to consistently produce high quality chestnut seedlings in bare-root nurseries, it is necessary to understand what factors affect chestnut seedling quality. Kormanik
et al. [
10] found that spacing, fertilization and irrigation are among the most important nursery cultural practices that influence oak seedling quality. The impacts of seed size, but not spacing and fertilization, on chestnut growth in the nursery have been tested. Clark
et al. [
11] compared two nut size classes for differences in seedling quality for American, Chinese and hybrid chestnuts grown in two commercial nurseries. They found that the large nut size class produced taller seedlings than the small nut size class and that mean nut weight of family × nut size class treatment was strongly related to seedling height.
This study extends the study of Clark
et al. [
11] by testing additional families and using individual nut weight, in addition to the mean nut weight of the treatments, to predict seedling quality, and by comparing early growth of chestnut to that of northern red oak. The main goal of the study is to better understand factors affecting growth and survival of chestnuts in a nursery setting in order to develop methods for producing high quality backcross chestnuts that will lead to outplanting success for restoration of the species. The study has three primary objectives: (1) Nut weight and seedling growth: evaluates the relationships among nut size (classified visually), nut weight and seedling growth using both seed plot mean and individual seed or seedling data; (2) Germination patterns: evaluates the relationships among timing of germination, generation, family, survival and growth at lifting; and (3) Temporal growth patterns: examines the temporal height growth patterns among chestnut parental species, various breeding generations, and northern red oak from a bulked (multiple genetic families) seedlot.
2. Materials and Methods
2.1. Experimental Materials
Chestnuts sown came from one American family, one Chinese family, one BC
3F
2 hybrid family and ten BC
3F
3 hybrid families [
7] (
Table 1), all derived from open-pollinated crosses. American and Chinese chestnuts were included in this study in order to evaluate how the backcross chestnut families compare to their parental species in growth characteristics. The goal of the breeding programs is to produce a hybrid chestnut with the growth characteristics of the American chestnut, and with only blight-resistance traits from the Chinese chestnut parental species.
Table 1.
Number of chestnut nuts sown and seedlings lifted within each parental species, breeding generation, and family. All chestnut families were used in the nut weight and growth and germination patterns experiments. Families noted with an asterisks (*) were used in the seasonal growth patterns study and counts in parentheses are the number of seedlings used in the seasonal growth patterns study at the date of the last measurement before lifting.
Table 1.
Number of chestnut nuts sown and seedlings lifted within each parental species, breeding generation, and family. All chestnut families were used in the nut weight and growth and germination patterns experiments. Families noted with an asterisks (*) were used in the seasonal growth patterns study and counts in parentheses are the number of seedlings used in the seasonal growth patterns study at the date of the last measurement before lifting.
| Parental Species or Generation | Family | Number of Nuts Sown | Number of Seedlings at Lifting | Germination by May 25 (Percent) | Seedling Survival (Percent) |
|---|
| 100% American | Plummer4 * | 356 | 245 (120) | 91 | 69 |
| 100% Chinese | CD * | 374 | 236 (138) | 64 | 63 |
| BC3F2 (94% American) | CH283 * | 179 | 136 (158) | 84 | 76 |
| BC3F3 (94% American) | D3 * | 181 | 140 (156) | 81 | 77 |
| D12 * | 180 | 157 (174) | 97 | 87 |
| D13 | 181 | 144 | 86 | 80 |
| D14 | 180 | 123 | 72 | 68 |
| D15 | 176 | 62 | 39 | 35 |
| D16 | 175 | 71 | 42 | 41 |
| D17 | 172 | 60 | 38 | 35 |
| D18 | 179 | 105 | 65 | 59 |
| D19 * | 182 | 163 (170) | 90 | 90 |
| D20 | 176 | 99 | 60 | 56 |
| | All | 1782 | 1124 | 67 | 63 |
| Total chestnut | | 2691 | 1741 | 70 | 64 |
| Northern red oak | | | (939) | | |
The chestnuts were harvested at TACF’s Meadowview Research Farms, Meadowview, VA, USA, in the fall of 2009. As the fertilization and irrigation regime used in this study was originally developed to grow high quality northern red oak and because a considerable amount of research on hardwood artificial regeneration has focused on this species [
16], we included northern red oak in the study to evaluate if these nursery practices produce similar results with chestnut. Additionally, northern red oak is one of the hardwood species most commonly planted in forested settings and is likely to be included in mixed species plantings with hybrid chestnuts; therefore, understanding how the two species differ in their early growth is of interest. The northern red oak came from a bulked seedlot from several mother trees at an east Tennessee seed orchard. Prior to sowing, nuts and acorns were stored in sealed plastic bags that contained peat moss in cold rooms at 1 °C. Chestnuts and acorns were manually sown at the East Tennessee State Nursery in Delano, TN, USA (35°14′27″ N, 84°34′21″ W) on 19 February 2010 at a density of 65 seeds m
2. Within the nursery bed, chestnuts were sown in the inner two rows and northern red oaks in the outer two rows. Northern red oak served to shelter the chestnut seedlings from wind and to provide shade to the outer sides of the chestnuts to produce uniform growing conditions. Fertilization and irrigation of the seedlings followed guidelines developed by Kormanik
et al. [
10]. Prior to sowing, each chestnut family was visually separated into large and small nut size classes, each with the same number of nuts. Each size class per family was split into two replicates that were equal in number and in total weight, to the nearest 0.1 g (
Table 2).
Table 2.
Descriptive statistics (standard error in parenthesis) of mean chestnut nut weight, seedling growth characteristics for whole population and for two nut size classes. FOLR stands for first order lateral roots and RCD stands for root collar diameter.
Table 2.
Descriptive statistics (standard error in parenthesis) of mean chestnut nut weight, seedling growth characteristics for whole population and for two nut size classes. FOLR stands for first order lateral roots and RCD stands for root collar diameter.
| Nut and seedling characteristics | Total Population | Nut Size Class 1 |
|---|
| Mean | Range | n | Large | n | Small |
|---|
| Nut weight (g) | 3.2 (0.03) | 0.6–14.9 | 1343 | 4.3 (0.03) a | 1348 | 3.0 (0.03) b |
| Height (cm) | 136 (1.06) | 7–259 | 885 | 141 (5.82) a | 856 | 132 (5.82) b |
| RCD (mm) | 13.9 (0.12) | 1.4–35.2 | 885 | 14.6 (0.39) a | 856 | 13.3 (0.40) b |
| FOLR number 2 | 13 (0.16) | 0–37 | 803 | 13 (0.38) a | 786 | 12 (0.42) b |
| Missing tap root (percent) | 9 (0.68) | -- | 885 | 4 (0.01) a | 856 | 3 (0.01) a |
| Stem forking (percent) | 2 (0.37) | -- | 885 | 0.002 (0.03) a | 856 | 0.4 (7.8) b |
2.2. Experimental Design
The nuts were planted in a randomized complete block design, with a nested and split plot treatment arrangement. Generation treatments were whole plots. For the BC3F3 generation, family treatments were nested within the whole plot. Nut size class was the sub-plot. Each family × nut size class treatment was replicated twice and replicates were blocked to reduce bias from environmental variation within the planting beds. Samples from each seed plot were randomly chosen and used for individual nut measurements.
2.3. Measurements
Prior to sowing, each individual nut was weighed and used to calculate mean and variation of nut weights within each family × nut size class treatment for each replicate (hereafter referred to as the seed plot). Weights for each seed plot were recorded; individual nut weights were not. Additionally, a random sample of 86–92 nuts from each seed plot was individually weighed and assigned a unique number to maintain individual nut and seedling identities throughout the study. A small pin flag with a tag labelled with the assigned number was placed next to each of these individually identified nuts at sowing. The tag was transferred to the stem after the seedling grew approximately 10 cm in height to ensure proper identification.
Germination was tallied for each individually tagged nut and germination counts were made for all nuts within each seed plot on five dates: 23 April, 30 April, 4 May, 14 May and 25 May 2010. No new seedlings emerged after 25 May. Height of Chinese, American, BC3F2, three of the BC3F3 chestnut families and the associated northern red oak were measured on nine dates (17 May, 1 June, 14 June, 28 June, 6 July, 2 August, 23 August, 13 September and 4 October 2010) during the growing season.
All seedlings were lifted from the nursery on 8 March 2011. They were stored in a cold room (~1 °C) until measured. Height to the terminal bud and root collar diameter (RCD) of all seedlings were measured. The number of FOLR, lateral roots originating from the taproot and having at least 1 mm at the proximal end, for each chestnut seedling was counted. To reduce bias, the same person counted FOLR on all seedlings. Chestnut seedlings that lacked a taproot or had stem forks were recorded. By definition seedlings without taproots did not have FOLR. A stem fork was defined as a lateral branch beginning at or near the root collar and extending at least half the length of the main stem.
2.4. Statistical Analysis
Analyses for each objective below were processed using SAS software [
24], and diagnostics such as normality and equal variance were monitored and corrected for all analyses. Pearson correlations were used to assess associations among variables.
2.4.1. Nut Weight and Seedling Growth
Seedlings with signs of disease caused by
Phytophthora cinnamomi (such as presence of black roots), heavy insect damage on stems, or other disease symptoms were excluded from any analyses. Fewer than 10 trees total were removed from the study. Seedlings with missing taproots were excluded from FOLR analysis.
T-tests were conducted to determine if pre-treatment differences in mean nut weight existed between replications within each family by nut size class treatment. Mixed model analysis of variance (ANOVA) and DandA.sas macros [
25] were used to test nut size, generation and family, and their interaction effects on height, RCD and FOLR for all chestnut seedlings. Any significant main effects or interactions (α = 0.05) were further analyzed using Tukey’s mean separation method.
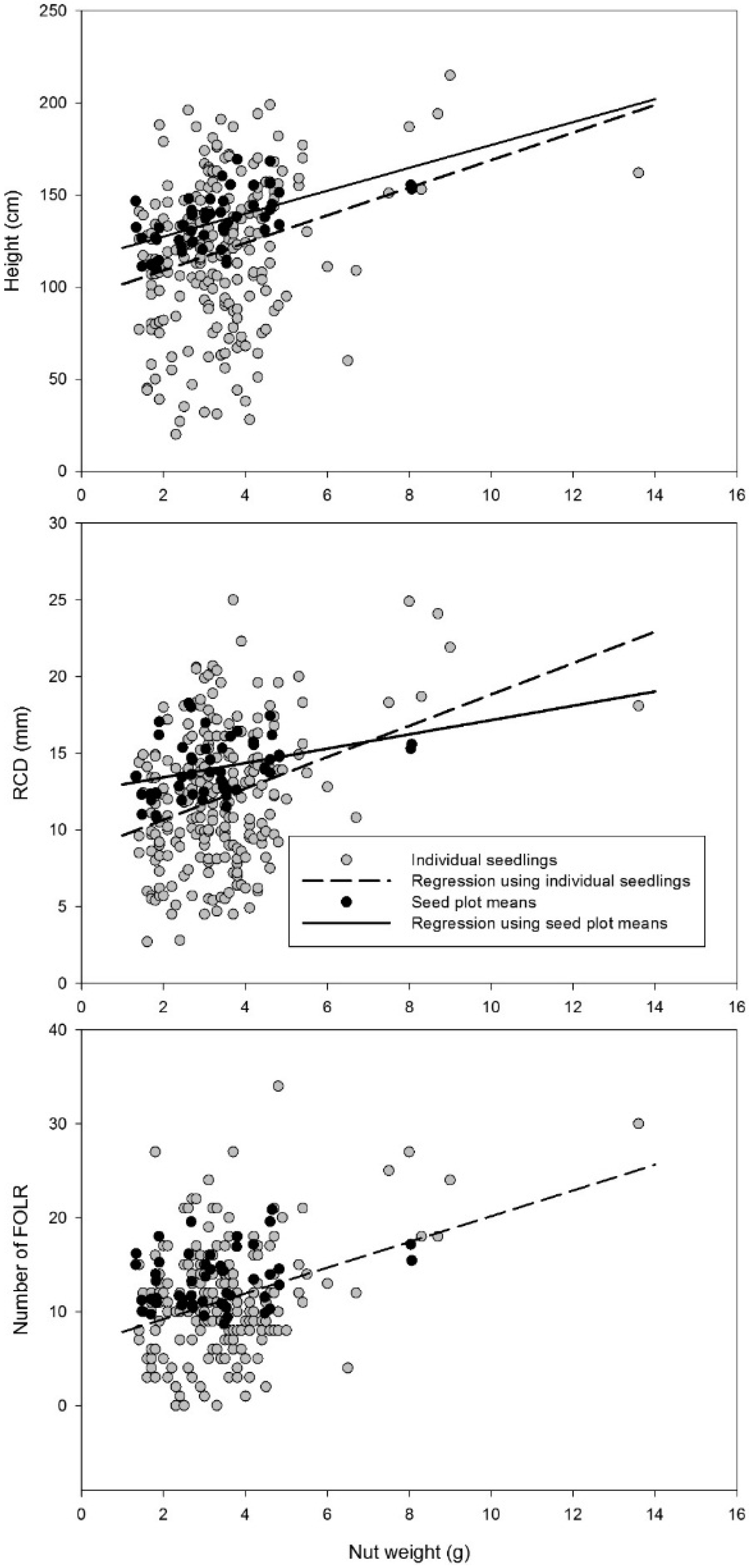
Indicator variable regression (PROC GLM) was used to test both the ability of the mean nut weight of the seed plot and, separately, the nut weights of sampled seeds, to predict seedling height, RCD and the number of FOLR [
11].
Logistic regression (PROC LOGISTIC) was used to determine if the probability of having a missing taproot or stem fork was influenced by family, nut size class, or nut weight. Dummy variables were used for categorical independent variables [
26] and the final model was selected by conducting chi-square tests on Akaike information criterion values. The most parsimonious model was selected as the final model. Hosmer-Lemeshow goodness of fit statistic was used to test that the model adequately explained the variation in the data.
2.4.2. Germination Patterns
Seed plot means were used in indicator variable regression (PROC GLM) to determine if nut weight within family could predict final percentage of germination on 25 May 2010, and if final percentage of germination of the seed plot influenced height, RCD and number of FOLR of resulting seedlings. Odds ratios were produced to compare seedlings from each family with those from the Chinese chestnut family. An odds ratio of 1 or greater indicates that the family had a greater chance of germination compared to the Chinese chestnut family, while a ratio of less than 1 indicates the family had a lower chance of germination compared to the Chinese chestnut family. Indicator variable regression was also used to determine if growing-degree days at the time of germination could predict seedling height, RCD or number of FOLR. The five germination dates were converted to growing-degree days using daily temperature data from the National Climatic Data Center for the two weather stations closest to the nursery, stations 13,891 and 13,882 [
27]. Daily maximum and minimum temperatures for each day starting from 1 January 2010 were used to calculate growing-degree days for each weather station (then averaged) using the formula:
where Tmax was maximum daily temperature, Tmin was minimum daily temperature and Tbase was set to 10 °C. Tmax and Tmin were set to 10 °C if less than the Tbase.
Logistic regression was used to determine if growing-degree days at the time of germination, nut weight, nut size class and family could be used to predict seedling survival until lifting, and final (25 May) percent germination.
2.4.3. Temporal Growth Patterns
Mixed model ANOVA and DandA.sas macros with an autoregressive covariance structure for repeated measures were used to test family, nut size and growth period effects on daily height growth. Differences among treatment means were examined if main effects and interactions were significant (α = 0.05) using Tukey’s mean separation method.
4. Discussion
4.1. Nut Weight and Seedling Growth
Results from the nut weight and seedling growth study and the temporal growth pattern study show that seed plots with larger chestnut seeds produced higher-quality seedlings compared to seed plots with smaller nuts. Seedling quality includes growth variables such as daily height growth, end of season height and RCD growth and number of FOLR. These results generally agree with Clark
et al.’s [
11] findings. Improvements in seedling quality were reliably made across all breeding generations and parental species through a course visual grade based on nut size. However, predictions for increases in seedling quality from individual nuts were not reliable, as indicated by the low R
2 values. These findings indicate that managers will see improvements if grading nuts by distinguishable size class groupings, and not by relatively small gradations within a seedlot. However, as suggested by Clark
et al. [
11], selecting only the largest nuts may lead to indirectly selecting individuals with additional Chinese chestnut traits, as Chinese chestnuts have larger seeds than Americans.
Our findings were similar to a previous study that found seedlings with larger height and RCD were produced from large size-class chestnut seed plots and found a 3 cm increase in height and no significant relationship with RCD for every 1-gm increase in nut weight [
11]. The similarity of results is particularly meaningful because different families were used in each study, indicating a general trend among backcross chestnut hybrids, not just certain families. Our results also agree with several studies that have found a relationship between acorn size and seedling development [
28,
29]. Our results suggest that the occurrences of missing taproots and stem forking reduced height and RCD. Missing taproots and forking are presumably caused, in part, by damage to young radicle tips by biotic (insects) or abiotic factors. These seedlings then develop adventitious roots that look like lateral roots from the base of remaining radicles. Sung
et al. [
30] reported more white oak (
Q. alba L.) seedlings had forking root systems after half or whole radicles were surgically removed before sowing, suggesting forked root systems are caused by loss of the radicle. Like white oak, emergence of the radicle tip can occur with chestnuts even during cold storage of the nuts. Planting nuts before radicle emergence, therefore, is important to reduce the occurrence of forking or missing taproots.
The stronger relationship between seed plot mean nut weight and growth variables compared to individual nut weights and growth variables was expected given that variability among individual nuts/seedlings is higher than variability among seed plots. However, the lower explanatory power of individual nuts/seedlings could also be due to the fact that we marked individual nuts with pin flags. These flags flapped against the seedlings as they sprouted from nuts, noticeably injuring many of them and presumably reducing their growth, which would weaken a relationship between nut size and growth. Nonetheless, results do indicate that a high degree of variability could be expected in American chestnut nursery seedlings within the same family with similar nut sizes, or weights or both, grown in relatively uniform conditions of a commercial nursery bed. The large variation within families may have occurred partially because these nuts were from open-pollinated trees or because of damage to nuts from weevils [
31].
4.2. Species and Family Treatments
After the growth period between 2 and 14 June, Chinese chestnuts outperformed other species/generations. Clark
et al. [
11] also reported better growth by Chinese chestnut. Although Chinese chestnut is generally not a good competitor after outplanting [
32], the ideal growth conditions in the nursery beds may be conducive to superior growth of this Asian species due in part to its larger nut size. Families of the BC
3F
3 generation and American chestnuts were similar in growth including a slow growth period between 15 and 28 June. These results support the expectations of TACF’s backcross breeding program. The differences in mean height and RCD of some BC
3F
3 families compared to the American family indicate the importance of genetic influence on growth, and that genetic selection of superior families in the nursery may eventually be possible. Significant family differences were also reported in other studies for backcross chestnut families [
11,
12].
In general, the BC
3F
2 family, CH283, yielded inferior growth results compared to the other chestnut families tested. Although the BC
3F
2 generation might be expected to behave similarly to the BC
3F
3 generation, the same BC
3F
2 family, CH283, grew less in height and RCD compared with BC
3F
3, American and Chinese families in Clark
et al.’s 2012 chestnut nursery study [
11]. These results suggest a genetic effect, but because only one BC
3F
2 family was tested, we cannot determine if the inferior growth is related to a family traits as opposed to a generational (
i.e., breeding). Nevertheless, these shared results indicate uniformity in growth patterns between years. Daily growth rate was greater for all chestnut families than for northern red oak. Northern red oak, which was planted in the outer nursery rows, may have been stunted due to high wind conditions of the nursery; however, several other studies have demonstrated its inferior growth as compared to American chestnut [
33,
34]. Northern red oak, a mid-shade tolerant species [
35], has been found to have a low growth potential in high-resource environments [
36], compared to shade-intolerant species such as trembling aspen (
Populus tremuloides Michx.) [
37]. Chestnut, however, is most productive in high-light, high-nutrient environments [
13,
15]. These results suggest the need for nursery protocols designed specifically for chestnut, such as increasing fertilization rates to enhance growth.
4.3. Germination Patterns
This study found no relationship between nut size or weight and percent germination among the chestnuts, except for BC3F3 family D16, which also had the lowest nut weight of any family in the study. This outcome suggests that germination may be reduced for families with small chestnut seeds, but more research is needed before strong inferences can be made.
Regardless of generation and family, seed plots with higher germination averaged fewer FOLR and smaller RCD than those with lower germination, suggesting chestnuts in seed plots with lower germination faced less competition and therefore had greater amounts of resources, such as light, soil water and nutrients. The American parental species had both the best germination rate and the greatest mortality between germination and lifting. The higher mortality may have been caused by tighter spacing, and therefore increased competition, from high germination. Furthermore, the later into the spring a chestnut germinated, the less chance it had to survive and the fewer FOLR it developed, suggesting that those that germinated later faced more intense competition. If we had started measuring germination earlier in the season, (before 23 April), we may have seen a relationship between growing-degree days and height and RCD growth, and an improved relationship with number of FOLR.
This study found differences in probability of germination among chestnut generations and families, with the Chinese chestnut having lower germination compared to the American family, but the BC3F3 families having generally similar germination compared to the American chestnut. Differences may be due to generation or family effects; for instance, perhaps weevils prefer Chinese chestnuts to American chestnuts, decreasing the Chinese chestnut germination rate. Differences in germination, however, may be related to other factors. For example, unknown differences in seed collection or handling practices, such as variation in the amount of time seeds sat on the ground before collection, could be a contributing factor. Because percent germination did affect the growth of the seedlings, specifically RCD and FOLR, future studies should take germination rate into account when looking at differences in growth among species or families.
4.4. Temporal Growth Patterns
Daily growth rate did not display a consistent pattern from date to date (
Figure 2). The highest growth rates for American and BC
3F
3 families took place between 7 July and 2 August with a distinct drop in growth rate between 15 and 28 June, whereas Chinese chestnut seedlings did not show the same drop in growth rate for this period. No environmental conditions can be directly connected with the decreased growth rate for the American and four BC
3F
3 chestnut families. Seedlings were irrigated and fertilized regularly and temperatures were not abnormal during this period. It is possible that the variation in daily growth rates between Chinese chestnuts and American and backcross chestnuts over the growing season can be explained by the distribution of carbohydrate from sources to sinks. We hypothesize that American and backcross chestnut seedlings had exhausted most of the carbohydrate stored in their seed cotyledons by 28 June, causing a distinct decrease in daily growth. Chinese chestnuts, however, have larger seeds, which presumably continued to function as a source of carbohydrates. After June, we hypothesize the carbohydrate available from photosynthesis was greatly increased as the first flush of leaves matured and started to export photosynthate. No drop in growth was found in Chinese chestnuts, presumably because by the time the carbohydrates from their seeds was exhausted, they had already begun exporting large amounts of photosynthate from newly matured leaves.
Figure 2.
Family × period interaction for daily height growth. Values for BC3F3 were means of D2, D12 and D19 families. Growth among families differed for all periods except 9/14–10/4.
Figure 2.
Family × period interaction for daily height growth. Values for BC3F3 were means of D2, D12 and D19 families. Growth among families differed for all periods except 9/14–10/4.
Except for the drop in daily height growth among American and backcross families between 15 and 28 June, and the steady drop in daily height growth among all chestnut families after July, chestnut families exhibited fairly smooth growth patterns over their first growing season (see
Figure 2). In contrast, northern red oak exhibited several small increases in growth over the growing season, possibly associated with periods of episodic flushing. Northern red oak is a determinate species, meaning it exhibits distinct flushes of shoot expansion, followed by periods of growth lag [
38], whereas American chestnut is an indeterminate species.
5. Conclusions
Successful restoration of American chestnut will require the production of high quality seedlings that can overcome multiple challenges to seedling establishment, including competition from sprouts and other seedlings and browsing by deer [
39]. Poor-quality seedlings and direct seeded chestnuts have been found to be less successful than high quality seedlings in withstanding the competition present in recently-harvested sites [
39]. This study aimed to refine methods for producing high quality bare-root chestnut seedlings in nurseries. Results corroborate findings by Clark
et al. [
11] that larger chestnuts generally produced larger chestnut seedlings. This should not, however, lead nursery managers to discard smaller chestnuts before planting, as this practice may indirectly select for trees with greater Chinese heritage. More testing is needed to link field performance for desired American traits to nut size class before specific recommendations can be made.
The results of this study suggest that, overall, chestnuts from the BC3F3 generation grow similarly to American chestnuts. There is, nonetheless, substantial variation in growth variables among and within BC3F3 chestnut families. Chestnut breeders should keep track of family differences, and use these data to select superior performing families with good blight resistance for outplanting.
Results suggest that chestnuts that germinated earlier in the season have a better chance of survival than those that germinate later than mid-May. The late germinating seedlings may be shaded by the early germinating seedlings, or the early germinating seedlings may have more space to grow. The results also suggest that wider spacing in a nursery bed leads to larger chestnut seedlings, based on the number of FOLR and RCD. Further research is needed to refine seed spacing for chestnut growth.
Daily growth patterns showed that growth in American and backcross chestnuts slowed in the latter part of June. It may pay for nursery managers to adjust their fertilization regime to maximize mid-season growth in chestnuts.