Commercializing Biorefinery Technology: A Case for the Multi-Product Pathway to a Viable Biorefinery
Abstract
: While there may be many reasons why very interesting science ideas never reach commercial practice, one of the more prevalent is that the reaction or process, which is scientifically possible, cannot be made efficient enough to achieve economic viability. One pathway to economic viability for many business sectors is the multi-product portfolio. Research, development, and deployment of viable biorefinery technology must meld sound science with engineering and business economics. It is virtually axiomatic that increased value can be generated by isolating relatively pure substances from heterogeneous raw materials. Woody biomass is a heterogeneous raw material consisting of the major structural components, cellulose, lignin, and hemicelluloses, as well as minor components, such as extractives and ash. Cellulose is a linear homopolymer of D-glucopyrano-units with β-D(1→ 4) connections and is the wood component most resistant to chemical and biological degradation. Lignin is a macromolecule of phenylpropanoid units, second to cellulose in bio-resistance, and is the key component that is sought for removal from woody biomass in chemical pulping. Hemicelluloses are a collection of heteropolysaccharides, comprised mainly of 5- and 6-carbon sugars. Extractives, some of which have high commercial value, are a collection of low molecular weight organic and inorganic woody materials that can be removed, to some extent, under mild conditions. Applied Biorefinery Sciences, LLC (a private, New York, USA based company) is commercializing a value-optimization pathway (the ABS Process™) for generating a multi-product portfolio by isolating and recovering homogeneous substances from each of the above mentioned major and minor woody biomass components. The ABS Process™ incorporates the patent pending, core biorefinery technology, “hot water extraction”, as developed at the State University of New York College of Environmental Science and Forestry (SUNY-ESF). Hot water extraction in the absence of mineral acids and bases is preferred because of its ability to generate multiple high value output products without chemical input, recovery, or disposal costs. Instead of added chemicals in the cooking phase, the ABS Process™ relies upon an autocatalytic reaction in which acetyl groups, bound through an ester linkage to hemicellulose chains, are hydrolyzed at high temperature in water. The resulting acidic conditions (final pH ∼3.5) and temperatures of 160–170 °C permit further solubilization and diffusion of oligomeric 5- and 6-carbon sugars, acetic acid, aromatic substances, monomeric sugars, and other trace compounds into the extract solution. These conditions also avoid extensive degradation of monosaccharides, enabling membrane fractionation and other chemical separation techniques to be used in the following separations. A range of separation techniques are applied on the extract solution to isolate and purify fermentable sugars, acetic acid, lignin, furfural, formic acid, other hemicellulose related compounds, lignin, lignin degradation products, and phenolic extractives for commercial sale. The extracted lignocellulosic biomass, with reduced hemicellulose content and is thus less heterogeneous, carries the value-added advantages of: (1) enhanced product characteristics, and (2) reduced energy and chemical manufacturing costs. Thus, by fractionating woody biomass into more homogeneous substances, the ABS Process™ holds potential as an economically viable pathway for capturing sustainable, renewable value not currently realized from lignocellulosic biomass.1. Introduction
Woody biomass is the most abundant organic source on Earth, with an annual production in the biosphere of about 5.64 × 1016 g of carbon [1]. Of the annual biomass production each year, only 4.8% or 2.7 × 1015 g of C/year were utilized by mankind, including food (1.7 × 1015 g of carbon); pulp, paper, energy, furniture and construction materials (0.9 × 1015 g of carbon); and the rest as clothing and chemicals [2]. Tapping into the chemical energy of woody biomass and reclaiming the historically important position of woody biomass as feedstock for chemicals, energy and transportation is imperative to sustainability of the world economy. With respect to productivity, forest resources are unparalleled in comparison to other plant biomass systems. Forests cover about 9.5% of Earth's surface or about 32% of the land area, but account for 89.3% of the total standing biomass. A distant second are savanna and grassland ecosystems, which account for 11% of total biomass production. When measured in energy terms, forests synthesize 1.09 × 1021 J/year [3], equivalent to more than double the world's total primary energy consumption of about 4.85 × 1020 J in 2005 [4]. Plants produce biomass through a natural process that uses solar energy to synthesize compounds from raw materials assimilated from Earth's ecosystem. Woody biomass is a sustainable resource in that plant systems readily regenerate in native ecosystems, as well as in human influenced systems such as dedicated, managed wood product and energy crops (forest, agriforest, and/or agricultural biomass). Once synthesized in nature, biomass related compounds can be fractionated back into chemicals and energy that mankind can utilize. Unlike the centuries of evolution during which humans lived in a primarily agrarian society, today's world population is significantly larger (more than doubled in the past 50 years), and tends to dwell in urban or suburban settings where it is impractical for most people to grow their own food and fuel. Instead, food, fuel, and other products are now provided through an economically viable industrial manufacturing and supply chain. The “biorefinery” is a manufacturing facility that transforms raw plant biomass into some of the products humans use.
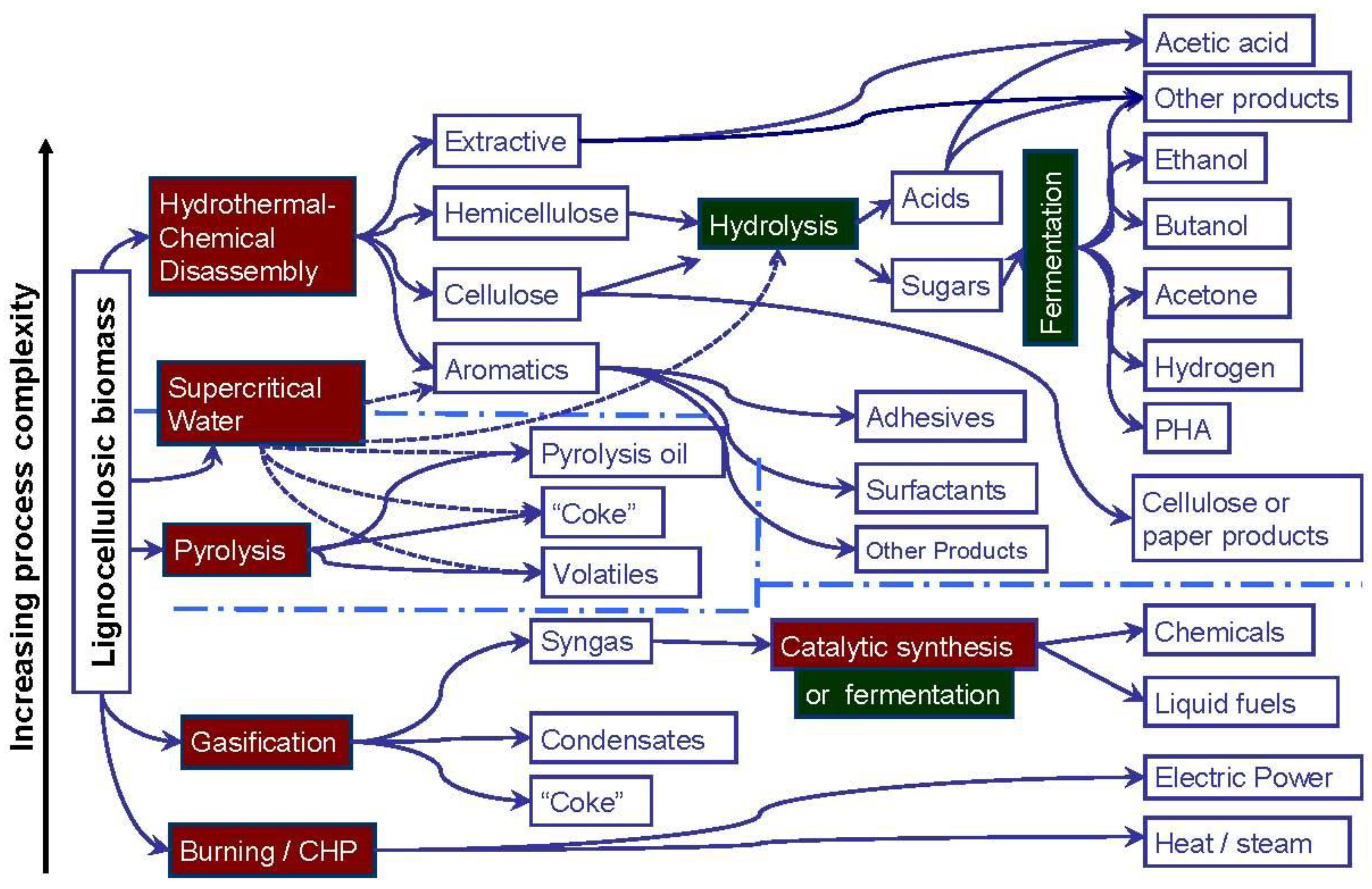
Biorefinery processes that convert woody biomass to chemicals, energy and materials can be nominally classified as biochemical, thermochemical, and hydrothermal routes, although this division is increasingly blurred as biological and thermal processes are mixed in many current-day applications (Figure 1). At SUNY ESF, active research includes thermal and/or chemical disassembly followed by biological conversion. Pyrolysis, gasification, and burning may take place after hydrothermal or chemical disassembly. Supercritical water technologies are currently being developed, and they may, depending on how applied, play a role in future disassembly processes leading to products from hydrolysis and/or pyrolysis.
Applied Biorefinery Sciences, LLC, or “ABS”, is a private R&D, consulting, and IP licensing company established to research, develop, and deploy biorefinery technology, and/or as a conduit for transferring licensed technology such as that invented at SUNY-ESF, and described in the article by Amidon et al. [4]. The objective of this paper is to outline the process options and potential product portfolios from biorefinery technologies being commercialized by ABS. In particular, the hydro-thermal process lineage, (shown in Figure 1) is currently the process category most actively championed by ABS for commercial practice.
2. Chemical Composition of Woody Biomass
Woody biomass is a complex and non-uniform natural product with respect to anatomical and chemical properties. Within each plant, different cell types perform mechanical support, water transport, and storage of reserve food supplies. These cells differ in anatomical structure and chemical composition, and also exhibit differences in different parts of the plant: stem, branches, roots, tops, and bark. A notable variation of chemical composition occurs in the radial direction with alternating layers of earlywood and latewood along with the variation of the distribution of chemical constituents in different layers of cell walls and middle lamella. Wood chemical composition also depends on tree age, with juvenile wood deposited in the annual rings during the earliest 15–20 years of growth. Chemical composition also varies in the presence of reaction wood; reaction wood is a special type of tissue formed in response to external stresses and forces which enable the return of bent stems to a vertical orientation (tension wood in hardwoods forms on the upper side of inclined stems and compression wood in softwoods forms on the underside of leaning stems). In general, wood consists of approximately two-thirds of polysaccharides (cellulose and hemicelluloses), with significant differences in the ratio of cellulose to hemicelluloses. In addition, depending on the age and type of tissue, wood may contain other polysaccharides including pectin and xyloglucan. The content of the third main wood component, lignin, varies widely (18–33%) among species, with higher lignin content characteristic for softwoods compared to lower lignin content for hardwood species. Extractives are minor wood components represented by diverse individual compounds ranging from terpenoids and steroids, fats, and waxes to phenolic compounds including stilbenes, tannins, and flavonoids. The inorganic constituents of wood are almost entirely contained in the ash, the residue after burning the organic matter. Even though ash is a minor component of wood (0.1–1.0% in wood of temperate zones), these inorganic compounds play an essential role in the growth of trees [6].
2.1. Polysaccharides
Polysaccharides or carbohydrates include cellulose and hemicelluloses, and are the main components in woody biomass. Because they are constructed from monosaccharides, they revert to monosaccharides by acid or enzymatic hydrolysis.
2.1.1. Cellulose
Cellulose is a linear polymer of β-d-glucopyranose units linked to each other by (1→4)-glycosidic bonds. The linear cellulose chains have a strong tendency to form intra- and inter-molecular hydrogen bonds, which promote aggregation of parallel chains into elementary microfibrils. Celluloses from plant sources are blends or composites of the two distinct forms of cellulose, Iα, which is a single-chain triclinic unit cell and Iβ, which is a two-chain monoclinic unit cell. Celluloses from higher plants, such as wood, are dominantly of the Iβ crystalline form. Most wood species contain 40–45% cellulose based on oven-dry (OD) wood. Degree of polymerization (DP) of cellulose is the number of glucopyranose units in the cellulose chain. The DP of wood cellulose varies from 500 (low-molecular-weight chains at the surface of microfibrils) to 10,000, with the primary wall containing cellulose of a lower DP than the secondary wall. Compression wood of softwoods contains less crystalline cellulose than non-compression wood. For hardwoods, a higher order of cellulose crystallinity has been observed in tension wood compared to that of cellulose in non-tension wood [6].
2.1.2. Hemicelluloses
In contrast to cellulose, hemicelluloses are heterogeneous polysaccharides of much lower DP, typically 50–300 and are essentially amorphous. Hemicelluloses are frequently branched and hence, are relatively soluble in water. Hemicelluloses are easily hydrolyzed, in the presence of acids to their monomeric constituents consisting mainly of d-glucose, d-mannose, d-xylose, l-arabinose, and d-galactose and small amounts of other monosaccharides and derivatives including 4-O-methyl-d-glucuronic acid, l-rhamnose, and d-galacturonic acid. The main hemicelluloses of softwoods are galactoglucomannans (∼20%) and arabinoglucuronoxylans (5–10%), whereas in hardwoods they are glucuronoxylans (15–30%) and glucomannans (2–5%) [6]. Knowing the details on the chemical composition of hemicelluloses in softwoods ((galacto)glucomannans and arabinoglucuronoxylans) and hardwoods (glucomannans and glucuronoxylans) are important because the constitutive monosaccharides and functional groups as well as their way of bonding to the main chain and branches, significantly influence the autohydrolysis results, including rate, composition, and yield of dissolved wood constituents.
(Galacto)glucomannans: Softwood galactoglucomannans can be of low or high galactose content, with the Gal:Glu:Man ratio of 0.1:1:4 and 1:1:3, respectively [6]. The α-d-galactopyranose is linked by a (1→ 6) bond as a single-unit side chain to the main backbone of (1→4) linked β-d-glucopyranose (β-d-Glcp) and β-d-mannopyranose (β-d-Manp). OH's on C2 and C3 positions of glucose and mannose units are partially substituted by acetyl groups (OAc), generally in a ratio of one Ac group per 3–4 hexose units. Glucomannans of hardwoods vary in the glucose to mannose ratio between 1:2 and 1:1, and they may be acetylated (one OAc group per 2–3 hexose units) [6]. However, recent studies have indicated O-acetylation at the C2 or C3 position of some mannose residues of low-molecular weight fractions of aspen and birch glucomannans (DP of ∼16) with a random distribution of acetyl groups and a degree of acetylation of ∼0.3 [7].
Xylans: Softwood arabinoglucuronoxylans are not acetylated, and contain on the average 1.3 (1→3)-linked α-arabinofuranose units and 1.4 (1→2)-linked 4-O-methyl-α-d-glucuronic acid groups (4-O-MeGlcA) per ten xylose units. Because of their furanoside structure, the arabinose side chains are easily hydrolyzed by acids [6]. In contrast to non-acetylated softwood xylan, most of the xylose residues in hardwood glucuronoxylan contain an OAc group at C2 or C3 (∼7 Ac per 10 Xyl units) [8]. The xylan backbone carries on average one 4-O-MeGlcA per 10 Xyl units ((1→2)-linked 4-O-methyl-α-d-glucuronic acid residues). The uronic acid residues have been reported to be distributed irregularly along the hardwood xylan chain, whereas they seem to be periodically distributed in softwood xylans [9]. The glucuronide linkages between the xylose units and the 4-O-MeGlcA substituents have been shown to be more stable toward acid hydrolysis than the β(1→4) linkages between the xylose residues in the xylan backbone. In one study on the stability of glucuronoxylans subjected to hydrolysis with trifluroacetic acid, the MeGlcA-residues remained attached to the backbone of both hardwood and softwood xylans/xylooligosaccharides. Free 4-O-methylglucuronic acid was not detected throughout the entire period of hydrolysis (up to 30 days), whereas xylose and neutral xylooligosaccharides with DP ≤ 4 were released into the solution [9].
2.1.3. Distribution of Polysaccharides
Studies on the distribution of hemicelluloses in different softwood species indicate that even though the composition of different cell wall layers is similar, glucomannan content increases from the outer parts of the cell wall to the inner parts, whereas the arabino-4-O-methylglucuronoxylan content is very high in the S3 layer (inner layer of secondary wall). In the case of hardwood species, the content of glucomannan is uniformly distributed along the cell wall layers, and the S1 (outer layer of S secondary wall) and the outer part of the S2 (middle layer of secondary wall) layers have a very high content of 4-O-methylglucuronoxylan. For softwoods and hardwoods, the cellulose content is lowest in the compound middle lamella (CML equals middle lamella plus primary wall), which contains a high percentage of pectin and xyloglucan. The main polysaccharide in pectin is polygalacturonan consisting of (1→4)-α-linked galacturonic acid units, which may be different in degree of methyl esterification [10]. Even though the pectin content in mature wood has been estimated to be relatively low (up to 5% of walls in woody tissue) methylated galacturonan can be easily demethylated yielding carboxylic groups which may affect wood processing [10]. Xyloglucan is composed of 1,4-linked-β-d-glucopyranosyl residues in the main chain, and is an important hemicellulose in primary walls of higher plants. Some of the β-d-glucopyranosyl residues are substituted on C6 with α-d-xylopyranosyl side groups which may be β-(1→ 2) linked to d-galactopyranosyl unit which may be α-(1→2) linked to l-fucopyranosyl unit. O-acetyl substituents have been also detected, mostly on the galactosyl residues. The notable decrease in xyloglucan content during growth and a rapid degradation after auxin treatment have been observed; these changes suggest that xyloglucan is important in the mechanism of cell enlargement [11]. Recently, xyloglucan has been recognized as the most significant non-cellulosic component of the predominantly cellulose-containing gelatinous layer, a thick specialized cell wall layer inside the secondary cell wall layer of tension wood. Arabinogalactan appears also in tension wood [12]. Compression wood unlike normal softwood contains significant amounts of galactan. β(1→4)-galactan is a very good biochemical marker for compression wood and it is involved in the formation of lignin-carbohydrate bonds in highly lignified cell wall layers of compression wood [13].
2.2. Lignin
Lignin strengthens stems and vascular tissue, allowing upward growth and permitting water and minerals to be conducted through the xylem under negative pressure without collapse of the tissue. In addition to mechanical support, lignin contributes to protective functions in plants by, for example, increasing resistance to biodegradation and environmental stresses such as changes in humidity and water balance. Derived primarily from p-hydroxycinnamyl alcohols (p-HCAs: coumaryl, coniferyl, and sinapyl alcohol; when incorporated into lignin HCAs convert to the p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) unit, respectively), lignin results from the radical-coupling polymerization reactions in the wall after polysaccharides have been laid down. Lignin provides a matrix in which polysaccharides are embedded and possibly cross-linked (lignin-carbohydrate complex, LCC). The result is a naturally occurring composite material, which imparts strength and rigidity to the cell wall and this strength allows organized plants to grow upward [6]. The structure and characteristics of lignin that provide advantages in living plants make lignin problematic in manufacturing some products. Lignin is the component sought for removal in chemical pulping, and it is a major barrier in processing of lignocellulosics to fuels and chemicals. Presently lignin is separated by (primarily the kraft) chemical pulping process. The vast majority of this lignin is burned (heating value of 23.3–25.6 kJ/g for lignin) in recovery boilers (during the regeneration of kraft chemicals (NaOH and Na2S)) providing relatively low cost fuel for pulp mills. Lignin is the most abundant natural aromatic polymer, however, despite this natural abundance, a minor amount of the total available lignin is used in higher-value products or specialty commodity applications [14].
Lignin polymerization proceeds with the coupling of different resonance structures of phenoxy radicals resulting from enzyme-initiated dehydrogenation of p-HCAs. The phenoxy oxygen and the Cβ are considered the most reactive in these resonance structures, readily coupling into aryl-ether linkages. This may account for the high abundance of the β-O-4 inter-unit linkages in lignin, estimated to be as high as 50% in softwoods and almost 60% in hardwoods. More than two-thirds of the phenylpropanoid units in lignins are linked by ether bonds and the rest by carbon-carbon bonds. Different contribution of p-HCAs in the biosynthesis of hardwood (SG-lignin) and softwood (G-lignin) lignins causes significant differences in the lignin structure, including the character and content of different bonds between phenylpropanoid units and the main functional groups (for example, methoxyl, phenolic hydroxyl, aliphatic hydroxyl, carbonyl, and carboxyl groups). The differences in structure cause differences in the reactivity among different lignin types with hardwood lignin being less resistant against microbiological attack, and more reactive and less prone to condense during chemical processing [6,15].
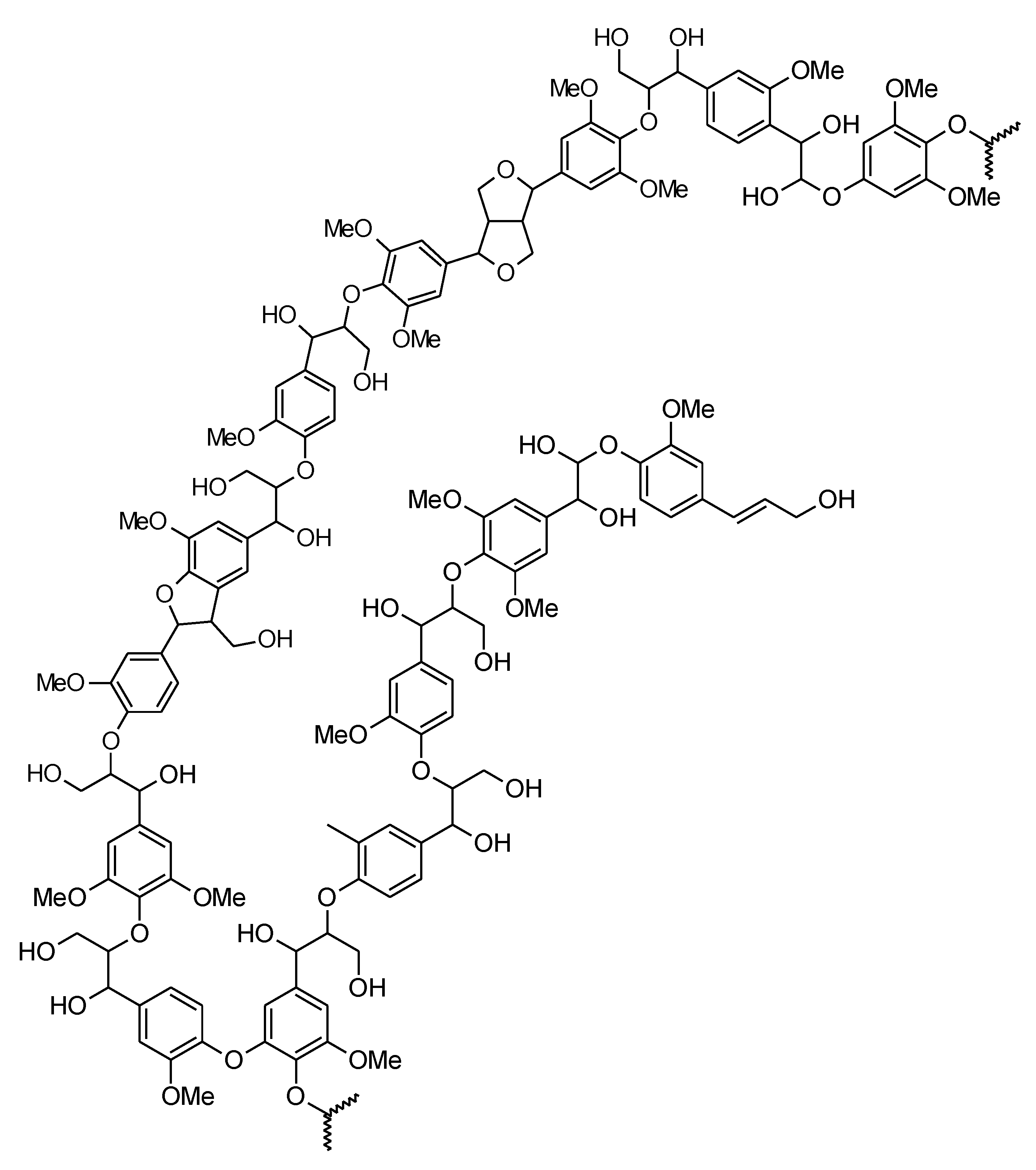
A small fraction of lignin building blocks are not phenylpropanoid units. These units may be aromatic aldehydes (e.g., vanillin and coniferaldehyde) or aromatic carboxyl acids (e.g., vanillic and ferulic acid). They are proposed to be linked to lignin by ether bonds as lignin end units. Some lignins are esterified with phenolic acids; for example, aspen lignin is esterified with p-hydroxybenzoic acid. Also, dihydrocinnamyl alcohols, arylpropane-1,3-diol, and arylglycerol are sporadically incorporated into the lignin structure [15]. A suggested model of hardwood lignin is presented in Figure 2.
Lignin that is representative of protolignin is isolated by the MWL (milled-wood lignin) method after ball-milling of extractive-free wood and extraction with dioxane-water. Purification of extracted lignin includes its precipitation in water from an acetic acid solution. The yield is usually ∼25%, based on the Klason lignin content (the most commonly used method in determination of the content of lignin using the two-step acid hydrolysis of polysaccharides with 72% followed by 3% H2SO4). In spite of purification, MWL is not carbohydrate-free. This is an important indicator of the presence of lignin-carbohydrate bonds in wood. It is notable that MWLs from hardwoods contain larger amounts of carbohydrates (a few percent) than MWLs from softwoods (traces) [16].
Even though the lignin concentration is high in the middle lamella and it decreases in the lumen direction, at least 70% of the total lignin in wood is located in the secondary wall due to the thickness of that wall. For example, the lignin concentration in the secondary wall, middle lamella, and cell corners of tracheids in the earlywood of black spruce is 23%, 50%, and 80%, respectively, with more than 70% of the total lignin located in the secondary wall, which is 87% of the total wood tissue volume [6]. There are species and morphological variations in lignin distribution. For example, ray parenchyma cells, which constitute about 5% by weight of xylem in softwoods, contain significantly higher lignin concentration than the whole wood, whereas in hardwood species, ray parenchyma cells have lower lignin concentration than fibers which are, in turn, of lower lignin content than vessels. The S/G (syringyl-to-guaiacyl) ratio of lignin is different in different morphological regions of hardwoods. Principally, the SW of fibers contains predominantly S units, whereas SW of vessels consists mostly of G units [6]. Compression wood is of higher lignin content than normal softwood and contains more H- and fewer G-units [13]. In contrast to compression wood, in hardwoods, tension wood is of lower lignin content than non-tension wood because the gelatinous layer in tension fibers is almost devoid of lignin [17].
2.3. Lignin-Carbohydrate Complex, LCC
The difficulties in separation of lignin and carbohydrates during isolation of MWL and production of pulp have frequently been attributed to the presence of the lignin-carbohydrate bonds in native wood [18]. The question of whether lignin is chemically bound to polysaccharides in the plant cell wall has been critical to understanding the architecture of the cell wall and the hierarchy of its biosynthesis. The results of extensive studies have shown that covalent bonds between lignin and carbohydrates (LC bonds) are present in lignified cell walls. Four types of native LC bonds have been proposed: benzylether, benzylester, phenylglycoside and acetal bonds; of these, benzylether and benzylester have been considered to be the most probable LC bonds in native wood [19]. There are different methods in the isolation of LCC from wood, and all of them aim at preparing representative LCC; that is, they are aimed at excluding both the cleavage of the native bonds and the formation of artificial bonds. Isolated LCC samples have been analyzed to define the type of LC bonds by a wide variety of indirect methods, including acid and alkali treatment, sodium borohydride reduction, and methylation, as well as lignin-specific methods such as DDQ oxidation (DDQ: 2,3-dichloro-5,6-dicyano-1,4-benzoquinone) [19]. Recently, powerful 2D NMR techniques have been suggested as an efficient method of LC bond analysis because NMR analysis excludes tedious analytical steps and the possibility of chemical change to the LCC [20].
2.4. Extractives
Extractives represent a minor fraction of the total wood components and are composed of a wide array of individual hydrophilic and hydrophobic compounds. Related to issues of sustainability and more efficient utilization of natural resources such as wood, extractives are receiving increased attention for potential recovery and sale as low-volume, but high-value, products. The content of extractives is usually less than 10%, but can vary from trace amounts to 40% of the total dry wood weight. Various parts of the same tree can have varying content and composition of extractives. Extractives are usually present in certain morphological sites in wood structure. Resin acids for example are located in resin canals whereas fats are in the ray parenchyma cells and phenolic extractives are present in heartwood and bark [6]. Extractives are necessary to maintain and perform a diversified set of functions in trees. Fats are known as an energy source of wood cells whereas terpenoids, resin acids, and phenolics protect the tree against microorganism attack. Extractives are not only useful and important for understanding the taxonomy and biochemistry of the trees, but also are important when considering the technological aspects of wood usage in industry; e.g., pulping and papermaking. In the chemical recovery and regeneration process, tall oil and turpentine are recovered as traditionally the most important by-products of the kraft pulp industry. These are utilized primarily in the manufacture of specialty chemicals and a wide range of industrial products, including solvents, adhesives, polymers, emulsifiers, coatings, and paper sizing. For example, polyphenolics, valued for their high antioxidizing efficiency, have been examined and different processes for isolation from wood have been suggested. Exploitation of extractive-rich knotwood (if separated from wood prior to pulping) has been suggested as a pathway for recovering lignans from softwoods and flavonoids from hardwoods. Mineral components of extractives (Table 1) are important as they may impact processing of wood. Therefore, reduction of mineral content during hot-water extraction (4) is desirable.
The major constituents of woody biomass (cellulose, hemicelluloses and lignin) cannot be simultaneously isolated as polymers and several processes must be employed involving the degradation of at least one of the polymers. Of the three major components, hemicelluloses are the easiest to degrade, while cellulose is the most difficult to degrade. Therefore, an ideal method would be to separate hemicelluloses from woody biomass first and convert cellulose as the last step, leading to an incremental disassembly of woody biomass. Table 1 shows a summary of the components of hemicellulose and extractives. These components provide opportunity for building a diversified biorefinery product portfolio from lignocellulosic biomass.
At high temperature during hot-water extraction, autohydrolysis of wood takes place. Autohydrolysis is catalyzed by the hydronium ions (H+) originating from water and acidic compounds generated in situ. Acetic acid (pKa = 4.76) is formed during deacetylation and promotes random cleavage of branched hemicellulosic chains resulting in a mixture of oligosaccharides and monosaccharides. Being stable in acid conditions, glycuronide bonds, i.e., 4-O-MeGlcA residues stabilize xylans against hydrolysis [6,22]. Accordingly, higher contents of acetylated and less acidic xylans and more acid-soluble SG lignin make hardwoods more amenable to hot-water extraction than softwoods. This translates into higher quantities of valuable products, such as acetic acid and lignin from hardwoods. Significant differences in the properties and chemical composition between normal and reaction wood, including lower contents of hemicelluloses in both compression and tension wood in softwoods and hardwoods, respectively [23] affect quantity and quality of extracts produced during hydrothermal treatments. These differences require selection of normal wood for careful chemical research. Therefore, this report primarily elaborates the results from hot-water extraction of hardwood pulpwood which has little or no reaction wood.
3. Economically Viable Deconstruction and Utilization of Woody Biomass
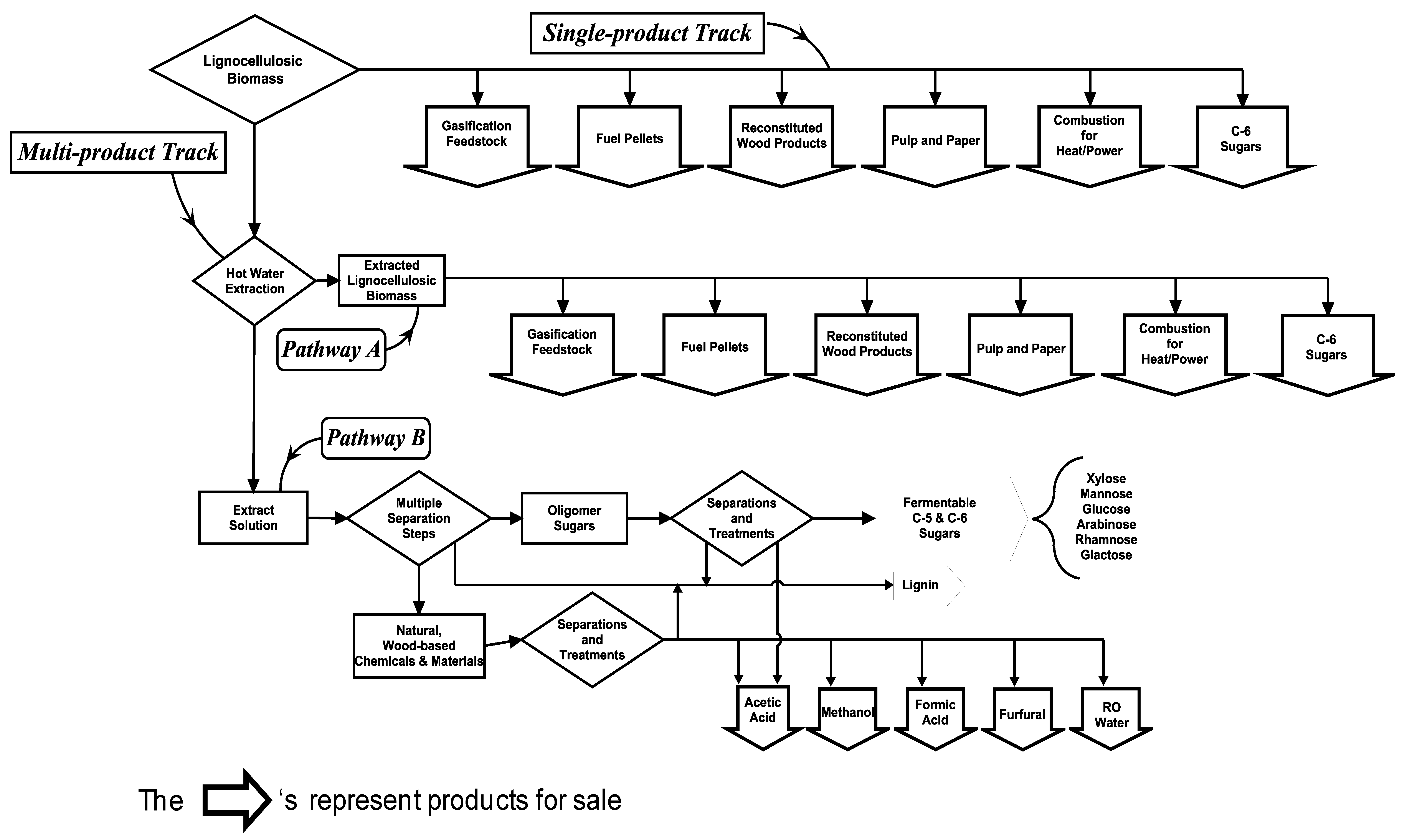
Lignocellulosic biomass is a heterogeneous raw material consisting of the major structural components, cellulose, lignin, and hemicelluloses, as well as the minor components, extractives and ash. Figure 3 shows contrasting generalized process flows for a single-product biorefinery versus a multi-product biorefinery; each of which might process lignocellulosic biomass with particular emphasis on “wood”. The diagram contains diamond-shaped, “decision boxes” depicting junctures where the process flow bifurcates at a separation/purification step. Since, it is virtually axiomatic that increased value can be generated by isolating relatively pure substances from heterogeneous raw materials, it follows then that a greater number of “decision boxes” (purification opportunities) in a biorefinery process flow, should result in greater total value recovered.
The Single-product Track shows traditional uses for lignocellulosic biomass. Note that there are no separation/purification opportunities along the Single-product Track. In each single-use case “cellulose” is always a target, and, generally in manufacturing these products, there is no significant volume or value of co-products produced. The various single-product cases can be divided into two groups based on whether cellulose is “purified” by substantially removing the other components (as is the case for pulp and paper), or whether some or all the components remain with the cellulose through to the end product (as is generally the case with products such as fuel pellets and fiberboard). Either way, under a single-product scenario, it is difficult to claim that substantive, multi-component value is recovered from woody biomass. Moreover, in cases where nearly pure cellulose is required for the end product, purification is often accomplished by transforming, contaminating, or disassembling the other woody components into low value materials the disposal of which is accomplished through costly sub-operations for energy recovery or wastewater treatment. For example, delignification of cellulose for pulp is mostly accomplished by kraft cooking which creates sulphur-containing kraft lignin, thusly turning a product which might sell for “dollars” per pound (in pure form) into a contaminated product that has little more than recovery boiler heat value.
On the other hand, the Multi-product Track contains several separation/purification steps in which components of lignocellulosic biomass are “purified”, and therefore increased in value as saleable products [24-26]. Also note that, since the structural components of woody biomass (cellulose and lignin) remain largely intact during and after hot water extraction, the Multi-product Track contains the same wood product manufacturing opportunities as the Single-product Track. Traditionally, lignin has been treated more as a component that needed to be “gotten out of the way” because there was no technology for recovering lignin as a high value product. Now, under new non-sulfur processes allowing recovery of high value lignin, a very significant improvement in profitability in biorefinery operations is possible [14]. Along Pathway A of the Multi-product Track, items manufactured from residual, extracted woody biomass are less costly to produce, and/or are improved in quality or function compared to the same goods manufactured using woody biomass untreated by hot water extraction. This is true because, with the hemicellulose components mostly removed, among other advantages, the residual woody biomass is more chemically reactive and substantially less hydrophilic. These two properties alone result in reduced processing time, reduced chemical costs, increased digester yield, and increased hydro-stability [4,27,28].
The preceding discussion assumes the following caveats. To make sound choices about which woody biomass components to target for commercial production, each separation/purification opportunity must be conceived from a deep understanding of lignocellulosic biomass chemistry, bioprocess engineering, and demand for marketable products. Then, the entire separation sequence must function to meet production goals within an industrially viable time-frame.
4. The ABS Process™
The heterogeneous chemical composition of woody biomass gives rise to opportunities for a multiple-product-portfolio when incremental deconstruction of woody biomass is implemented. The ABS Process™ is a biorefinery technology that cooks (using Hot Water Extraction™, or “HWE™”) lignocellulosic biomass, in water only, to extract and purify naturally occurring chemicals which can serve as renewable alternatives to fuels and industrial chemicals currently derived from petroleum, coal, and natural gas [4,21,24-31]. However, in addition to producing multiple platform chemicals, HWE™ leaves the structural portion of the extracted biomass still largely intact, but improved, through creation of several key, HWE Advantaged™ properties which add significant value. For example, wood chips subjected to HWE™ are more energy dense (Btu/ton), lighter (per unit of equal volume), lower in ash content, and markedly less hydrophilic. These improvements have significant implications for HWE™ applications in the manufacture of conventional wood derived products such as paper, fiberboard, chipboard, and fuel pellets [27,28,32,33]. Similarly, agricultural residues become more chemically reactive following HWE™, and cellulose has been reported to be more readily processed into derivatives including C-6 sugars available for chemical conversion [34,35]. By virtue of these multiple, platform-product pathways, HWE™-based biorefinery technology can serve at least three global industrial market opportunities: wood derived products, fermentation products, and commercial chemicals.
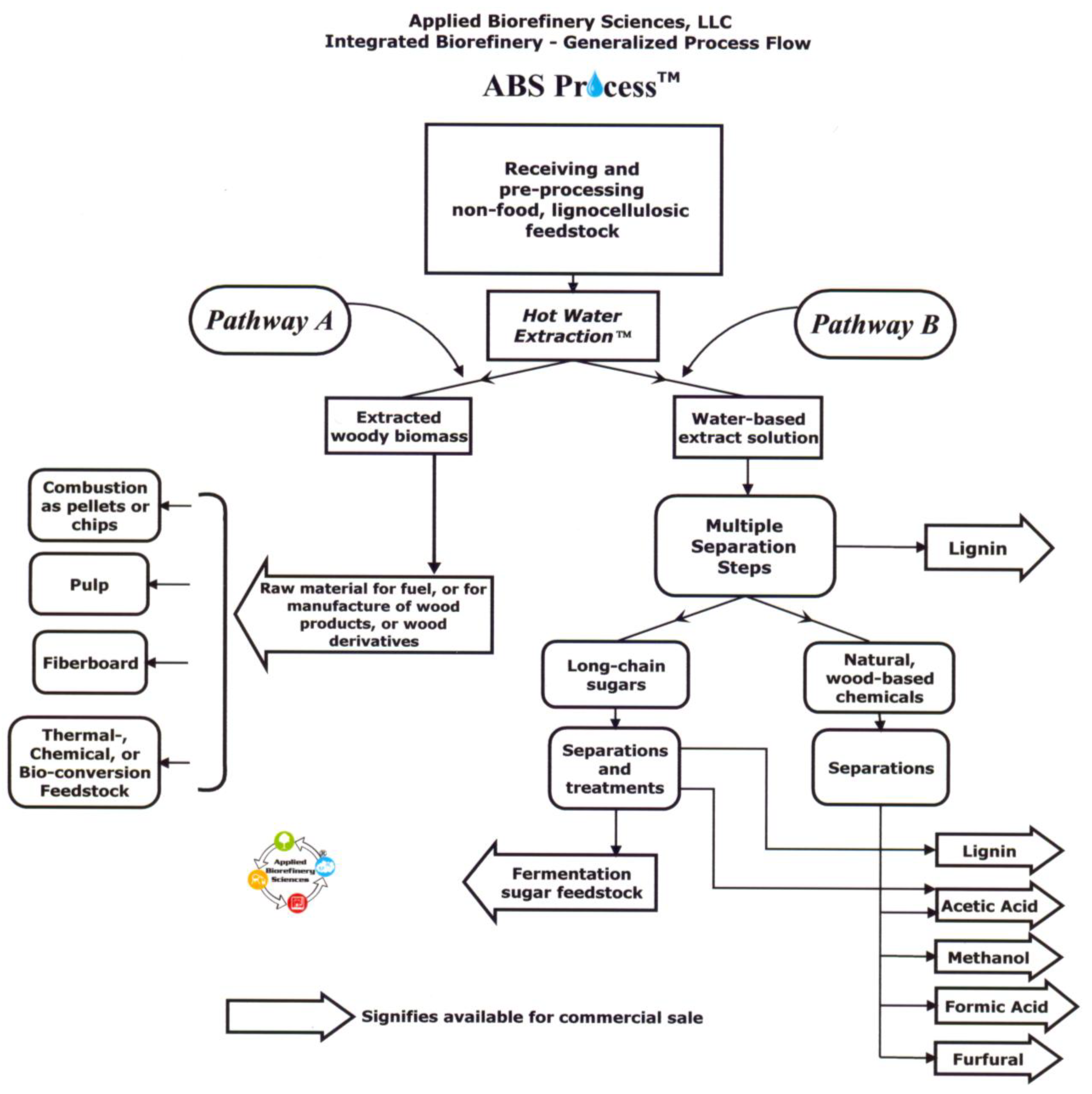
The patent pending ABS Process™ is an over-arching biorefinery sequence that encompasses steps from pretreatment of lignocellulosic raw material through to purification and preparation of multiple platform materials for commercial sale. As can be seen in Figure 4, the main ABS Process™ subsystems include comminution, screening, and cleaning of incoming raw plant biomass, followed by Hot Water Extraction™, followed by numerous steps to separate, recover, and concentrate output products for transport.
HWE™ in the absence of mineral acids and bases is preferred because of its ability to generate multiple high value output products without chemical input, recovery, or disposal costs. Instead of added chemicals in the cooking phase, the ABS Process™ relies upon an autocatalytically driven reaction in which deacetylation of hemicelluloses occurs in liquid water at temperatures from 160 to 170 °C. The resulting acidic conditions (final pH ∼3.5) permit further solubilization and hydrolysis of hemicelluloses, but the relatively mild temperatures used avoid extensive acid degradation of monosaccharides. Oligomeric 5- and 6-carbon sugars, acetic acid, aromatic compounds, monomer sugars and other trace compounds diffuse into the extract solution (leading to the right branch of processes in Figure 4, or Pathway B). With few degradation products in the extract (especially furfural), subsequent separation processes are facilitated. Membrane fractionation and other chemical separation techniques are applied to the extract solution to isolate and purify fermentable sugars, acetic acid, lignin, furfural, formic acid, and other hemicellulose related compounds for sale commercially. In Pathway A (or the left branch of processes in Figure 4), extracted lignocellulosic fiber, with reduced hemicellulose content, carries the advantage of expanded product opportunities, as well as enhanced product characteristics at reduced energy and chemical manufacturing costs.
5. Partial List of Product Potentials
Platform chemical and material outputs from the ABS Process™ will serve at least three global, industrial markets: wood derived products, fermentation products, as well as commercial chemicals and materials. Following are specific examples of potential products in each of these markets.
5.1. Wood Derived Products
Many conventional wood derived products can be improved when produced from woody biomass subjected to HWE™. Following hot-water extraction the residual woody material contains predominantly cellulose and lignin. Cellulose is an excellent biopolymer of wide applications. High heat content lignin is an aromatic polymer with a wide range of valuable commercial applications. Following are examples emphasizing the benefits of HWE™.
Pulp and paper: Following removal of most hemicellulose related compounds through HWE™, residual sugar maple chips are reduced in mass typically by 23%. These HWE Advantaged™ chips, with reduced hemicellulose content, higher porosity (lower density), and with lignin of higher reactivity and weaker LCC, can improve pulping and bleaching, improve net energy efficiencies, and can offer lower chemical use, increased pulp viscosity, as well as higher bulk, absorbance, and stiffness of pulp [4]. The paper has lower tensile strength which can be recovered by the application of green additives such as the conventional dry-strength agent, starch and recently evaluated polylactic acid (PLA) while retaining the advantages associated with higher bulk [32].
Fuel Pellets: ABS research on fuel pellets produced from HWE Advantaged™ woody materials showed that these pellets have: (1) reduced ash content, (2) increased Btu/lb content, (3) increased hydro-stability, and (4) increased physical durability. HWE™ induced ash reduction (property #1) is well documented and properties 2, 3, and 4 are consistent with higher relative lignin and lower hemicellulose contents. All these improved properties combine to create superior fuel pellets [27,28,33].
Fiberboard: Decreased woodchip bulk density (after hot-water extraction) leads to lighter products, decreased hydrophilicity, increased weather resistance, increased dimensional stability, increased mold resistance. So this could be ideal material for reconstituted wood products. A project was underway in joint collaboration between SUNY ESF and Washington State University [27].
Carboxymethylated Cellulose Hot water pre-extraction of wood significantly enhanced the reactivity of the cellulose component in the residual woody material and could facilitate the preparation of the carboxymethyl-cellulose (CMC) as the bulk of hemicelluloses are removed [35].
Nanocellulose: Nanocellulose is a high value product that can be obtained by acid hydrolysis of cellulose. Removal of hemicellulose material through HWE™ makes the residual lignocellulosic material more chemically reactive and amenable to delignification [36]. In addition, the increased relative cellulose content, along with more accessible regions of amorphous cellulose, in combination is expected to improve recovery of nanocellulose.
5.2. Fermentation Products
Up until the issuance of the Energy Independence and Security Act (“EISA”) in 2007, processes increasing utility of non-food plant biomass generally, as a group, targeted depolymerization of cellulose into glucose for fermentation to ethanol. Per stipulations in EISA 2007, the United States Department of Energy (“DOE”) began to include other sugar-based alcohol and biodiesel fuels as acceptable end products for biomass based processes, but fuels derived from sugar fermentation still dominate the R&D objectives of government grants, and therefore both public and private sector agendas. Ethanol is the most common product of fermentation of sugars to chemicals, materials, and fuel [26,29]. Examples of other products which have been extensively studied are butanol, propane-diol, hydrogen, higher alkanes, polyhydroxyalkanoates (PHA), and lactic acid. Xylitol, fermented specifically from 5-carbon (xylose) extracted sugars, is used as a sweetener.
5.3. Commercial Chemicals and Materials
As shown in Figure 4, the ABS Process™ extracts, separates, and recovers naturally occurring, water soluble hemicellulose related products such as sugars, acetic acid, formic acid, furfural, and methanol. All chemicals in this group are commercially saleable, and/or may be converted to numerous commodity chemicals and materials. Lignin recovered by the ABS Process™ is more valuable because it is sulfur-free, and contaminating carbohydrates may be removed in a separate step if desired. A wide range of products based on this lignin may be suggested and is under study, including adhesives, thermoplastics, and oxygenated phenolics [37].
6. Feedstock Supply
Unanticipated supply chain issues can be a fatal bottleneck in any business. For biorefinery operations, one critical supply chain element is “feedstock”. Biorefineries must be located and scaled with full consideration and acceptance of the fact that raw biomass cannot be economically transported long distances. In agricultural and forested regions of the United States, with its well developed road system, raw agricultural biomass may be transported up to a maximum 50–60 miles, while for more dense biomass from trees the distance may be 75–100 miles. Beyond these distances, fuel and time costs become increasingly prohibitive, and threaten long-term viability. In addition to distance factors, feedstock supply may be subject to seasonal variability. Biomass from trees is available most times of the year except for the transition period from winter to spring. Historically, since wood can be stored for months without deterioration, forest product industries have circumvented this issue by adopting systems for stockpiling raw material sufficient to span the annual periods when wet soil conditions may prohibit access by logging equipment. Agricultural materials, different from trees, grow seasonally, and spoil more quickly. Therefore, biorefinery processes utilizing agricultural materials as feedstock must either adjust production schedules to accommodate different feedstock sources or develop low-cost storage systems, which arrest or deter spoilage. Woody feedstock is subject to potential increased competition from alternative uses that require project developers to have very good local business knowledge and significant local consensus on the desirability of a biorefinery project. While some biomass will continue use as a source of energy for transportation and heat, it is the authors' opinion that the highest and best use of lignocellulosic biomass is primarily as a renewable substitute for chemicals and polymeric materials currently derived from fossil sources.
7. Conclusions and Discussions
Petroleum is an unsustainable energy source; even the most conservative projections indicate crude oil reserves will “peak” (half of the known reserves consumed) by 2030, and the crude oil will be depleted by the year 2100 [38]. Tapping back into the potential of biomass as a renewable source of materials and cleaner energy is imperative to facilitate the sustainability of human civilization. Further development and deployment of lignocellulosic-based biorefinery technology, making full use of all available forest materials, woody biomass crops, perennial grasses, and agricultural residues, will be a valuable contribution to sustainable world development.
From a manufacturing perspective, assuming an adequate, cost-conscious feedstock supply, economic viability is enhanced by optimizing potential product value from raw materials. In this regard, similar to a petroleum refinery, a biorefinery should be designed with processes that extract as much high-value product from raw material as is cost-effectively possible. Analyses have been conducted showing positive ROI, but discussion on biorefinery economics is outside the scope of this article [28]. For biorefineries, woody biomass presents both challenge and opportunity since it is heterogeneous with at least four major constituents: cellulose, hemicellulose, lignin and extractives. Extractives and hemicellulose are the easiest components to degrade either chemically or biologically, while cellulose and lignin are significantly more resistant. Therefore, to optimize value from lignocellulosic biomass, biorefinery modification of woody material into usable products will necessarily require different designs or sequences to reflect the differing requirements for component conversion. Such value-optimization pathways are available through a biorefinery technology (the ABS Process™) being commercialized by Applied Biorefinery Sciences, LLC (“ABS”). For example, a manufacturing process flow using ABS hardwood centric, water-based biorefinery technology might include: an initial hot-water extraction (HWE™), followed by two, sub-branched pathways. Along Path A, the extracted lignocellulosic fiber is used for manufacturing traditional wood products including pulp and paper, fuel pellets, and fiberboard. Along Path B, the extract solution undergoes modification to isolate extracted hemicellulose compounds for sale as fermentable sugars, chemicals, and materials.
Hot water extraction in the absence of mineral acids and bases is preferred because of its ability to generate multiple high value output products without chemical input, recovery, or disposal costs. Instead of added chemicals in the cooking phase, the ABS Process™ relies upon an autocatalytically driven reaction in which acetyl groups inside the woody biomass are solubilized under heat and pressure. The resulting acidic conditions (final pH ∼3.5) permit further solubilization and diffusion of oligomeric 5- and 6-carbon sugars, acetic acid, aromatic substances, monomer sugars and other trace compounds into the extract solution. In Path A (above), the extracted lignocellulosic fiber, with reduced hemicellulose content, carries the advantage of enhanced product characteristics at reduced energy and chemical manufacturing costs. Then, (via Path B above) membrane fractionation and other chemical separation techniques are applied to the extract solution to isolate and purify fermentable sugars, acetic acid, lignin, furfural, formic acid, and other hemicellulose related compounds for commercial sale. As described heretofore, when commercially deployed, the biorefinery technology contained in the ABS Process™ will bridge the gap between science and an economically viable pathway for capturing, sustainable, renewable value not currently realized from lignocellulosic biomass.
| Type | Distribution, wt % | ||
|---|---|---|---|
| Soft-woods | Hard-woods | ||
| Hemicellulose | 25 ∼ 30 | 25 ∼ 35 | |
| Galactoglucomannan (1:1:3) |  i.e., R = CH3CO (Ac) or H i.e., R = CH3CO (Ac) or H | 5 ∼ 8 | 0 |
| (Galacto)glucomannan (0.1: 1:4) | 10 ∼ 15 | 0 | |
| Glucomannan (1:2–1:1) |  | 0 | 2 ∼ 5 |
| Arabinoglucuronoxylan | 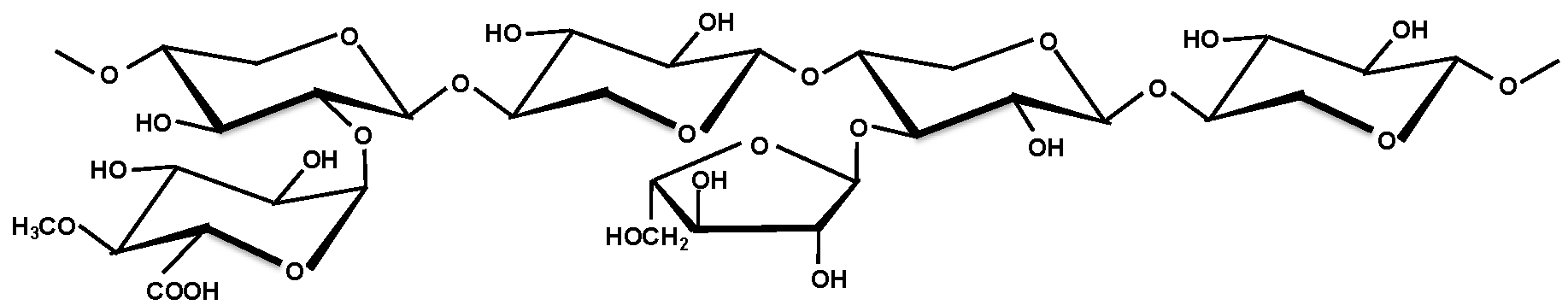 | 7 ∼ 10 | Trace |
| Glucuronoxylan | 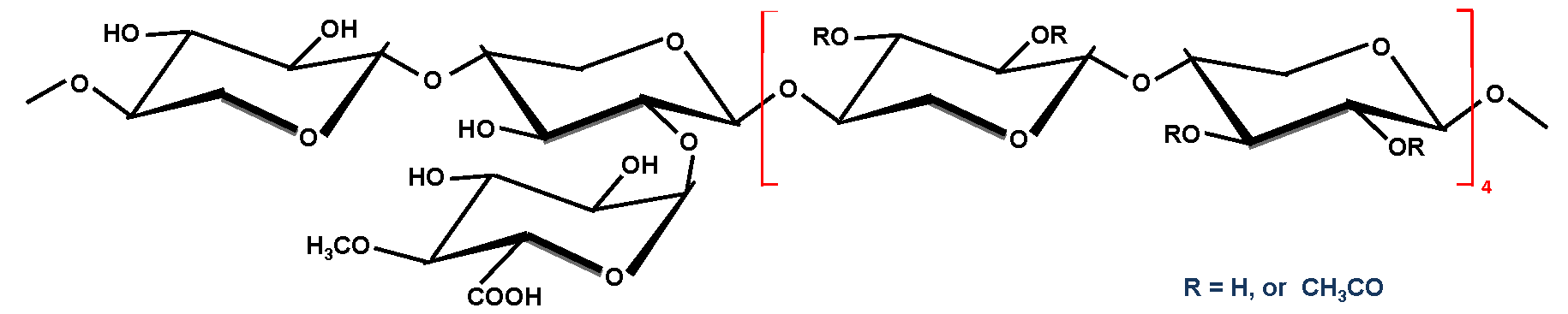 i.e., R = CH3CO (Ac) or H i.e., R = CH3CO (Ac) or H | Trace | 15 ∼ 30 |
| Extractives | 5 ∼ 8 | 2 ∼ 4 | |
| Aliphatic and alicyclic | terpenes, terpenoids, esters, fatty acids, alcohols,… | ||
| Phenolics | phenols, stilbenes, lignans, isoflavones,… | ||
| Carbohydrates | arabinose, galactose, glucose, xylose, starch, pectic material | ||
| Others | cyclitols, tropolones, amino acids, protein, alkaloids, … | ||
| Ash | Ca, K, Mg, Mn, Na, P, Fe, Si, etc. | 0.2 ∼ 0.5 | 0.2 ∼ 0.8 |
References
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–240. [Google Scholar]
- Thoen, J.; Busch, R. Industrial chemicals from biomass—Industrial concepts. In Biorefineries— Industrial Processes and Products: Status Quo and Future Directions; Kamm, B., Gruber, P.R., Kamm, M., Eds.; Wiley-VCH: Weinheim, Germany, 2006; Volume 2. [Google Scholar]
- Klass, D. Biomass for Renewable Energy, Fuels, and Chemicals; Academic Press: New York, NY, USA,, 1998. [Google Scholar]
- Amidon, T.E.; Wood, C.D.; Shupe, A.M.; Wang, Y.; Graves, M.; Liu, S.J. Biorefinery: Conversion of woody biomass to chemicals, energy and materials. J. Biobased Mater. Bioenergy 2008, 2, 100–120. [Google Scholar]
- Liu, S.J.; Deng, Y.L. A special issue on the biorefinery: Employing biomass to relieve our dependence on fossil sources. J. Biobased Mater. Bioenergy 2008, 2, 97–99. [Google Scholar]
- Sjötröm, E. Wood Chemistry. Fundamentals and Applications, 2nd ed.; Academic Press: San Diego, CA, USA, 1993. [Google Scholar]
- Teleman, A.; Nordtsröm, M.; Tenkanen, M.; Jacobs, A.; Dajlman, O. Isolation and characterization of O-acetylated glucomannans from aspen and birch wood. Carbohydr. Res. 2003, 338, 807–815. [Google Scholar]
- Teleman, A.; Lundqvist, J.; Tjerneld, F.; Stålbrand, H.; Dahlman, O. Characterization of 4-O-methylglucuronoxylan isolated from aspen employing 1H and 13C NMR spectroscopy. Carbohydr. Res. 2000, 329, 807–815. [Google Scholar]
- Jacobs, A.; Larsson, P.T.; Dahlman, O. Distribution of uronic acid in xylans from various species of soft- and hardwood as determined by MALDI mass spectrometry. Biomacromolecules 2001, 2, 979–990. [Google Scholar]
- Hafrén, J.; Westermark, U. Distribution of acidic and esterified polygalacturonons in sapwood of spruce, birch and aspen. Nord. Pulp Paper Res. J. 2001, 16, 284–289. [Google Scholar]
- Hayashi, T. Xyloglucans in the primary cell wall. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 139–168. [Google Scholar]
- Mellerowicz, E.J.; Bausher, M.; Sundberg, B.; Boerjan, W. Unravelling cell wall formation in the woody dicot stem. Plant Mol. Biol. 2001, 47, 239–274. [Google Scholar]
- Timell, T.E. Recent progress in the chemistry and topochemistry of compression wood. Wood Sci. Technol. 1982, 16, 83–122. [Google Scholar]
- Meister, J.J. Modification of lignin. J. Macromol. Sci. Polym. Rev. 2002, C42, 235–289. [Google Scholar]
- Ralph, J.; Brunow, G.; Boerjan, W. Lignins. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; Available online: www.els.net (accessed on 8 November 2011). [Google Scholar]
- Lin, S.Y.; Dence, C.W. Methods in Lignin Chemistry; Springer: Berlin, Germany, 1992. [Google Scholar]
- Timell, T.E. The chemical composition of tension wood. Sven. Papperstidning 1969, 72, 173–181. [Google Scholar]
- Björkman, A. Studies on finely divided wood. II Extraction of lignin-carbohydrate complexes with neutral solvents. Sven. Papperstidning 1957, 60, 243–251. [Google Scholar]
- Koshijima, T.; Watanabe, T. Association between Lignin and Carbohydrates in Wood and Other Plant Tissues Springer Series in Wood Science; Springer: Berlin, Germany, 2003. [Google Scholar]
- Balakshin, M.Y.; Capanema, E.A.; Chang, H.-M. MWL fraction with high concentration of lignin carbohydrate linkages: Isolation and 2D NMR spectroscopic analysis. Holzforschung 2007, 61, 1–7. [Google Scholar]
- Liu, S.J. Woody biomass: Niche position as a source of sustainable renewable chemicals and energy and kinetics of hot-water extraction/hydrolysis. J. Biotech. Adv. 2010, 28, 563–582. [Google Scholar]
- Springer, E.L.; Zoch, L.L. Hydrolysis of xylan in different species of hardwoods. Tappi 1968, 5, 214–218. [Google Scholar]
- Sjöström, E.; Alén, R. Analytical Methods in Wood Chemistry, Pulping, and Papermaking; Springer: Berlin, Germany; New York, NY, USA, 1999. [Google Scholar]
- Hu, R.F.; Lin, L.; Liu, T.J.; Liu, S.J. Dilute sulfuric acid hydrolysis of sugar maple wood extract at atmospheric pressure. Bioresour. Technol. 2010, 101, 3586–3594. [Google Scholar]
- Liu, S.; Amidon, T.E. Wood CD membrane filtration: Concentration and purification of hydrolyzates from biomass. J. Biobased Mater. Bioenergy 2008, 2, 121–134. [Google Scholar]
- Liu, T.J.; Lin, L.; Sun, Z.J.; Hu, H.F.; Liu, S.J. Bioethanol fermentation by robust recombinant E. coli FBHW using hot-water wood extract hydrolyzate as substrate. J. Biotech. Adv. 2010, 28, 602–608. [Google Scholar]
- Amidon, T.; Liu, S.; Howard, J.; Yadama, V. Biofuels from woody biomass and potential integration into existing wood fuel, wood products, and pulp and paper products, Presented at the Energy, Utility & Environment Conference (EUEC) 2010, Phoenix, AZ, USA, 1–3 February 2010.
- Amidon, T.E.; Howard, J.R.; Wood, C.D.; Liu, S. Business case for a 300 dtpd *HWE™ based commercial hardwood biorefinery in the USA (*Hot Water Extraction). Proceedings of the PIRA's Biorefining & Fibre Engineering Conferences 2011: Biorefining for the Pulp & Paper Industry, Barcelona, Spain, 24–26 May 2011.
- Amidon, T.E.; Liu, S.J. Water-based woody biorefinery. Biotechnol. Adv. 2009, 27, 542–550. [Google Scholar]
- Liu, S.J. A kinetic model on autocatalytic reactions in woody biomass hydrolysis. J. Biobased Mater. Bioenergy 2008, 2, 135–147. [Google Scholar]
- Liu, S.J.; Mishra, G.; Amidon, T.E.; Gratien, K. Effect of hot-water extraction of woodchips on the kraft pulping of eucalyptus woodchips. J. Biobased Mater. Bioenergy 2009, 3, 363–372. [Google Scholar]
- Hasan, A.; Bujanovic, B.; Amidon, T.E. Strength properties of kraft pulp produced from hot-water extracted woodchips within the biorefinery. J. Biobased Mater. Bioenergy 2009, 4, 1–7. [Google Scholar]
- Chaffee, T. Potential for Enhanced Properties of Wood Products by Hot Water Extraction of Low-Value, Undebarked Ponderosa Pine. MS Thesis, SUNY College of Environmental Science & Forestry, Syracuse, NY, USA, 2011. [Google Scholar]
- Jahan, M.S.; Saeed, A.; Ni, Y.H.; He, Z.B. Pre-extraction and its impact on the alkaline pulping of bagasse. J. Biobased Mater. Bioenergy 2009, 3, 380–385. [Google Scholar]
- Chien, S.N.; Ren, H.; Aoyagi, M.; Amidon, T.E.; Lai, Y.Z. Fractionation of wood polymers by carboxymethylation-influence of reaction conditions. J. Biobased Mater. Bioenergy 2010, 4, 40–45. [Google Scholar]
- Gong, C.; Goundalkar, M.; Bujanovic, B.; Amidon, T.E. Evaluation of different sulfur-free delignification methods for hot-water extracted hardwood. J. Wood Chem. Technol. 2011. in press. [Google Scholar]
- Ye, P.X.; Cheng, L.; Ma, H.; Bujanovic, B.; Goundalkar, M.; Amidon, T. Biorefinery with water. In The Role of Green Chemistry in Biomass processing and Conversion; Xie, H., Gathergood, N., Eds.; Wiley John & Sons: Hoboken, NJ, USA, 2011; in press. [Google Scholar]
- Roberts, P. The End of Oil: On the Edge of a Perilous New World; Houghton Mifflin Co: New York, NY, USA, 2004. [Google Scholar]
- Conflict of Interest: The authors declare no conflict of interest.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Amidon, T.E.; Bujanovic, B.; Liu, S.; Howard, J.R. Commercializing Biorefinery Technology: A Case for the Multi-Product Pathway to a Viable Biorefinery. Forests 2011, 2, 929-947. https://doi.org/10.3390/f2040929
Amidon TE, Bujanovic B, Liu S, Howard JR. Commercializing Biorefinery Technology: A Case for the Multi-Product Pathway to a Viable Biorefinery. Forests. 2011; 2(4):929-947. https://doi.org/10.3390/f2040929
Chicago/Turabian StyleAmidon, Thomas E., Biljana Bujanovic, Shijie Liu, and Joel R. Howard. 2011. "Commercializing Biorefinery Technology: A Case for the Multi-Product Pathway to a Viable Biorefinery" Forests 2, no. 4: 929-947. https://doi.org/10.3390/f2040929





