1. Introduction
Organisms residing in the litter layer and within the upper layers of the soil in forests comprise a substantial portion of the biodiversity of these ecosystems [
1], and are engaged in such vital ecosystem functions as soil formation, decomposition, nutrient mobilization, regulation of fungal populations, predation, and soil mixing [
2]. Natural and anthropogenic disturbances can alter the abundance of these organisms, thereby altering the natural functions these organisms are responsible for in these ecosystems [
3,
4]. There is growing recognition of the potential utility of managing soil biota to develop a desirable soil structure [
5] and to maintain ecosystem services provided by healthy forests [
2]. However, surprisingly little is known of how the structure and function of the food webs that maintain soil productivity are affected by forest management practices that alter physical disturbances, change the composition of organic matter inputs, or modify the physical properties of soils [
1,
6].
The community composition of organisms in the litter layer and upper layers of the soil are mediated by numerous mechanisms including the supply of resources (
i.e., the timing, amount, and quality of inputs), the heterogeneity of the habitat, the interactions among organisms across trophic levels, factors that encourage or thwart the vertical migration of arthropods within the substrate, and abiotic factors [
1,
7]. Reductions in the amount of basal resources entering managed forests has been shown to decrease the diversity or abundance of arthropods that process organic inputs [
8,
9], initiating a negative feedback cycle toward lower site productivity [
6,
10,
11]. Forest management activities such as litter removal, may also abruptly shift trophic interactions, altering the dominance of species and changing the functioning of ecosystem processes [
12].
The need to understand how disturbances influence long-term resiliency in modified forest ecosystems is growing more urgent [
6]. Human-modified ecosystems are typically characterized by reduced resiliency to disturbances relative to natural ecosystems, because they are simplified and less functionally redundant [
13,
14]. Monoculture pine plantations, with lower biodiversity and less functional redundancy than naturally occurring ecosystems, are likely to be more prone to degradation in the wake of disturbances, with diminished capabilities of generating the ecosystem services for which they are maintained [
3,
4,
15,
16].
An increasingly common practice in the Southeastern U.S. is harvesting pine litter from plantation forests for sale to the nursery industry [
17,
18,
19]. Pine litter (called “pine straw”) is considered an attractive mulching material for landscaping because it reduces soil erosion, preserves soil moisture, moderates soil temperature, provides organic matter, and inhibits weed growth where applied [
17]. However, sites from which pine straw is harvested may suffer adverse effects. The practice of litter removal was banned from temperate European forests more than half a century ago following the discovery of the detrimental impacts this activity had on soil formation and nutrient cycling [
20]. In contrast, pine straw harvesting is currently growing in popularity in the U.S. For example, between 2000 and 2010, timber revenues dropped over 40% in the state of Georgia, while income received from pine straw collection increased five-fold [
21]. Raking contracts typically begin when stands are 6 to 10 years old, and are often negotiated for 5 years, providing a steady annual income to forest owners during the period prior to thinning [
21]. Despite the growing popularity of repeatedly raking pine stands, we are aware of no research on the effects of repeated litter removal on soil fauna.
Pine straw raking typically involves the removal of the upper layer of needles shortly after peak annual needlefall, to minimize the collection of partially decayed needles. Raking typically commences at the time of canopy closure, 6–10 years after stand establishment for most southern pine species [
22]. Removal of the protective layer of fresh needles exposes the soil in the harvested stand for nearly an entire year. Thus, pine straw raking not only removes large quantities of organic matter and nutrients [
23], but also leaves the soil exposed for many months, which can lead to increased soil erosion, reduced soil moisture, and increased soil bulk density [
24,
25,
26]. Raking for three consecutive years can cause near complete removal of organic residues at the soil surface, increased rain water runoff volume, and decreased infiltration rates [
17].
The purpose of this study is to examine the effects of repeated pine litter removal on soil fauna in north Florida, a region where the pine litter harvest is growing in popularity. Because changes to the terrestrial arthropod community were apparent following a single raking event in this region [
27], we predict that repeatedly raking pine straw will cause more extreme alterations to arthropod communities.
Our objectives are to (1) examine the effects of litter removal on the biodiversity of terrestrial arthropod communities in pine plantations; (2) compare how these effects differ among arthropod groups; (3) determine if these effects differ among plantations with different species of pine; and (4) assess whether consistent trends over time are apparent after repeated rakings. Our analyses investigate faunal groups expected to differ in recovery ability (due to differences in life-history traits, such as dispersal and reproductive strategy) and emphasize organisms expected to play dominant roles in these ecosystems, including Araneae (mobile predators, expected to be highly responsive to changes in habitat structure), Collembola (the most abundant order of arthropods of the forest floor, responsible for decomposition and nutrient mobilization), Coleoptera (the order contributing the greatest biomass and species richness to the forest floor faunal community, responsible for a variety of roles ranging from predation to detritivory), and Hymenoptera (one of the most ubiquitous terrestrial arthropods).
2. Experimental Section
2.1. Study Site
The study site was located in the Southeastern Coastal Plain, at the Suwannee Valley Agricultural Extension Station in Live Oak, Florida, at 30°18' N and 82°54' W. Annual mean temperature during the study period was 19.2 °C (66.5 °F), and annual precipitation was 107 cm (42.0 in) (Florida Automated Weather Network). The soil in this area is a Foxworth Fine Sand association, which is a moderately well to somewhat excessively drained soil.
Three blocks of loblolly (Pinus taeda), longleaf (P. palustris) and slash (P. elliottii) pine stands were established in a former bahia-grass (Paspalum notatum) pasture in 2000. Each block was 63 m × 296 m (210 ft × 960 ft) and contained one stand of each pine species planted at a 3 m × 2.1 m (10 ft × 7 ft) spacing. The entire area was fertilized in 2002, 2005 and March 2007 (109 kg/ha or 97 lb/acre N). During early development, herbicide was also periodically administered in longleaf stands to reduce competition from herbaceous vegetation, because the pine canopy cover was sparse. We divided each of the nine stands in half (three stands per species of pine), and randomly selected which half of the resulting 18 plots would receive raking treatments. These stands were raked manually each winter after needlefall peaked (December 2007, December 2008, and February 2010).
2.2. Arthropod Sampling
In each of the 18 plots we installed a grid of four pitfall traps: two traps that were 9.5 cm in diameter and 11.5 cm in depth and two that were 8.0 cm in diameter and 10.5 cm in depth. One pitfall trap of each size was located within the rows of pines and the other between the rows. Each pitfall trap consisted of two cups placed in a hole in the soil with the rim of the upper cup flush with the soil surface. Only the upper cup was removed when arthropods were collected; the bottom cup remained in place to limit local soil disturbance.
Once per month during January, April, July, and October of each year (2008, 2009 and 2010), we filled each trap with a 50/50 mixture of propylene glycol/water to a depth of 3 cm. We installed a roof over the top of each trap using four long nails to support a ceramic tile to prevent rainwater from getting in and to reduce heating from direct sunlight. Traps remained open for five consecutive days, after which we strained arthropods from the antifreeze solution, rinsed them and placed them in a Whirl-Pak bag in a 50/50 solution of ethanol/water. We then placed a lid directly over each trap to prevent the capture of any organisms until the next sampling period began. The January, 2010, sampling period was skipped, due to unplanned delays in implementing the raking treatment this winter.
Although it is widely recognized that data from pitfall trapping can have biases, this technique is one of the most versatile, useful, and most frequently used arthropod sampling methods [
28]. It is commonly recognized that pitfall trap catches represent a composite measure of activity and abundance and, therefore, may not be useful for assessing differences in abundance between species with very different activity levels. However, pitfall trapping has been shown to provide reliable insight into changes in abundance within arthropod groups over time and, thus, was considered well suited for the research questions of interest in this study [
29,
30]. We took several steps to ensure our sampling acquired high quality data. First, we installed numerous traps per plot to ensure high rates of capture; capture rates are known to be higher with the use of several smaller traps rather than fewer large traps [
28,
30]. We used two different sized traps to increase the probability of catching invertebrates of all sizes; small traps are known to be more efficient at catching small species and larger traps at catching larger species [
31,
32]
. The size of both traps was selected so that it was near the modal size of the traps reported in the literature [
28], so that the results would be comparable with those of other studies. A killing/preserving solution was placed in each trap to prevent predation of smaller invertebrates and to reduce the probability of escape by more mobile individuals [
28].
We counted and sorted captured organisms to the level of order. Several ‘‘focal’’ orders were further sorted into lower taxonomic groupings (Araneae, Coleoptera, Collembola and Hymenoptera). These four focal orders were selected because of their dominance in the litter layer in terms of abundance and/or biomass, as well as the diversity of ecological roles in which they are involved. Araneae were categorized into two functional groups according to Ubick
et al. [
33]: wandering-hunters (Anyphaenidae, Corinnidae, Gnaphosidae, Lycosidae, Miturgidae, Oxyopidae, Pisauridae, and Salticidae), and web-weavers (Araneidae, Dictynidae, Linyphiidae, Mysmenidae, Theridiosomatidae, and Uloboridae). Coleoptera were categorized into four functional groups according to Lawrence
et al. [
34]: predators (Cantharidae, Carabidae, Cucujidae, Histeridae and Staphylinidae), fungivores (Anthribidae, Cryptophagidae and Leiodidae), detritivores (Nitidulidae, Scarabaeidae, Silvanidae and Tenebrionidae), and phytophages (Curculionidae, Elateridae, Lyctidae and Scolytidae). Collembola were sorted by family: Entomobryidae, Isotomidae, Sminthuridae and Tomoceridae. Hymenoptera in the family Formicidae were sorted by subfamily: Formicinae and Myrmicinae.
2.3. Statistical Analyses
2.3.1. Biodiversity and Abundance of Orders
We used linear mixed-effects models [
35] to evaluate the main and interactive influence of raking treatment, timber species, and year on three aspects of ground dwelling arthropod community composition and the abundance of 8 dominant orders. Raking treatment (yes or no), timber species (loblolly, longleaf or slash pine), year (categorical variable 1, 2 or 3) and their interaction terms were included as fixed effects in full models. For focal orders sorted to family, subfamily or functional group (
i.e., Araneae, Collembola, Coleoptera, and Hymenoptera) we included Finer Taxonomic Resolution (FTR) as a categorical term with the associated interaction terms as fixed effects in full models. Season, block and plot were modeled as random effects. Including random effects in the models allowed us to account for the split-plot design and repeated measurements taken across seasons. An iterative process was used to identify the most parsimonious model for each analysis. We compared the log-likelihood ratio from the full model to that from a reduced model from which the least informative interaction term or variable had been dropped (
i.e., the model with the smallest sum of squares). This process was repeated until the likelihood ratio test (henceforth LLRT) was significantly different, indicating the best model had been identified [
36]. All analyses were conducted in R version 3.0.2 [
37], using lme4 [
38]. Post-hoc Tukey’s honest significant differences (Tukey HSDs) were calculated using multcomp 1.3 [
39] to identify significant differences between raking treatment, years or timber type.
The two aspects of ground dwelling arthropod community composition used as response variables were ordinal diversity (Shannon’s Index) and ordinal richness. Community composition estimates were calculated from the ordinal abundance using R version 3.0.2 and vegan [
40]. Winged Diptera, Hymenoptera, Lepidoptera, Psocoptera, Thysanoptera and Trichoptera were excluded from the analysis as these were likely volant individuals assumed to be attracted to the antifreeze rather than residents of the litter layer. The abundance of the eight most dominant orders (Acari, Araneae, Blattodea, Coleoptera, Collembola, Hemiptera, Hymenoptera and Orthoptera) were also modeled as response variables. Because many traps contained no members of each order, we pooled data across the four traps within each plot to obtain a single value for each treatment plot each sampling period for all analyses. The abundance of Araneae, Collembola, Hemiptera, and Hymenoptera were log transformed to meet the assumptions of normality and modeled linearly. Because the abundance of Acari, Blattodea, Coleoptera, and Orthoptera could not be transformed to meet the assumption of normality for linear models, we modeled the presence/absence of these arthropods within the combined traps using logistic regression with binomial errors.
3. Results and Discussion
We collected 28,595 individual arthropods. The majority were Collembola (26%), Hymenoptera (16%), Araneae (15%), Diptera (13%), Coleoptera (10%) and Orthoptera (5%). Hemiptera, Blattodea, and Acari each represented <5% each of the total sample. Other orders collected included Dermaptera, Geophilomorpha, Isoptera, Lepidoptera, Lithobiomorpha, Mantodea, Microcoryphia, Neuroptera, Opiliones, Psocoptera, Scolopendromorpha, Spirobolida, Thysanoptera and Trichoptera.
3.1. Biodiversity
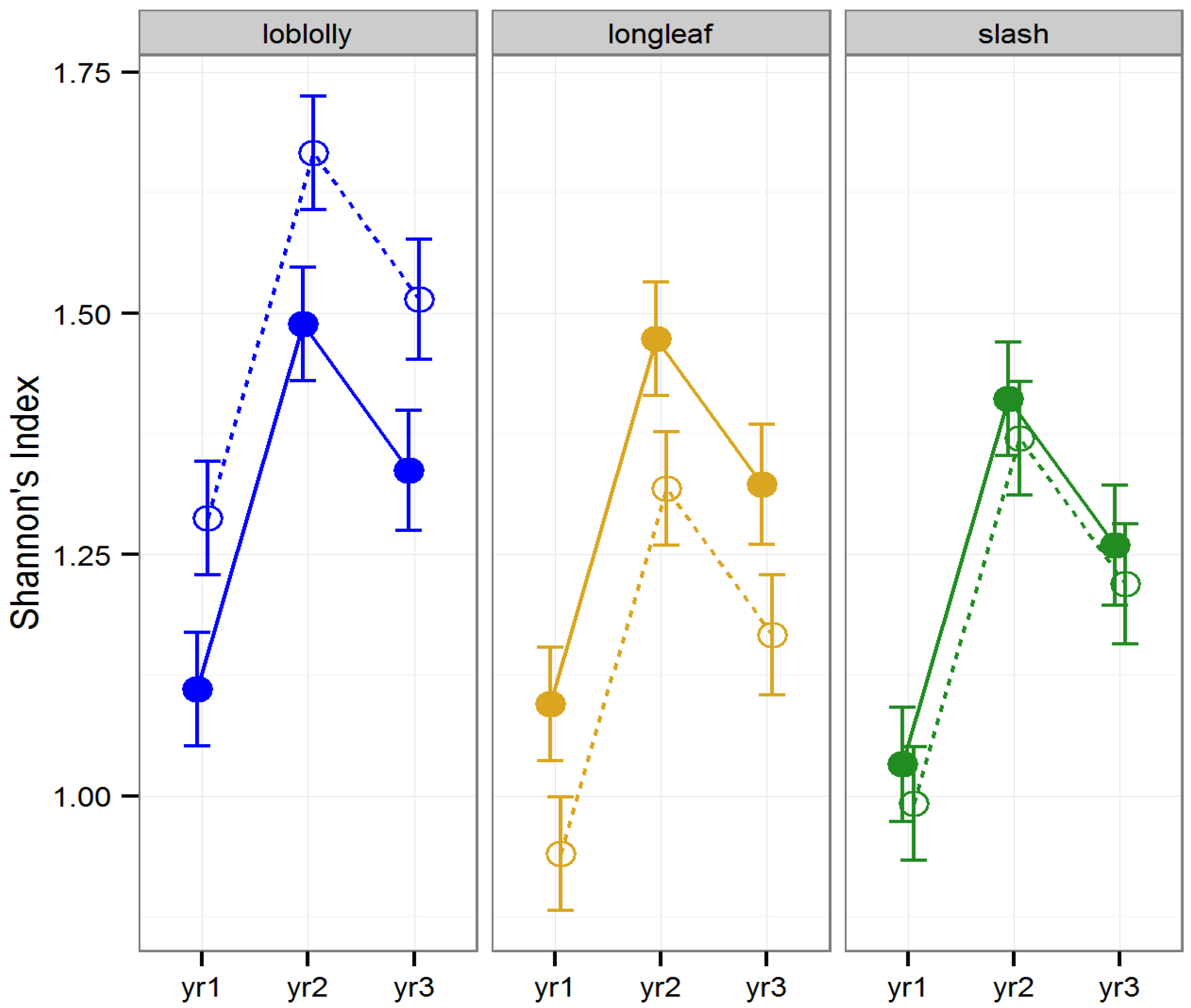
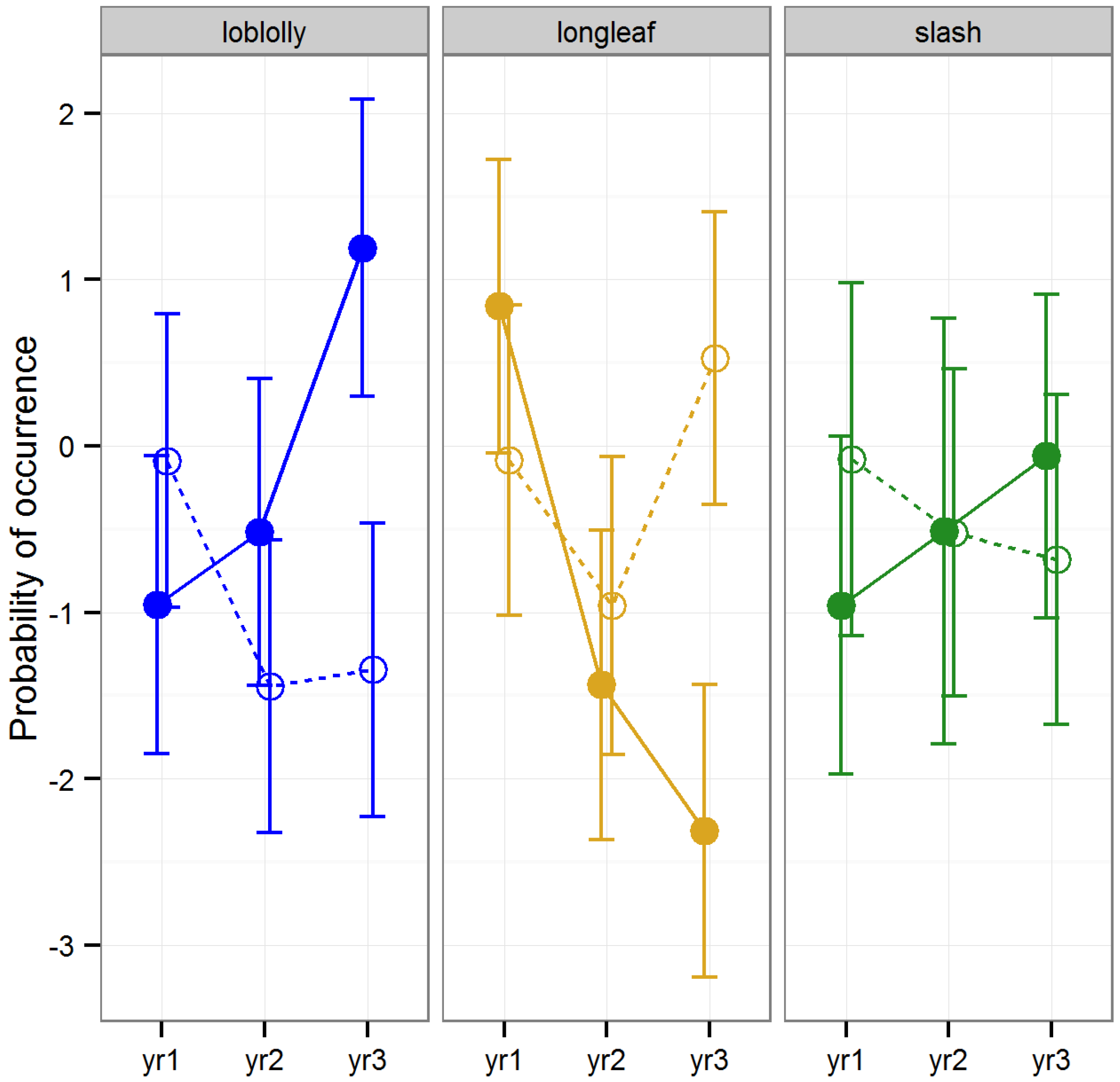
Diversity (as measured by Shannon’s Index) and ordinal richness were not altered by “raking (LLRT
p > 0.1). However, Shannon’s Index differed by timber type (
F2, 22 = 8.42,
p = 0.001) differed among years (
F2, 157 = 29.44,
p < 0.001), and the effect of raking on Shannon’s Index differed across years (
F2, 157 = 5.32,
p = 0.006;
Figure 1). Ordinal diversity did not differ among timber types (
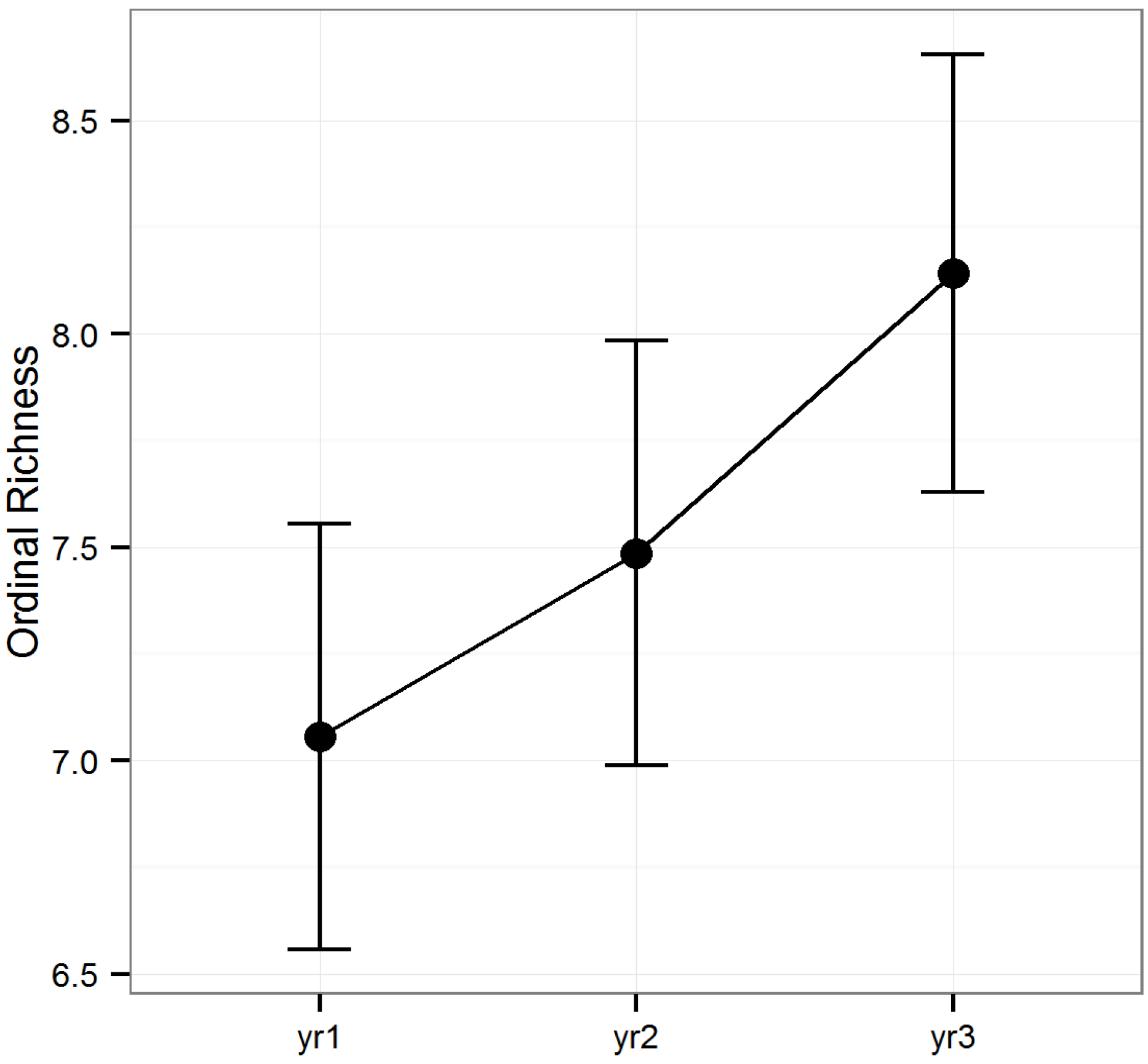
p > 0.1), but did differ among years (
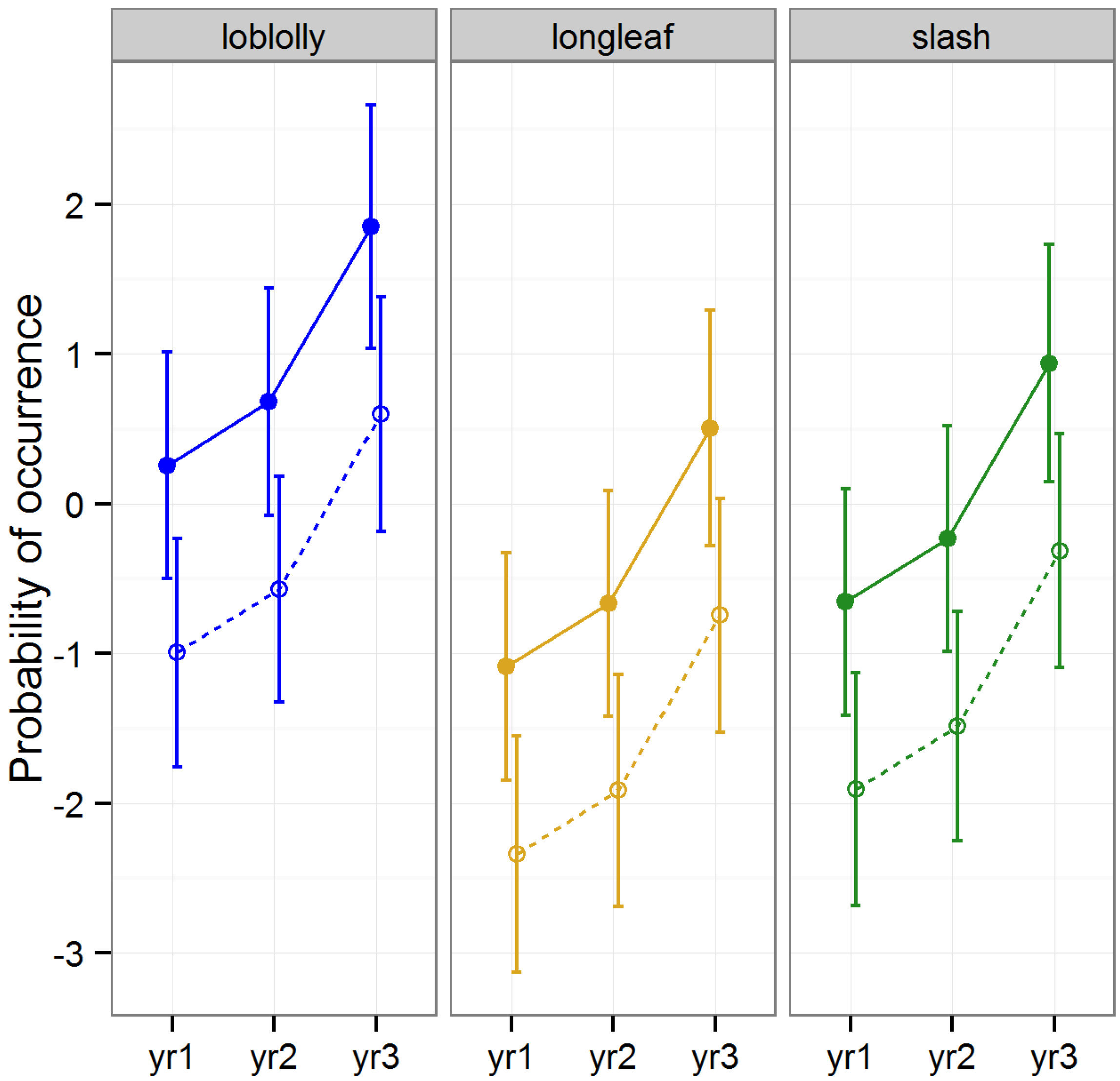
F2, 160 = 7.56,
p < 0.001;
Figure 2). Diversity peaked during year two, while ordinal richness increased gradually over time in all stands investigated.
The lack of influence of litter removal on general measures of arthropod biodiversity has been reported in another study involving manipulation of the litter layer [
43,
44]. Metrics that describe community composition or finer scales of taxonomic resolution often provide more insight into the effects of disturbances than these more general metrics, due to their greater sensitivity to small changes in low abundances of various taxa.
Figure 1.
Predicted means and standard errors for biodiversity of arthropods collected during three years of seasonal pitfall trapping in pine plantations in North Florida as measured by Shannon’s Index of diversity against fixed effects from the most parsimonious model based on log-likelihood ratio tests; raked stands are displayed with open circles and dashed lines, intact stands with solid circles and lines; 2008 is yr1, 2009 is yr2 and 2010 is yr3.
Figure 1.
Predicted means and standard errors for biodiversity of arthropods collected during three years of seasonal pitfall trapping in pine plantations in North Florida as measured by Shannon’s Index of diversity against fixed effects from the most parsimonious model based on log-likelihood ratio tests; raked stands are displayed with open circles and dashed lines, intact stands with solid circles and lines; 2008 is yr1, 2009 is yr2 and 2010 is yr3.
Figure 2.
Predicted means and standard errors for the biodiversity of arthropods collected during three years of seasonal pitfall trapping in pine plantations in North Florida as measured by ordinal richness against fixed effects from the most parsimonious model based on log-likelihood ratio tests; 2008 is yr1, 2009 is yr2 and 2010 is yr3.
Figure 2.
Predicted means and standard errors for the biodiversity of arthropods collected during three years of seasonal pitfall trapping in pine plantations in North Florida as measured by ordinal richness against fixed effects from the most parsimonious model based on log-likelihood ratio tests; 2008 is yr1, 2009 is yr2 and 2010 is yr3.
3.2. Abundance of Orders
Complex patterns became apparent when we teased apart changes in the individual orders that comprise the composite biodiversity measures. Removal of the litter layer affected the abundance or occurrence of seven of the eight dominant orders of arthropods (
Table 1 and
Table 2). In addition, the abundance or occurrence of arthropods differed among timber species for four of the eight orders investigated, and differences among functional groups, families, or subfamilies were significant for all four orders among which these patterns of higher taxonomic resolution were investigated (Araneae, Coleoptera, Collembola and Hymenoptera;
Table 1 and
Table 2).
3.2.1. Abundance of Araneae
The effect of raking on the abundance of Araneae varied according to year and functional groups (
Table 1,
Figure 3). The abundance of web weavers, the functional group less frequently encountered, was similar between raked and intact stands in all cases. Web-weaving spiders are low-mobility predators that use an energetically costly strategy to capture prey [
45]. Forests with a deep litter layer would be expected to furnish a quality habitat with a variety of attachment points for webs, whereas forests with limited quantities of litter would be expected to provide a lower quality habitat [
12]. Surprisingly, we found that web-weaver abundance did not respond strongly to pine litter removal. It has been posited that web-weavers consider structural features when making habitat selection decisions, but foraging success later dictates decisions to move away from a selected site [
45]. The manual raking we implemented may have left just enough litter that web-weavers were able to remain in raked stands and catch prey items that became more vulnerable in the open conditions caused by litter removal. Alternatively, the dispersal of unsuccessful web weavers in raked stands may have increased our capture rates in these stands relative to the capture rates of non-dispersing web weavers in stands with intact litter layers.
The abundance of the other major functional group, wandering-hunters, was significantly lower in raked than intact stands of all three species of pine during year 3 (Tukey HSD
p < 0.001). Wandering-hunters increased in abundance greatly across all pine stands with intact litter layers between years 2 and 3, but showed only a moderate increase in raked stands during this same time period. Spiders that engage in active hunting (
i.e., those that do not build webs) rely on structurally complex habitats to provide a diversity of prey items along with options to conceal themselves from potential prey [
46]. This may explain why the abundance of wandering-hunters increased at a faster rate in stands with intact layers of litter than those subject to repeated rakings.
Table 1.
ANOVA table for the most parsimonious model explaining the effects of pine straw raking on terrestrial arthropod abundance based on significant log-likelihood ratio tests (LLRT < 0.05); fixed effects (the source of variation), degrees of freedom (df), F-ratio (F), and significance levels for the main effects and interactions including raking (pine straw intact or raked), timber type (loblolly, slash or longleaf pine), year (2008, 2009, or 2010), and finer taxonomic resolution (FTR); significant effects are in bold.
Table 1.
ANOVA table for the most parsimonious model explaining the effects of pine straw raking on terrestrial arthropod abundance based on significant log-likelihood ratio tests (LLRT < 0.05); fixed effects (the source of variation), degrees of freedom (df), F-ratio (F), and significance levels for the main effects and interactions including raking (pine straw intact or raked), timber type (loblolly, slash or longleaf pine), year (2008, 2009, or 2010), and finer taxonomic resolution (FTR); significant effects are in bold.
| | Araneae | Collembola | Hemiptera | Hymenoptera |
|---|
| Source of variation | df | F | p | df | F | p | df | F | p | df | F | p |
| Raking | 1, 347 | 0.89 | 0.347 | 1, 727 | 4.78 | 0.029 | 1, 159 | 11.10 | 0.001 | 1, 341 | 58.73 | <0.001 |
| Timber type | 2, 22 | 2.51 | 0.104 | 2, 22 | 1.99 | 0.160 | . | . | . | 2, 22 | 17.90 | <0.001 |
| Year | 2, 347 | 22.45 | <0.001 | 2, 727 | 45.66 | <0.001 | 2, 159 | 17.46 | <0.001 | 2, 341 | 17.69 | <0.001 |
| FTR | 1, 347 | 158.23 | <0.001 | 3, 727 | 18.41 | <0.001 | . | . | . | 1, 341 | 148.41 | <0.001 |
| Raking × timber | . | . | . | . | . | . | . | . | . | 2, 341 | 7.63 | <0.001 |
| Raking × year | 2, 347 | 6.31 | 0.002 | 2, 727 | 2.43 | 0.089 | . | . | . | 2, 341 | 3.44 | 0.033 |
| Raking × FTR | 1, 347 | 5.85 | 0.016 | 3, 727 | 7.00 | <0.001 | . | . | . | 1, 341 | 22.24 | <0.001 |
| Timber × year | . | . | . | . | . | . | . | . | . | 4, 341 | 2.51 | 0.041 |
| Timber × FTR | 2, 347 | 3.21 | 0.042 | 6, 727 | 3.15 | 0.005 | . | . | . | 2, 341 | 9.45 | <0.001 |
| Year × FTR | 2, 347 | 22.63 | <0.001 | 6, 727 | 9.69 | <0.001 | . | . | . | 2, 341 | 6.97 | <0.001 |
| Rake × year × FTR | 2, 347 | 3.05 | 0.049 | 6, 727 | 3.08 | 0.006 | . | . | . | 2, 341 | 5.74 | 0.004 |
Table 2.
ANOVA table for the most parsimonious model explaining the effects of pine straw raking on terrestrial arthropod occurrence based on significant log-likelihood ratio tests (LLRT < 0.05); fixed effects (the source of variation), degrees of freedom (df), F-ratio (F), and significance levels for the main effects and interactions including raking (pine straw intact or raked), timber species (loblolly, slash or longleaf pine), year (2008, 2009, or 2010), and finer taxonomic resolution (FTR); significant effects are in bold.
Table 2.
ANOVA table for the most parsimonious model explaining the effects of pine straw raking on terrestrial arthropod occurrence based on significant log-likelihood ratio tests (LLRT < 0.05); fixed effects (the source of variation), degrees of freedom (df), F-ratio (F), and significance levels for the main effects and interactions including raking (pine straw intact or raked), timber species (loblolly, slash or longleaf pine), year (2008, 2009, or 2010), and finer taxonomic resolution (FTR); significant effects are in bold.
| | Blattodea | Coleoptera | Orthoptera |
|---|
| Source of variation | df | F | p | df | F | p | df | F | p |
| Raking | 1, 159 | 8.84 | 0.003 | 1, 750 | 3.42 | 0.065 | 1, 750 | 1.02 | 0.315 |
| Timber type | 2, 22 | 2.48 | 0.107 | . | . | . | 2, 22 | 2.02 | 0.156 |
| Year | 2, 159 | 6.39 | 0.002 | 2, 750 | 22.80 | <0.001 | 2, 750 | 30.84 | <0.001 |
| FTR | . | . | . | 3, 750 | 15.13 | <0.001 | . | . | . |
| Raking × timber | . | . | . | . | . | . | 2, 750 | 5.73 | 0.003 |
Figure 3.
Predicted means and standard errors of Araneae abundance (log transformed) during three years of seasonal pitfall trapping in pine plantations in North Florida against fixed effects from the most parsimonious model based on log-likelihood ratio tests; raked stands are displayed with open circles and dashed lines, intact stands with solid circles and lines; 2008 as yr1, 2009 as yr2 and 2010 as yr3.
Figure 3.
Predicted means and standard errors of Araneae abundance (log transformed) during three years of seasonal pitfall trapping in pine plantations in North Florida against fixed effects from the most parsimonious model based on log-likelihood ratio tests; raked stands are displayed with open circles and dashed lines, intact stands with solid circles and lines; 2008 as yr1, 2009 as yr2 and 2010 as yr3.
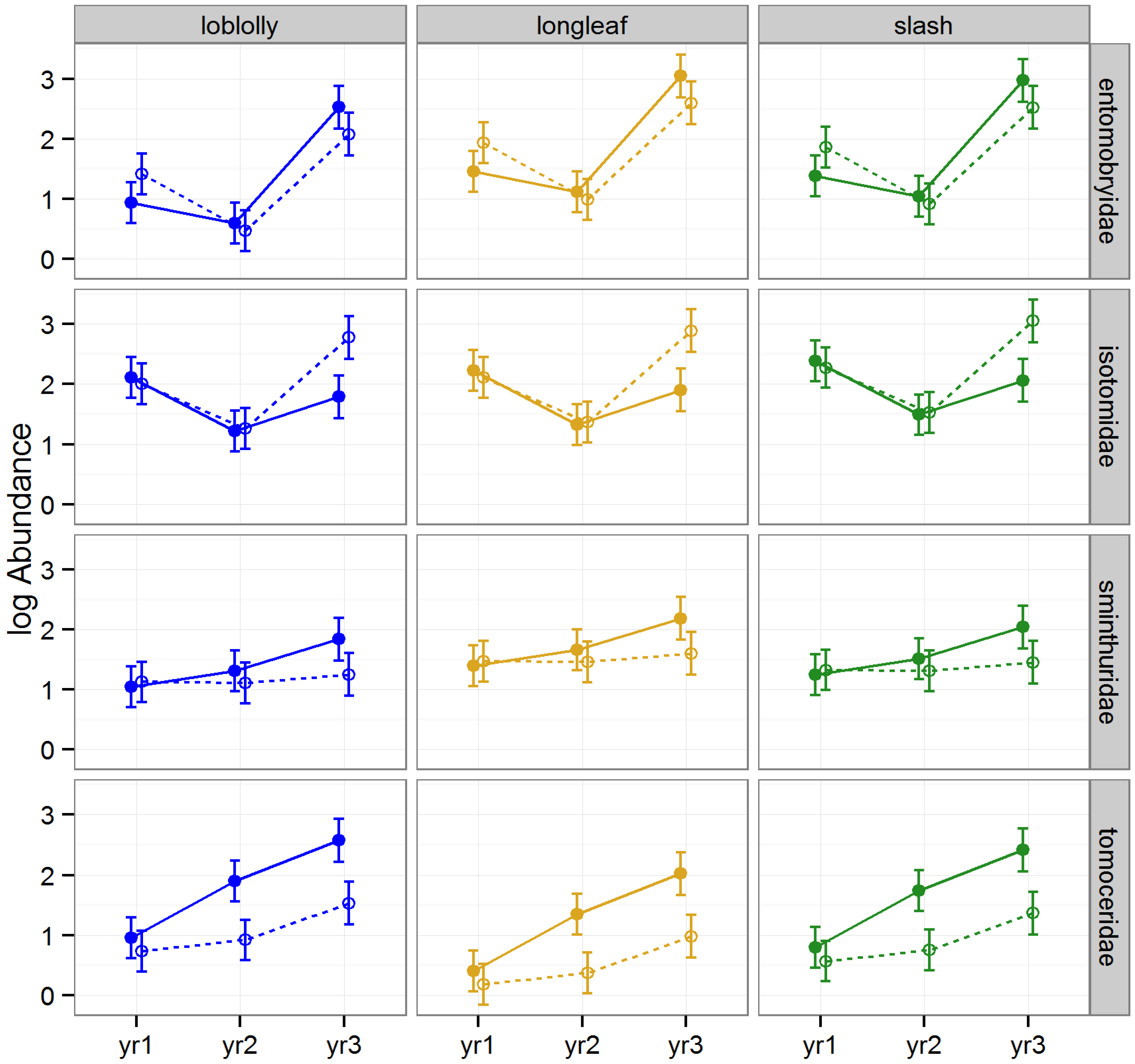
3.2.2. Abundance of Collembola
The effect of raking on the abundance of Collembola varied significantly by year, and amongst families (rake × year × FTR,
p = 0.006), but did not significantly differ among timber types (LLRT = 0.562;
Table 1,
Figure 4). Abundance was significantly lower in raked than intact stands for Tomoceridae in 2009 and 2010 (Tukey HSDs,
p = 0.001); in contrast, abundance was higher in raked than intact stands for Isotomidae in 2010 (Tukey HSD,
p = 0.017,
Figure 4).
Figure 4.
Predicted means and standard errors of Collembola abundance (log transformed) during three years of seasonal pitfall trapping in pine plantations in North Florida against fixed effects from the most parsimonious model based on log-likelihood ratio tests; raked stands are displayed with open circles and dashed lines, intact stands with solid circles and lines; 2008 as yr1, 2009 as yr2, and 2010 as yr3.
Figure 4.
Predicted means and standard errors of Collembola abundance (log transformed) during three years of seasonal pitfall trapping in pine plantations in North Florida against fixed effects from the most parsimonious model based on log-likelihood ratio tests; raked stands are displayed with open circles and dashed lines, intact stands with solid circles and lines; 2008 as yr1, 2009 as yr2, and 2010 as yr3.
Springtails are highly abundant, low-mobility microarthropods at the bottom of food webs [
47], where they are responsible for mineralizing soil nutrients. Because of their relative abundance and their role as decomposers, they have a high potential to influence site productivity. Short-term investigations have shown increases in springtail abundance in response to organic matter addition in agricultural areas [
48] and forests [
11,
12], as well as decreases in abundance in response to disturbances such as tillage in agricultural systems [
48] and litter removal in forest systems [
49,
50]. However, longer-term studies have demonstrated fairly quick recovery in abundance following disturbances [
51,
52]. The abundance of springtails in the litter layer is closely linked with soil moisture and temperature [
12], two factors that are likely to be altered by the removal of the litter layer.
We found that raking generally caused a decrease in abundance among springtails: the gap between springtail abundance in raked
versus intact stands was particularly evident among Tomoceridae during later years following repeated rakings, and although not yet significant, this gap seemed to be growing over time among Sminthuridae as well. This, coupled with the widening gap in abundance of Isotomidae evident by year three, with higher abundance in raked than intact stands, suggests that this order was undergoing shifts in dominance among families in response to the changing conditions imposed by repeated litter removal (
i.e., the abundance of Tomoceridae was increasing as the abundance of Isotomidae was decreasing). Contrary to our findings, Entomobryidae and Sminthuridae have previously been linked to disturbed forest litter (we found no significant differences in abundance between raked and intact stands during any year for these families) and Isotomidae to undisturbed forest litter [
53] (we found an increase in abundance in raked
versus intact stands by year 3). Entomobryidae has been considered less sensitive to disturbance than Isotomidae [
50], whereas our findings suggest that repeated disturbance actually increased the abundance of Isotomidae. Clearly, additional research is needed on the effects of litter removal on this enigmatic taxon.
3.2.3. Abundance of Hemiptera
The abundance of Hemiptera varied by raking treatment and year, but was similar across timber types (LLRT = 0.851;
Table 1,
Figure 5). The abundance was significantly higher in raked than intact stands (Tukey HSD
p < 0.001) and increased significantly as the years progressed (Tukey HSDs
p < 0.012).
Hemiptera typically occupy mid-trophic levels, as they are primarily phytophagous. Their increase in abundance in response to raking is somewhat surprising, given their tendency to remain constant in the wake of disturbances, such as gap creation due to wind damage or salvage logging [
54] or to a decline in the wake of repeated disturbances to the litter layer [
55].
Figure 5.
Predicted means and standard errors of Hemiptera abundance during three years of seasonal pitfall trapping in pine plantations in North Florida against fixed effects from the most parsimonious model based on log-likelihood ratio tests; raked stands are displayed with open circles and dashed lines, intact stands with solid circles and lines; 2008 as yr1, 2009 as yr2 and 2010 as yr3.
Figure 5.
Predicted means and standard errors of Hemiptera abundance during three years of seasonal pitfall trapping in pine plantations in North Florida against fixed effects from the most parsimonious model based on log-likelihood ratio tests; raked stands are displayed with open circles and dashed lines, intact stands with solid circles and lines; 2008 as yr1, 2009 as yr2 and 2010 as yr3.
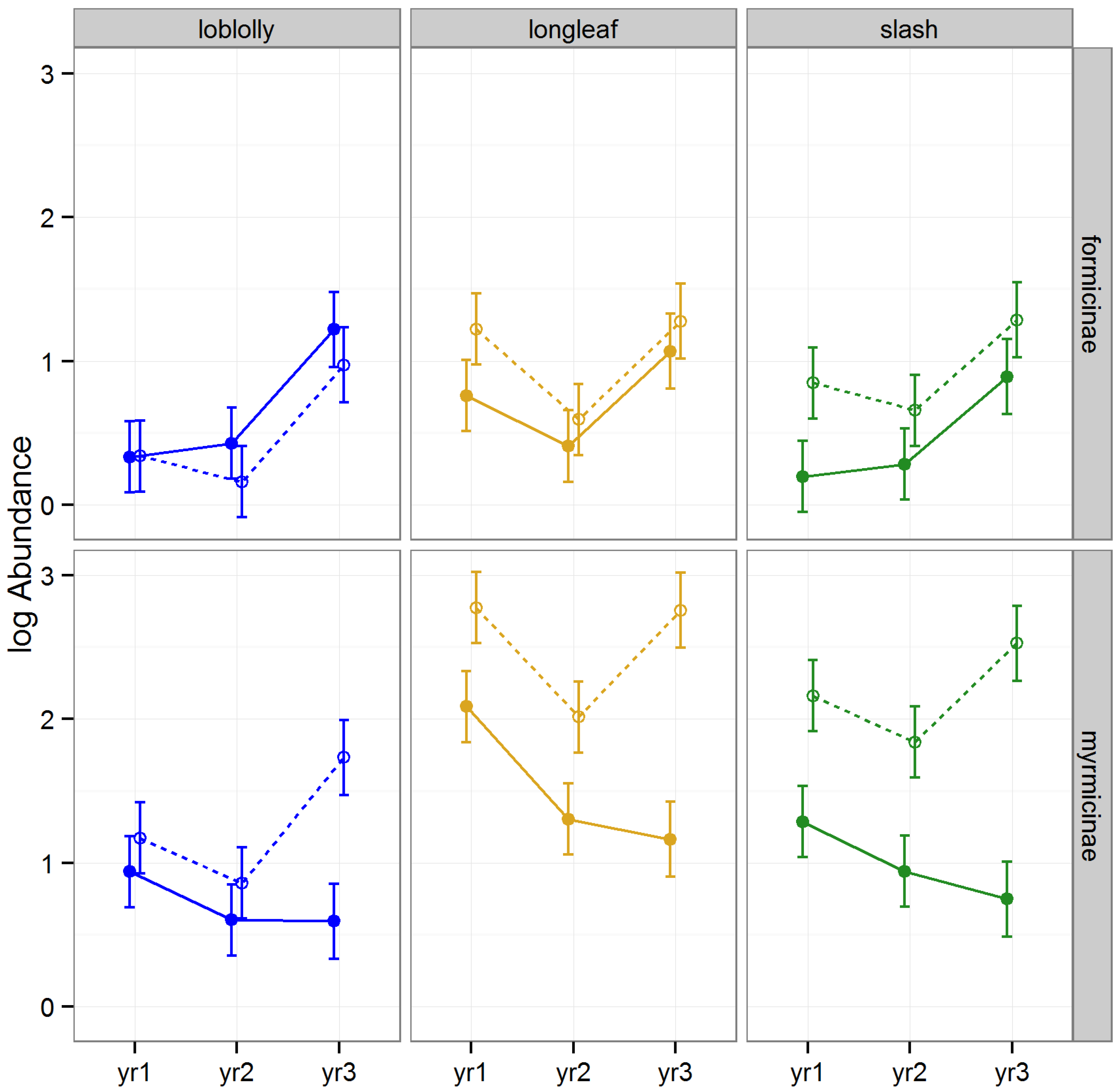
3.2.4. Abundance of Hymenoptera
The abundance of Hymenoptera was altered by raking, differed among timber species, differed among subfamilies, and the effect of raking varied among years, subfamilies, and timber types (
Table 1,
Figure 6). The abundance of Myrmicinae was significantly higher in raked than intact stands in slash during 2008 and 2010 (Tukey HSD,
p < 0.01), and during 2010 in longleaf (Tukey HSDs,
p < 0.01).
Previous research has demonstrated increases in ant abundance following repeated forest disturbance [
56]. Many taxa within hymenoptera have traits that promote tolerance of disturbance [
57], and these traits may explain why raking may cause increased abundance of Hymenoptera. First, the removal of litter may have increased the ability of ants to forage efficiently by enhancing their ability to locate and capture food resources in raked stands [
58]. Second, temperature variation plays an important role in determining the abundance, distribution, and diversity of ants [
59]. An increase in sunlight at the soil surface following raking may have been largely beneficial to ants, causing an increase in activity and abundance [
60]. This seems likely, given the fact that the increase in abundance of Myrmicinae following raking was greater in the two species of pine with more open conditions (longleaf and slash) than in the better shaded loblolly stands.
Figure 6.
Predicted means and standard errors of Hymenoptera abundance (log transformed) during three years of seasonal pitfall trapping in pine plantations in North Florida against fixed effects from the most parsimonious model based on log-likelihood ratio tests; raked stands are displayed with open circles and dashed lines, intact stands with solid circles and lines; 2008 as yr1, 2009 as yr2 and 2010 as yr3.
Figure 6.
Predicted means and standard errors of Hymenoptera abundance (log transformed) during three years of seasonal pitfall trapping in pine plantations in North Florida against fixed effects from the most parsimonious model based on log-likelihood ratio tests; raked stands are displayed with open circles and dashed lines, intact stands with solid circles and lines; 2008 as yr1, 2009 as yr2 and 2010 as yr3.
3.2.5. Occurrence of Acari
Raking had no significant effect on the occurrence of Acari (LLRT,
p = 0.88). Furthermore, the occurrence of Acari did not vary consistently among timber type (LLRT,
p = 0.24) or among years (LLRT,
p = 0.98,
Figure 7).
Our ability to detect clear patterns in the response of Acari occurrence to raking may have been obscured by the fact that mites are a trophically diverse group with some species functioning as predators and others as fungivores. For example, the abundance of some species that function as fungivores are positively influenced by soil moisture and would therefore be expected to decrease in response to raking, whereas predatory species are less influenced by soil moisture and would therefore be expected to show little response to raking [
12,
51]. Reductions in the abundance of fungivorous mites in response to the removal of the litter layer has been demonstrated for other forest types [
50].
The first time pine straw is raked from a stand, the protective mulch layer formed through years of accumulated pine needles matted together by hyphal fungi is removed, drastically altering the local microclimate and removing a wide variety of fungal food sources. Frequent, subsequent removal of recently accumulated litter would thereafter have less drastic effects on microclimate and food sources, because the fungal mat would be less well developed. Raking pine straw for consecutive years has been shown to reduce fungus in the litter layer, and several years with no raking are required before fungal growth begins to accumulate on freshly fallen pine straw [
17]. The decline in food availability for fungivores as the study progressed or the change in microclimate associated with the loss of fungus may explain the slight declining trend over time that we observed in Acari occurrence in both loblolly and slash pine stands.
Figure 7.
Predicted mean and standard errors of Acari occurrence during three years of seasonal pitfall trapping in pine plantations in North Florida against fixed-effects from the full model to display trends; raked stands are displayed with open circles and dashed lines, intact stands with solid circles and lines; 2008 as yr1, 2009 as yr2 and 2010 as yr3.
Figure 7.
Predicted mean and standard errors of Acari occurrence during three years of seasonal pitfall trapping in pine plantations in North Florida against fixed-effects from the full model to display trends; raked stands are displayed with open circles and dashed lines, intact stands with solid circles and lines; 2008 as yr1, 2009 as yr2 and 2010 as yr3.
3.2.6. Abundance of Blattodea
The occurrence of Blattodea was significantly higher in intact
versus raked stands across all three timber types (Tukey HSD,
p <0.01), and gradually increased over the three year period (Tukey HSD, all years,
p < 0.03;
Table 2,
Figure 8). Timber type did not explain a significant amount of variation overall (
p = 0.107), but occurrence was significantly higher in loblolly than longleaf pine stands (Tukey HSD,
p = 0.01).
Cockroaches are large and highly mobile; such taxa are expected to respond quicker to reductions in habitat quality than less mobile taxa due to their heightened dispersal abilities [
51]. Cockroaches have been shown to decrease in abundance shortly after forest disturbances, such as gap creation, as a result of wind damage, salvage logging, burning, and litter removal, [
27,
54,
61]. In addition, abundance may remain depressed for many years following disturbance [
61]. Our results corroborate the idea that Blattodea respond quickly to forest disturbance, and given that they are one of only two orders that showed a consistent negative effect of litter raking from year to year with no indication of recovery over time, our results also corroborate the idea that they do not rebound quickly following disturbance.
Figure 8.
Predicted means and standard errors of Blattodea occurrence during three years of seasonal pitfall trapping in pine plantations in North Florida against fixed effects from the most parsimonious model based on log-likelihood ratio tests; raked stands are displayed with open circles and dashed lines, intact stands with solid circles and lines; 2008 as yr1, 2009 as yr2 and 2010 as yr3.
Figure 8.
Predicted means and standard errors of Blattodea occurrence during three years of seasonal pitfall trapping in pine plantations in North Florida against fixed effects from the most parsimonious model based on log-likelihood ratio tests; raked stands are displayed with open circles and dashed lines, intact stands with solid circles and lines; 2008 as yr1, 2009 as yr2 and 2010 as yr3.
3.2.7. Abundance of Coleoptera
The occurrence of Coleoptera differed slightly between raked and intact stands and differed significantly among years and functional groups, but did not differ among timber types (
Table 2,
Figure 9). Occurrence increased significantly from 2008 to 2009 (Tukey HSD,
p < 0.001) and remained higher for 2010 (Tukey HSD 2008
vs. 2010,
p < 0.001, 2009
vs. 2010,
p = 1.00). Predators and detritivores occurred significantly more often than fungivores and phytophages (all Tukey HSDs <0.001,
Figure 9). Timber type was not significantly related to occurrence and therefore had no effect on any of the aforementioned patterns (LLRT,
p > 0.1). Even though raking treatment did not explain a significant amount of variability in the selected model (
p = 0.065), the probability of occurrence was significantly lower in raked than intact pine stands (Tukey HSD,
p = 0.015,
Figure 5).
Beetles are a diverse taxa that span a range of trophic positions [
62,
63] and typically show a range of responses to disturbances [
64]. A rapid response to changes in habitat conditions is expected from nearly all Coleoptera, given their high mobility. This order consistently experiences reductions in abundance following disturbances of the ground layer in forests, including a decrease in abundance following salvage logging operations and gap creation due to wind damage [
65], a decrease in abundance in response to fire [
55,
66], and a decrease of predatory beetles in response to the removal of the litter layer [
44]. Our results similarly show that raking had a negative effect on the presence of beetles regardless of timber type or functional group. Raking can be assumed to impact fungivorous and detritivorous beetles through a reduction of food resources, especially following the initial raking which removes the thick mulch layer formed through years of accumulated pine needles matted together by hyphal fungi. Raking may impact predaceous beetles through a reduction of prey (deeper, more complex litter layers can support a greater number of taxa by providing a greater diversity of microhabitats than shallower, less complex litter layers).
Figure 9.
Predicted means and standard errors of Coleoptera occurrence during three years of seasonal pitfall trapping in pine plantations in North Florida against fixed effects from the most parsimonious model based on log-likelihood ratio tests; raked stands are displayed with open circles and dashed lines, intact stands with solid circles and lines; 2008 as yr1, 2009 as yr2 and 2010 as yr3.
Figure 9.
Predicted means and standard errors of Coleoptera occurrence during three years of seasonal pitfall trapping in pine plantations in North Florida against fixed effects from the most parsimonious model based on log-likelihood ratio tests; raked stands are displayed with open circles and dashed lines, intact stands with solid circles and lines; 2008 as yr1, 2009 as yr2 and 2010 as yr3.
3.2.8. Abundance of Orthoptera
The effect of raking on the occurrence of Orthoptera varied by timber species, and the occurrence varied by year (
Table 2,
Figure 10). The gap between occurrence in intact
versus raked stands was pronounced only in longleaf stands, with occurrence significantly lower in raked stands (Tukey HSD,
p = 0.014). The occurrence of Orthoptera also significantly increased each year as the study progressed in all timber types (Tukey HSDs,
p < 0.001).
Grasshoppers and crickets are relatively low-mobility arthropods that occupy an intermediate tropic position, serving primarily as herbivores [
47]. Previous research has found mixed responses of this taxa to disturbances, with some families increasing in abundance [
65] and others decreasing, often followed by a fairly quick recovery [
43]. Our finding that the removal of the litter layer is more detrimental to Orthoptera in longleaf stands than loblolly or slash stands may be due to the fact that these forests were characterized by longest needle length (Minogue, personal-communication), which likely means longleaf stands have the least compacted litter layer, making conditions between raked and intact stands more different for arthropods here than in stands of other pine species. It is important to note that longleaf are also the stands that are most commonly subjected to pine straw raking [
21].
Figure 10.
Predicted means and standard errors of Orthoptera occurrence during three years of seasonal pitfall trapping in pine plantations in North Florida against fixed effects from the most parsimonious model based on log-likelihood ratio tests; raked stands are displayed with open circles and dashed lines, intact stands with solid circles and lines; 2008 as yr1, 2009 as yr2 and 2010 as yr3.
Figure 10.
Predicted means and standard errors of Orthoptera occurrence during three years of seasonal pitfall trapping in pine plantations in North Florida against fixed effects from the most parsimonious model based on log-likelihood ratio tests; raked stands are displayed with open circles and dashed lines, intact stands with solid circles and lines; 2008 as yr1, 2009 as yr2 and 2010 as yr3.
4. Conclusions
Understanding the effects of litter raking on terrestrial arthropod communities is challenging, because litter removal causes a complex chain of events involving both physical and biological changes [
7]. A single pine straw harvest in longleaf pine can reduce litter biomass for the next three years [
67]. Consecutive years of pine litter raking exacerbate physical changes at the soil surface, causing decreased infiltration rates, increased runoff, increased erosion, decreased porosity and increased bulk density when compared with stands raked less frequently [
17,
68]. Several years must pass without raking before fungal growth begins accumulating on pine straw [
17], to feed organisms at the base of food webs.
Litter plays two vital roles for terrestrial arthropods in forest ecosystems: inputs that form the basis for nutrient cycling, and regulation of microclimatic conditions at the soil surface [
20,
69]. Changes to the amount of litter available for decomposers and changes to the structure of the litter layer would be expected to change terrestrial arthropod species abundances, trophic relationships and, ultimately, community structure. Our results suggest that arthropod community structure did change as a result of three consecutive years of litter removal in two of the three pine plantations investigated (loblolly and longleaf). Blattodea and Coleoptera showed consistent patterns of reduced abundance or occurrence in raked
versus intact stands, and Orthoptera showed these patterns in longleaf pine stands as well. Over the course of the three-year study, the gap in abundance of wandering-hunter spiders and Tomoceridae springtails between raked and intact stands was widening, with significantly lower abundances in raked stands by the end of the study. Conversely, Hemiptera showed consistent patterns of increased abundance in response to raking, and the gap in abundance of Isotomidae springtails and Myrmicinae ants between raked and intact stands was widening, with significantly higher abundances in raked stands by the end of the study. Clearly, arthropod communities were affected by litter removal, and communities were becoming more disparate as the number of rakings increased.
Pine straw is currently raked in the Southeastern U.S. from all three species of pine we investigated. The rank order of preference in the nursery industry (and therefore, revenue generation) is longleaf followed by slash followed by loblolly, due to the length, color retention, and rate of deterioration of the needles [
22]. In contrast, pine straw yield tends to be highest for loblolly, intermediate for slash and lowest for longleaf [
22]. The result of these contrasting patterns of preference
versus yield is that all three species are frequently subjected to raking, which makes our findings from all three pine species highly relevant. In an investigation of the short term-effects of pine straw raking on terrestrial arthropods we found that many taxa showed differences in response to raking among the three types of pines [
27]. In general, reductions in arthropod abundances were most apparent in loblolly, and increases were most apparent in longleaf and slash stands [
27]. The present longer-term investigation found fewer taxa that responded differently to raking among the three types of pines, but taxa-specific reductions were apparent in longleaf and loblolly and increases in longleaf. The long-term investigation of community composition showed that repeated raking caused stronger shifts in community composition in longleaf and loblolly than in slash. These results are somewhat surprising, given that slash could be considered intermediate between longleaf and loblolly in several characteristics expected to affect the physical structure of the litter layer (
i.e., needle length, amount of sunlight at the soil surface).
Overall, we found few clear patterns in response to raking among orders or among taxa of different orders occupying similar trophic positions. A limitation of our work was the taxonomic resolution of our data. Treatment effects may have been more apparent at lower orders of taxonomic resolution than we were able to investigate, with shifts in relative abundances among species or genera in response to disturbances obscuring our ability to discern patterns at the family and ordinal levels [
70]. Regardless, litter removal altered the abundances of some arthropods that play essential roles in ecosystem processes, which could have negative consequences on ecosystem health and functioning. Our results highlight the role of pine litter in shaping terrestrial arthropod communities in plantations and imply that repeated removal of pine straw during consecutive years is likely to have unintended consequences on arthropod communities that inhabit the forest floor. Considering that these organisms are indicators of soil quality [
2], continuing to ignore the effect repeated pine straw raking has on them seems to be at odds with calls to manage forests in the Southeastern U.S. in a manner that promotes healthy forest ecosystems [
6].