Impact of Forest Fragmentation on Patterns of Mountain Pine Beetle-Caused Tree Mortality
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Site and Data

2.1.1. Mountain Pine Beetle Survey Data

2.1.2. Land Cover Classification

2.2. Methods
2.2.1. Measure of Forest Fragmentation
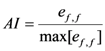

2.2.2. Regression Model

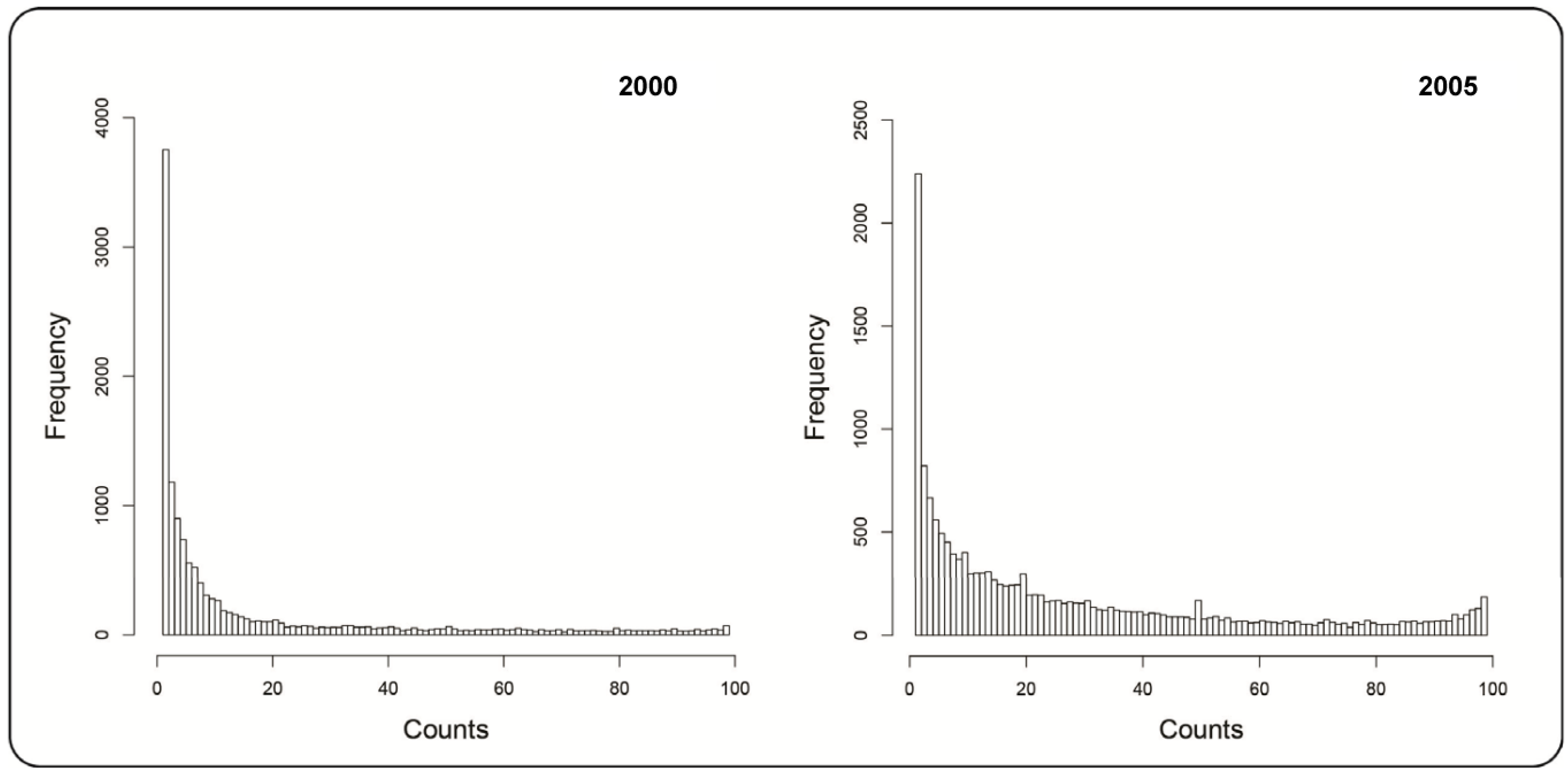
3. Results and Discussion
| Estimate | Standard Error | z value | p | |
|---|---|---|---|---|
| (Intercept) | 3.2107 | 0.1094 | 29.352 | <0.00001 |
| AI | −0.3351 | 0.1157 | −2.896 | 0.00378 |
| Estimate | Standard Error | z value | p | |
|---|---|---|---|---|
| (Intercept) | 5.29606 | 0.05467 | 96.87 | <0.0001 |
| AI | 2.21167 * | 0.06006 | −36.82 | 0.0001 |
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Mountain Pine Beetle Infestation Information; British Columbia Ministry of Forests, Lands and Natural Resource Operations: Victoria, Canada, 2013. Available online: http://www.for.gov.bc.ca/hfp/mountain_pine_beetle/facts.htm (accessed on 17 January 2013).
- Pederson, L. How Serious is the Mountain Pine Beetle Problem? From a Timber Supply Perspective. In Proceedings of the Mountain Pine Beetle Symposium: Challenges and Solutions, Kelowna, Canada, 30–31 October 2003; Shore, T.L., Brooks, J.E., Stone, J.E., Eds.; Canadian Forest Service: Victoria, Canada, 2004. BC-X-398. pp. 10–20. [Google Scholar]
- De la Giroday, H.-M.C.; Carroll, A.B.; Aukema, B.H. Breach of the northern Rocky Mountain geoclimatic barrier: Initiation of range expansion by the mountain pine beetle. J. Biogeogr. 2012, 39, 1112–1123. [Google Scholar] [CrossRef]
- Cullingham, C.I.; Cooke, J.E.K.; Dang, S.; Davis, C.S.; Cooke, B.J.; Coltman, D.W. Mountain pine beetle host-range expansion threatens the boreal forest. Mol. Ecol. 2011, 20, 2157–2171. [Google Scholar]
- Safranyik, L.; Carroll, A.L.; Régnière, J.; Langor, D.W.; Riel, W.G.; Shore, T.L.; Peter, B.; Cooke, B.J.; Nealis, V.G.; Taylor, S.W. Potential for range expansion of mountain pine beetle into the boreal forests of North America. Can. Entomol. 2010, 142, 415–442. [Google Scholar] [CrossRef]
- Meddens, A.J.H.; Hicke, J.A.; Ferguson, C.A. Spatiotemporal patterns of observed bark beetle-caused tree mortality in British Columbia and the western United States. Ecol. Appl. 2012, 22, 1876–1891. [Google Scholar] [CrossRef]
- Trzcinski, M.K.; Reid, M.L. Effect of management on the spatial spread of mountain pine beetle (Dendroctonus ponderosae) in Banff National Park. For. Ecol. Manag. 2008, 256, 1418–1426. [Google Scholar] [CrossRef]
- Amman, G.D. Mountain pine beetle brood production in relation to thickness of lodgepole pine phloem. J. Econ. Entomol. 1972, 65, 138–140. [Google Scholar]
- Reid, R.W. Biology of the mountain pine beetle, Dentroctonus ponderosae Hopkins, in the east Kootenay region of British Columbia. III. Interaction between the beetle and its host, with emphasis on brood mortality and survival. Can. Entomol. 1963, 95, 225–238. [Google Scholar]
- Safranyik, L.; Shrimpton, D.M.; Whitney, H.S. Management of Lodgepole Pine to Reduce Losses to Mountain Pine Beetle; Canadian Forest Service: Victoria, Canada, 1974; p. 24. [Google Scholar]
- Kautz, M.; Schopf, R.; Osher, J. The “sun-effect”: Microclimatic alterations predispose forest edges to bark beetle infestations. Eur. J. For. Res. 2013, in press. [Google Scholar]
- Borden, J.H.; Ryker, L.J.; Chong, L.J.; Pierce, H.D.; Johnson, B.D.; Oehlschlager, A.C. Response of the mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera:Scolytidae), to five semiochemicals in British Columbia lodgepole pine forests. Can. J. For. Res. 1987, 17, 118–128. [Google Scholar] [CrossRef]
- Wood, D.L. The role of pheromones, kairomones, and allomones in the host selection and colonization behavior of bark beetles. Ann. Rev.Entomol. 1982, 27, 411–446. [Google Scholar] [CrossRef]
- Raffa, K.F.; Aukema, B.H.; Erbilgin, N.; Klepzig, K.D.; Walling, K.F. Interactions among conifer terpenoids and bark beetles across multiple levels of scale: An attempt to understand links between population patterns and physiological processes. Adv. Phytochem. 2005, 39, 80–118. [Google Scholar]
- Carroll, A.L.; Safranyik, L. The Bionomics of the Mountain Pine Beetle in Lodgepole Pine Forests: Establishing a Context. In Proceedings of the Mountain Pine Beetle Symposium: Challenges and Solutions, Kelowna, Canada, 30–31 October 2003; Shore, T.L., Brooks, J.E., Stone, J.E., Eds.; Canadian Forest Service: Victoria, Canada, 2004. BC-X-398. pp. 21–32. [Google Scholar]
- Rudinsky, J.A.; Morgan, M.E.; Libbey, L.M.; Putnam, T.B. Antiaggregative-rivalry pheromone of the mountain pine beetle, and a new arrestant of the southern pine beetle. Env. Entomol. 1974, 3, 90–98. [Google Scholar]
- Safranyik, L.; Caroll, A.C. The Biology and Epidemiology of the Mountain Pine Beetle in Lodgepole Pine Forests. In The Mountain Pine Beetle: A Synthesis of Biology, Management, and Impacts on Lodgepole Pine; Safranyik, L., Wilson, W.R., Eds.; Canadian Forest Service: Victoria, Canada, 2006; pp. 3–66. [Google Scholar]
- Boone, C.K.; Aukema, B.H.; Bohlmann, J.; Carroll, A.L.; Raffa, K.F. Efficacy of tree defense physiology varies with bark beetle population density: A basis for positive feedback in eruptive species. Can. J. For. Res. 2011, 41, 1174–1188. [Google Scholar] [CrossRef]
- Waring, R. Characteristics of trees predisposed to die. Bioscience 1987, 37, 569–574. [Google Scholar] [CrossRef]
- Raffa, K.F.; Aukema, B.H.; Bentz, B.; Carroll, A.L.; Hicke, J.A.; Turner, M.G.; Romme, W. Cross-scale drivers of natural disturbances prone to anthropogenic amplification: The dynamics of bark beetle eruptions. Bioscience 2008, 58, 501–517. [Google Scholar]
- Berryman, A.A. Theoretical explanation of mountain pine beetle dynamics in lodgepole pine forests. Env. Entomol. 1976, 5, 1225–1233. [Google Scholar]
- Bone, C.; Dragicevic, S.; Roberts, A. Integrating high resolution RS, GIS and fuzzy set theory for identifying susceptibility areas of forest insect infestation. Int. J. Remote Sens. 2005, 26, 4809–4828. [Google Scholar]
- Larsson, S.; Oren, R.; Waring, R.H.; Barrett, J.W. Attacks of mountain pine beetle as related to tree vigor of ponderosa pine. For. Sci. 1983, 29, 395–402. [Google Scholar]
- Waring, R.H.; Pitman, G.B. Modifying lodgepole pine stands to change susceptibility to mountain pine beetle attack. Ecology 1985, 66, 889–897. [Google Scholar]
- Raffa, K.F.; Berryman, A.A. Interacting selective pressures in conifer-bark beetle systems: A basis for reciprocal adaptations? Am. Nat. 1987, 129, 234–262. [Google Scholar]
- Hoffmeister, T.S.; Ver, L.E.; Biere, A.; Holsinger, K.; Filser, J. Ecological and evolutionary consequences of biological invasion and habitat fragmentation. Ecosystems 2005, 8, 657–667. [Google Scholar] [CrossRef]
- Cappuccino, N.; Martin, M.A. The birch tube-maker Acrobasis betulella in a fragmented habitat: The importance of patch isolation and edges. Oecoligca 1997, 110, 69–76. [Google Scholar] [CrossRef]
- Radeloff, V.C.; Mladenoff, D.J.; Boyce, M.S. The changing relation of landscape patterns and jack pine budworm populations during an outbreak. Oikos 2000, 90, 417–430. [Google Scholar]
- Cooke, B.J.; Roland, J. Spatial analysis of large-scale patterns of forest tent caterpillar outbreaks. Ecoscience 2000, 7, 410–422. [Google Scholar]
- Roland, J. Large-scale forest fragmentation increases the duration of tent caterpillar outbreak. Oecoligca 1993, 93, 25–30. [Google Scholar]
- Barclay, H.J.; Li, C.; Benson, L.; Taylor, S.; Shore, T. Effects of fire return rates on traversability of lodgepole pine forests for mountain pine beetle (Coleoptera:Scolytidae) and the use of patch metrics to estimate traversability. Can. Entomol. 2005, 137, 566–583. [Google Scholar] [CrossRef]
- Ryall, K.L.; Fahrig, L. Habitat loss decreases predator-prey ratios in a pine-bark beetle system. Oikos 2005, 110, 265–270. [Google Scholar]
- Aukema, B.H.; Carroll, A.L.; Zhu, J.; Raffa, K.F.; Sickley, T.A.; Taylor, S.W. Landscape level analysis of mountain pine beetle in British Columbia, Canada: A spatiotemporal development and spatial synchrony within the present outbreak. Ecography 2006, 29, 427–441. [Google Scholar] [CrossRef]
- Wulder, M.A.; White, J.C.; Grills, D.; Nelson, T.A.; Coops, N.C.; Ebata, T. Aerial overview survey of the mountain pine beetle epidemic in British Columbia: Communication of impacts. BC J. Ecosyst. Manag. 2009, 10, 45–58. [Google Scholar]
- British Columbia Ministry of Forests; Canadian Forest Service, Forest Health Aerial Overview Survey Standards for British Columbia: The BC Ministry of Forests Adaptation of the Canadian Forest Service’s FHN Report 97–1 “Overview Aerial Survey Standards for British Columbia and the Yukon”; Version 2.0; Resources Inventory Committee: Victoria, Canada, 2000.
- Wulder, M.A.; White, J.C.; Cranny, M.; Hall, R.J.; Luther, J.E.; Beaudoin, A.; Goodenough, D.G.; Dechka, J.A. Monitoring Canada’s forests. Part 1: Completion of the EOSD land cover project. Can. J. Remote Sens. 2008, 34, 549–562. [Google Scholar] [CrossRef]
- Wulder, M.A.; White, J.C.; Han, T.; Coops, N.C.; Cardille, J.A.; Holland, T.; Grills, D. Monitoring Canada’s forests. Part 2: National forest fragmentation and pattern. Can. J. Remote Sens. 2008, 34, 563–584. [Google Scholar] [CrossRef]
- Franklin, J.F.; Forman, R.T. Creating landscape patterns by forest cutting: Ecological consequences and principles. Landsc. Ecol. 1987, 1, 5–18. [Google Scholar] [CrossRef]
- Haines-Young, R.; Chopping, M. Quantifying landscape structure: A review of landscape indices and their application to forested landscapes. Prog. Phys. Geogr. 1996, 20, 418–445. [Google Scholar] [CrossRef]
- Gergel, S.E. New Directions in Landscape Pattern Analysis and Linkages with Remote Sensing. In Understanding Forest Disturbance and Spatial Pattern: Remote Sensing and GIS Approaches; Wulder, M.A., Franklin, S.E., Eds.; Taylor and Francis Group: Boca Raton, FL, USA, 2007; pp. 173–208. [Google Scholar]
- He, H.S.; DeZonia, B.E.; Mladenoff, D.J. An aggregation index (AI) to quantify spatial patterns of landscapes. Landsc. Ecol. 2000, 15, 591–601. [Google Scholar] [CrossRef]
- Neel, M.C.; McGarigal, K.; Cushman, S.A. Behavior of class-level landscape metrics across gradients of class aggregation and area. Landsc. Ecol. 2004, 19, 435–455. [Google Scholar] [CrossRef]
- Robertson, C.; Farmer, C.J.Q.; Nelson, T.A.; MacKenzie, I.K.; Wulder, M.A.; White, J.C. Determination of the compositional change (1999–2006) in the pine forests of British Columbia due to mountain pine beetle infestation. Env. Monit. Assess. 2009, 158, 593–608. [Google Scholar] [CrossRef]
- Shore, T.L.; Safranyik, L.; Lemieux, J.P. Susceptibility of lodgepole pine stands to the mountain pine beetle: Testing of a rating system. Can. J. For. Res. 2000, 30, 44–49. [Google Scholar] [CrossRef]
- Environmental Systems Research Institute (ESRI), ArcGIS Desktop: Release 9.3. ESRI: Redlands, CA, USA, 2008.
- R Development Core Team, R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011.
- Taylor, S.W.; Carroll, A.L. Disturbance, Forest Age, and Mountain Pine Beetle Outbreak Dynamics in BC: A Historical Perspective. In Proceedings of the Mountain Pine Beetle Symposium: Challenges and Solutions, Kelowna, Canada, 30–31 October 2003; Shore, T.L., Brooks, J.E., Stone, J.E., Eds.; Canadian Forest Service: Victoria, Canada, 2004. BC-X-398. pp. 41–51. [Google Scholar]
- Cudmore, T.J.; Bjorklund, N.; Carroll, A.L.; Lindgren, B.S. Climate change and range expansion of an aggressive bark beetle: Evidence of higher beetle reproduction in naive host tree populations. J. Appl. Ecol. 2010, 47, 1036–1043. [Google Scholar] [CrossRef]
- Lynch, H.J.; Renkin, R.A.; Crabtree, R.L.; Moorcroft, P.R. The influence of previous mountain pine beetle (Dentroctonus ponderosae) activity on the 1988 Yellowstone Fires. Ecosystems 2006, 9, 1318–1327. [Google Scholar] [CrossRef]
- Fares, Y.; Sharpe, P.J.H.; Magnuson, C.E. Pheromone dispersion in forests. J. Theor. Biol. 1980, 84, 335–359. [Google Scholar] [CrossRef]
- Bentz, B.J.; Logan, J.A.; Amman, G.D. Temperature-dependent development of the mountain pine beetle (Coleoptera:Scolytidae) and simulation of its phenology. Can. Entomol. 1991, 123, 1083–1094. [Google Scholar] [CrossRef]
- Régnière, J.; Bentz, B. Modeling cold tolerance in the mountain pine beetle, Dendroctonus ponderosae. J. Insect Physiol. 2007, 53, 559–572. [Google Scholar]
- Safranyik, L.; Linton, D.A. Mortality of mountain pine beetle larvae, Dendroctonus ponderosae (Coleoptera:Scolytidae) in logs of lodgepole pine (Pinus contorta var. latifolia) at constant low temperatures. J. Entomol. Soc. Br. Columbia 1998, 95, 81–87. [Google Scholar]
- Furnis, M.M.; Furnis, R.L. Scolytids (Coleoptera) on snowfields above timberline in Oregon and Washington. Can. Entomol. 1972, 104, 1471–1478. [Google Scholar] [CrossRef]
- Shore, T.L.; Safranyik, L. Susceptibility and Risk Rating Systems for the Mountain Pine Beetle in Lodgepole Pine Stands; Canadian Forest Service: Victoria, Canada, 1992; BC-X-336; p. 12. [Google Scholar]
- Geiszler, D.R.; Gallucci, V.F.; Gara, R.I. Modeling the dynamics of mountain pine beetle aggregation in a lodgepole pine stand. Oecologia 1980, 46, 244–253. [Google Scholar] [CrossRef]
- Elkin, C.M.; Reid, M.L. Shifts in breeding habitat selection behaviour in response to population density. Oikos 2010, 119, 1070–1080. [Google Scholar] [CrossRef]
- Wallin, K.F.; Raffa, K.F. Feedback between individual host selection behavior and population dynamics in an eruptive herbivore. Ecol. Monogr. 2004, 74, 101–116. [Google Scholar] [CrossRef]
- Amman, G.D.; McGregor, M.D.; Cahill, D.B.; Klein, W.H. Guidelines for Reducing Loss of Lodgepole Pine to the Mountain Pine Beetle in Unmanaged Stands in the Rocky Mountains; General Technical Report INT-36; USDA Forest Service: Ogden, UT, USA, 1978; p. 19.
- Berryman, A.A. A Synoptic Model of the Lodgepole Pine/Mountain Pine Beetle Interactions and Its Potential for Application in Forest Management. In Proceedings of a Symposium Theory and Practice of Mountain Pine Beetle Management in Lodgepole Pine Forests, Pullman, WA, USA, 25–27 April 1978; Berryman, A.A., Amman, G.D., Stark, R.W., Eds.; University of Idaho: Moscow, ID, USA, 1978; pp. 98–105. [Google Scholar]
- Mahoney, RL. Lodgepole Pine/Mountain Pine Beetle Risk Classification Methods and Their Application. In Proceedings of a Symposium Theory and Practice of Mountain Pine Beetle Management in Lodgepole Pine Forests, Pullman, WA, USA, 25–27 April 1978; Berryman, A.A., Amman, G.D., Stark, R.W., Eds.; University of Idaho: Moscow, ID, USA, 1978; pp. 106–110. [Google Scholar]
- Robertson, C.; Wulder, M.A.; Nelson, T.A.; White, J.C. Risk rating for mountain pine beetle infestation of lodgepole pine forests over large areas with ordinal regression. For. Ecol. Manag. 2008, 256, 900–912. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bone, C.; White, J.C.; Wulder, M.A.; Robertson, C.; Nelson, T.A. Impact of Forest Fragmentation on Patterns of Mountain Pine Beetle-Caused Tree Mortality. Forests 2013, 4, 279-295. https://doi.org/10.3390/f4020279
Bone C, White JC, Wulder MA, Robertson C, Nelson TA. Impact of Forest Fragmentation on Patterns of Mountain Pine Beetle-Caused Tree Mortality. Forests. 2013; 4(2):279-295. https://doi.org/10.3390/f4020279
Chicago/Turabian StyleBone, Christopher, Joanne C. White, Michael A. Wulder, Colin Robertson, and Trisalyn A. Nelson. 2013. "Impact of Forest Fragmentation on Patterns of Mountain Pine Beetle-Caused Tree Mortality" Forests 4, no. 2: 279-295. https://doi.org/10.3390/f4020279




