Impacts of Frequent Burning on Live Tree Carbon Biomass and Demography in Post-Harvest Regrowth Forest
Abstract
:1. Introduction
2. Methods
2.1. Study Area and Experimental Design
2.2. Biomass and Demographic Measurements
2.3. Statistical Analysis
3. Results
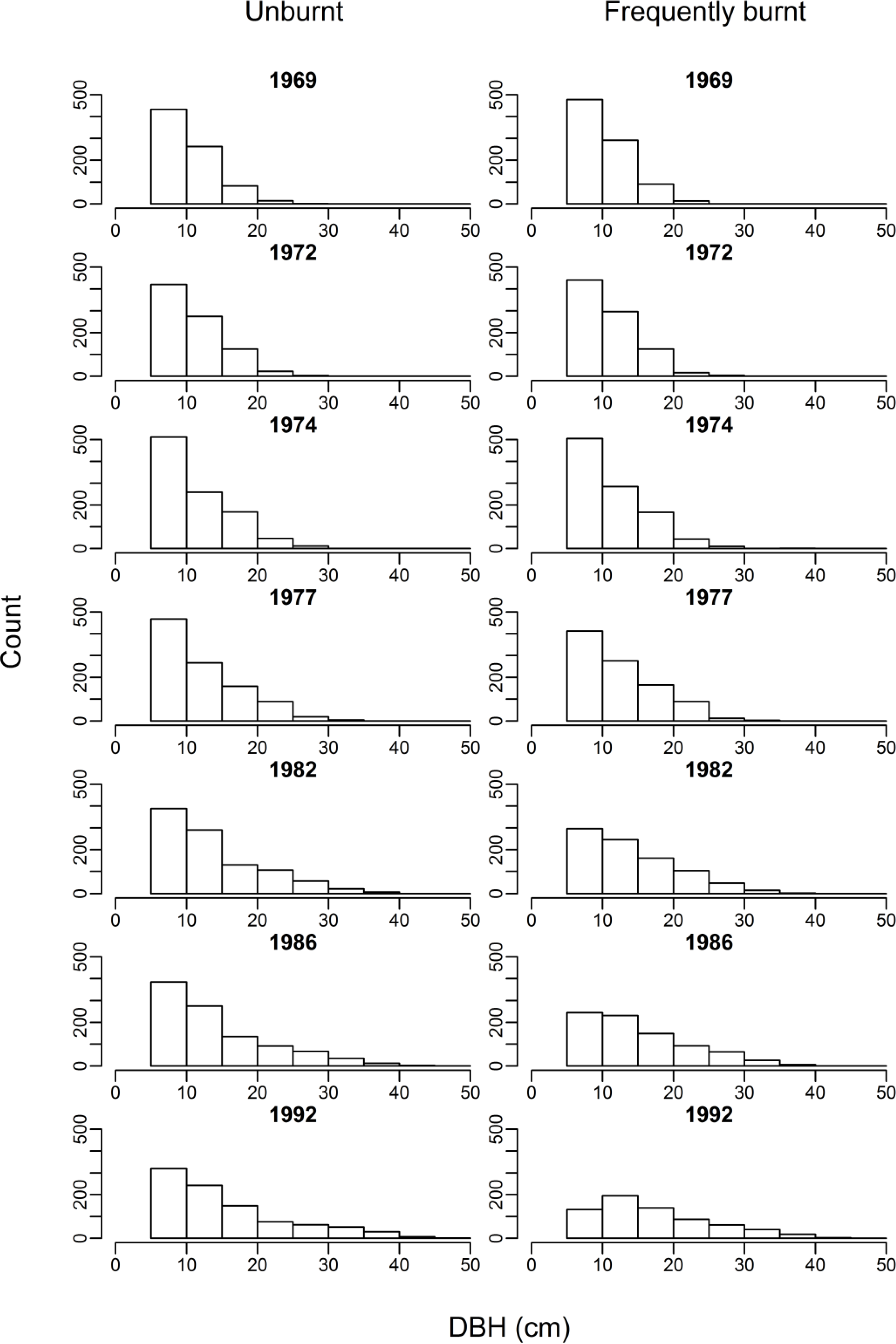
3.1. Biomass, Tree Density and Size


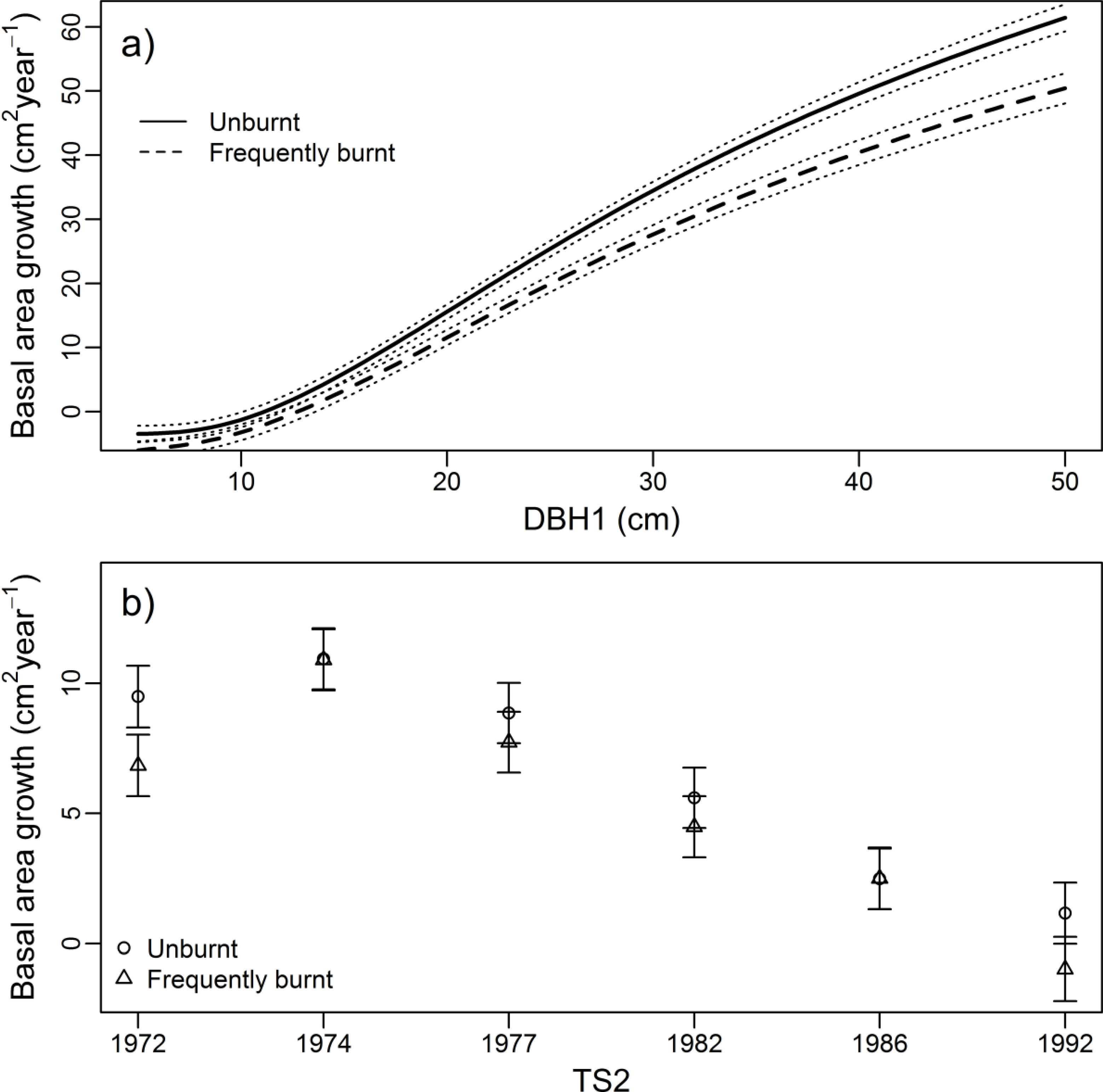
3.2. Growth Rate

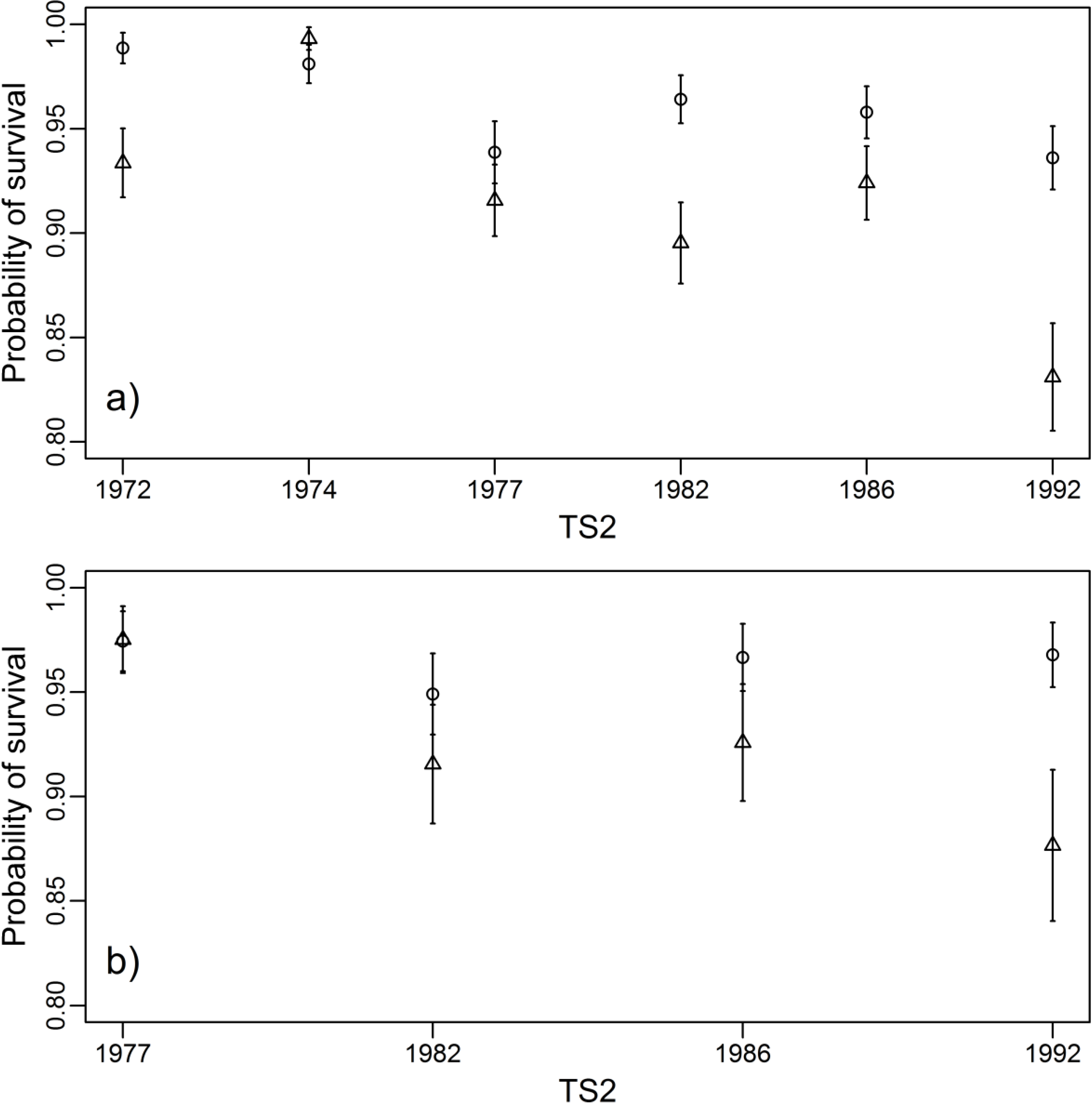
3.3. Survival
4. Discussion
4.1. Impacts of Frequent Burning on Carbon and Stand Structure
4.2. Implications of Fire Management and Future Fire Regimes for Carbon
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef]
- Luyssaert, S.; Schulze, E.D.; Borner, A.; Knohl, A.; Hessenmoller, D.; Law, B.E.; Ciais, P.; Grace, J. Old-growth forests as global carbon sinks. Nature 2008, 455, 213–215. [Google Scholar] [CrossRef]
- Roxburgh, S.H.; Wood, S.W.; Mackey, B.G.; Woldendorp, G.; Gibbons, P. Assessing the carbon sequestration potential of managed forests: A case study from temperate Australia. J. Appl. Ecol. 2006, 43, 1149–1159. [Google Scholar] [CrossRef]
- Williams, R.; Barrett, D.; Beringer, J.; Boer, M.; Bradstock, R.; Cary, G.; Cook, G.; Gill, A.; Hutley, L.; Keith, H.; et al. Fire regimes and carbon in Australian vegetation. In Flammable Australia: Fire Regimes, Biodiversity and Ecosystems in a Changing World; Bradstock, R., Gill, A., Williams, R., Eds.; CSIRO Publishing: Melbourne, Australia, 2012. [Google Scholar]
- Pekin, B.K.; Boer, M.M.; Macfarlane, C.; Grierson, P.F. Impacts of increased fire frequency and aridity on eucalypt forest structure, biomass and composition in southwest Australia. For. Ecol. Manag. 2009, 258, 2136–2142. [Google Scholar] [CrossRef]
- Bradstock, R.A. A biogeographic model of fire regimes in Australia: Current and future implications. Glob. Ecol. Biogeogr. 2010, 19, 145–158. [Google Scholar] [CrossRef]
- Archibald, S.; Lehmann, C.E.R.; Gómez-Dans, J.L.; Bradstock, R.A. Defining pyromes and global syndromes of fire regimes. Proc. Natl. Acad. Sci. USA 2013, 110, 6442–6447. [Google Scholar]
- Krawchuk, M.A.; Moritz, M.A. Constraints on global fire activity vary across a resource gradient. Ecology 2010, 92, 121–132. [Google Scholar] [CrossRef]
- Keeley, J.E.; Bond, W.J.; Bradstock, R.A.; Pausas, J.G.; Rundel, P.W. Fire in Mediterranean Ecosystems: Ecology, Evolution and Management. Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Bradstock, R.A.; Williams, J.E.; Gill, A.M. Flammable Australia: The Fire Regimes and Biodiversity of a Continent. Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Moritz, M.A. Spatiotemporal analysis of controls on shrubland fire regimes: Age dependency and fire hazard. Ecology 2003, 84, 351–361. [Google Scholar] [CrossRef]
- Gill, A.M.; Catling, P.C. Fire regimes and biodiversity of forested landscapes of southern Australia. In Flammable Australia: Fire Regimes and Biodiversity of a Continent; Bradstock, R.A., Williams, J.E., Gill, A.M., Eds.; Cambridge University Press: Cambridge, UK, 2002; pp. 351–369. [Google Scholar]
- Huston, M. Understanding the effects of fire and other mortality-causing disturbances on species diversity. In Fire in Ecosystems of South-west Western Australia: Impacts and Management; Abbott, I., Burrows, N., Eds.; Backhuys Publishers: Leiden, The Netherlands, 2003; pp. 37–70. [Google Scholar]
- Cary, G.J.; Bradstock, R.A.; Gill, A.M.; Williams, R.J. Global change and fire regimes in Australia. In Flammable Australia: Fire Regimes, Biodiversity and Ecosystems in a Changing World; Bradstock, R., Gill, A., Williams, R., Eds.; CSIRO Publishing: Collingwood, VIC, Canada, 2012. [Google Scholar]
- King, K.J.; de Ligt, R.M.; Cary, G.J. Fire and carbon dynamics under climate change in south-eastern Australia: Insights from FullCAM and FIRESCAPE modelling. Int. J. Wildland Fire 2011, 20, 563–577. [Google Scholar] [CrossRef]
- Penman, T.D.; Bradstock, R.A.; Price, O. Modelling the determinants of ignition in the Sydney Basin, Australia: Implications for future management. Int. J. Wildland Fire 2013, 22, 469–478. [Google Scholar] [CrossRef]
- Esplin, B.; Gill, A.M.; Enright, N.J. Report of the Inquiry into the 2002-2003 Victorian Bushfires. Department of Premier and Cabinet: Melbourne, Australia, 2003. [Google Scholar]
- Teague, B.; Mcleod, R.; Pascoe, S. 2009 Victorian Bushfires Royal Commission Final Report. Parliament of Victoria: Melbourne, Australia, 2010. [Google Scholar]
- Price, O.F.; Bradstock, R.A. Quantifying the influence of fuel age and weather on the annual extent of unplanned fires in the Sydney region of Australia. Int. J. Wildland Fire 2011, 20, 142–151. [Google Scholar] [CrossRef]
- Bradstock, R.A.; Cary, G.J.; Davies, I.; Lindenmayer, D.B.; Price, O.F.; Williams, R.J. Wildfires, fuel treatment and risk mitigation in Australian eucalypt forests: Insights from landscape-scale simulation. J. Environ. Manag. 2012, 105, 66–75. [Google Scholar] [CrossRef]
- Keith, D.A. Fire-driven extinction of plant populations: A synthesis of theory and review of evidence from Australian vegetation. Proc. Linn. Soc. N. S. W. 1996, 116, 37–78. [Google Scholar]
- Peterson, D.W.; Reich, P.B. Prescribed fire in oak savanna: Fire frequency effects on stand structure and dynamics. Ecol. Appl. 2001, 11, 914–927. [Google Scholar] [CrossRef]
- Murphy, B.P.; Russell-Smith, J.; Prior, L.D. Frequent fires reduce tree growth in northern Australian savannas: Implications for tree demography and carbon sequestration. Glob. Chang. Biol. 2010, 16, 331–343. [Google Scholar]
- Prior, L.D.; Brook, B.W.; Williams, R.J.; Werner, P.A.; Bradshaw, C.J.A.; Bowman, D.M.J.S. Environmental and allometric drivers of tree growth rates in a north Australian savanna. For. Ecol. Manag. 2006, 234, 164–180. [Google Scholar] [CrossRef]
- Bennett, L.T.; Aponte, C.; Tolhurst, K.G.; Löw, M.; Baker, T.G. Decreases in standing tree-based carbon stocks associated with repeated prescribed fires in a temperate mixed-species eucalypt forest. For. Ecol. Manag. 2013, 306, 243–255. [Google Scholar] [CrossRef]
- Guinto, D.F.; House, A.P.N.; Xu, Z.H.; Saffigna, P.G. Impacts of repeated fuel reduction burning on tree growth, mortality and recruitment in mixed species eucalypt forests of southeast Queensland, Australia. For. Ecol. Manag. 1999, 115, 13–27. [Google Scholar] [CrossRef]
- Williams, R.J.; Cook, G.D.; Gill, A.M.; Moore, P.H.R. Fire regime, fire intensity and tree survival in a tropical savanna in northern Australia. Aust. J. Ecol. 1999, 24, 50–59. [Google Scholar] [CrossRef]
- Ryan, C.M.; Williams, M. How does fire intensity and frequency affect miombo woodland tree populations and biomass? Ecol. Appl. 2010, 21, 48–60. [Google Scholar] [CrossRef]
- Penman, T.D.; York, A. Climate and recent fire history affect fuel loads in eucalyptus forests: Implications for fire management in a changing climate. For. Ecol. Manag. 2010, 260, 1791–1797. [Google Scholar] [CrossRef]
- Florence, R.G. Ecology and Silviculture of Eucalypt Forests. CSIRO Publishing: Collingwood, VIC, Australia, 1996; p. 413. [Google Scholar]
- Bradstock, R.A. Effects of large fires on biodiversity in south-eastern Australia: Disaster or template for diversity? Int. J. Wildland Fire 2008, 17, 809–822. [Google Scholar] [CrossRef]
- Gill, A.M. Bushfires and biodiversity in southern Australian forests. In Flammable Australia: Fire Regimes, Biodiversity and Ecosystems in a Changing World; Bradstock, R., Williams, R., Gill, A., Eds.; CSIRO Publishing: Melbourne, Australia, 2012. [Google Scholar]
- Port Macquarie Airport Station No. 060139. Available online: www.bom.gov.au (accessed on 11 March 2014).
- Christie, F.J.; York, A. No detectable impacts of frequent burning on foliar C and N or insect herbivory in an Australian eucalypt forest. Appl. Veg. Sci. 2009, 12, 376–384. [Google Scholar] [CrossRef]
- Keith, D. Ocean Shores to Desert Dunes: The Native Vegetation of New South Wales and the ACT. Department of Environment and Conservation: Sydney, Australia, 2004; p. 353. [Google Scholar]
- Collins, L.; Hawkesbury Institute for the Environment, University of Western Sydney, Richmond, NSW, Australia. Unpublished Work. 2013.
- Keith, H.; Barrett, D.; Keenan, R. Review of allometric relationships for estimating woody biomass for New South Wales, the Australian Capital Territory, Victoria, Tasmania and South Australia. In National Carbon Accounting System Technical Report No. 5b; Commonwealth of Australia: Canberra, Australia, 2000; p. 111. [Google Scholar]
- Chatto, K.; Bell, T.; Kellas, J. Effects of Repeated Low-intensity Fire on Tree Growth and Bark in a Mixed Eucalypt Foothill Forest in South-eastern Australia. Department of Sustainability and Environment: East Melbourne, Australia, 2003; p. 22. [Google Scholar]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists. Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Akaike, H. Information Theory as an extension of the maximum likelihood principle. In Proceedings of Second International Symposium on Information Theory; Akademiai Kiado: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-theoretic Approach. Springer: New York, NY, USA, 2002. [Google Scholar]
- Penman, T.D.; Lemckert, F.L.; Mahony, M.J. Meteorological effects on the activity of the giant burrowing frog (Heleioporus australiacus) in south-eastern Australia. Wildlife Res. 2006, 33, 35–40. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S-Plus. Springer Verlag: New York, NY, USA, 1994. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2010. [Google Scholar]
- Collins, L.; Hawkesbury Institute for the Environment, University of Western Sydney, Richmond, NSW, Australia. Unpublished Work. 2013.
- Murphy, B.P.; Lehmann, C.E.R.; Russell-Smith, J.; Lawes, M.J. Fire regimes and woody biomass dynamics in Australian savannas. J. Biogeogr. 2014, 41, 133–144. [Google Scholar] [CrossRef]
- Lawes, M.J.; Midgley, J.J.; Clarke, P.J. Costs and benefits of relative bark thickness in relation to fire damage: A savanna/forest contrast. J. Ecol. 2013, 101, 517–524. [Google Scholar] [CrossRef]
- Van Loon, A.P. Investigations into the Effects of Prescribed Burning on Young, Even-aged Blackbutt: Establishment and Preliminary Progress Report. Forestry Commission of New South Wales, Central Coast Research Centre: Taree, NSW, Australia, 1969; p. 49. [Google Scholar]
- Brennan, K.E.C.; Christie, F.J.; York, A. Global climate change and litter decomposition: More frequent fire slows decomposition and increases the functional importance of invertebrates. Glob. Chang. Biol. 2009, 15, 2958–2971. [Google Scholar] [CrossRef]
- Bastias, B.A.; Huang, Z.Q.; Blumfield, T.; Xu, Z.; Cairney, J.W.G. Influence of repeated prescribed burning on the soil fungal community in an eastern Australian wet sclerophyll forest. Soil Biol. Biochem. 2006, 38, 3492–3501. [Google Scholar] [CrossRef]
- Ximenes, F.; NSW Department of Primary Industries, North Parramatta, NSW, Australia. Unpublished Work. 2013.
- Bradstock, R.A.; Hammill, K.A.; Collins, L.; Price, O. Effects of weather, fuel and terrain on fire severity in topographically diverse landscapes of south-eastern Australia. Landsc. Ecol. 2010, 25, 607–619. [Google Scholar] [CrossRef]
- Montreal Process Implementation Group for Australia. Australia’s State of the Forests Report 2008. Bureau of Rural Sciences: Canberra, Australia, 2008; p. 250. [Google Scholar]
- Vivian, L.M.; Cary, G.J.; Bradstock, R.A.; Gill, A.M. Influence of fire severity on the regeneration, recruitment and distribution of eucalypts in the Cotter River Catchment, Australian Capital Territory. Austral Ecol. 2008, 33, 55–67. [Google Scholar] [CrossRef]
- Collins, L.; Bradstock, R.A.; Penman, T.D. Can precipitation influence landscape controls on wildfire severity? A case study within temperate eucalypt forests of south-eastern Australia. Int. J. Wildland Fire 2014, 23, 9–20. [Google Scholar] [CrossRef]
- Price, O.F.; Bradstock, R.A. The efficacy of fuel treatment in mitigating property loss during wildfires: Insights from analysis of the severity of the catastrophic fires in 2009 in Victoria, Australia. J. Environ. Manag. 2012, 113, 146–157. [Google Scholar] [CrossRef]
- Bradstock, R.A.; Boer, M.M.; Cary, G.J.; Price, O.F.; Williams, R.J.; Barrett, D.; Cook, G.; Gill, A.M.; Hutley, L.B.W.; Keith, H.; et al. Modelling the potential for prescribed burning to mitigate carbon emissions from wildfires in fire-prone forests of Australia. Int. J. Wildland Fire 2012, 21, 629–639. [Google Scholar] [CrossRef]
- Liedloff, A.C.; Cook, G.D. Modelling the effects of rainfall variability and fire on tree populations in an Australian tropical savanna with the flames simulation model. Ecol. Model. 2007, 201, 269–282. [Google Scholar] [CrossRef]
- Penman, T.D.; Kavanagh, R.P.; Binns, D.L.; Melick, D.R. Patchiness of prescribed burns in dry sclerophyll eucalypt forests in south-eastern Australia. For. Ecol. Manag. 2007, 252, 24–32. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Collins, L.; Penman, T.; Ximenes, F.D.A.; Binns, D.; York, A.; Bradstock, R. Impacts of Frequent Burning on Live Tree Carbon Biomass and Demography in Post-Harvest Regrowth Forest. Forests 2014, 5, 802-821. https://doi.org/10.3390/f5040802
Collins L, Penman T, Ximenes FDA, Binns D, York A, Bradstock R. Impacts of Frequent Burning on Live Tree Carbon Biomass and Demography in Post-Harvest Regrowth Forest. Forests. 2014; 5(4):802-821. https://doi.org/10.3390/f5040802
Chicago/Turabian StyleCollins, Luke, Trent Penman, Fabiano De Aquino Ximenes, Doug Binns, Alan York, and Ross Bradstock. 2014. "Impacts of Frequent Burning on Live Tree Carbon Biomass and Demography in Post-Harvest Regrowth Forest" Forests 5, no. 4: 802-821. https://doi.org/10.3390/f5040802
APA StyleCollins, L., Penman, T., Ximenes, F. D. A., Binns, D., York, A., & Bradstock, R. (2014). Impacts of Frequent Burning on Live Tree Carbon Biomass and Demography in Post-Harvest Regrowth Forest. Forests, 5(4), 802-821. https://doi.org/10.3390/f5040802




