Monthly Dynamical Patterns of Nitrogen and Phosphorus Resorption Efficiencies and C:N:P Stoichiometric Ratios in Castanopsis carlesii (Hemsl.) Hayata and Cunninghamia lanceolata (Lamb.) Hook. Plantations
Abstract
:1. Introduction
2. Materials and Methods
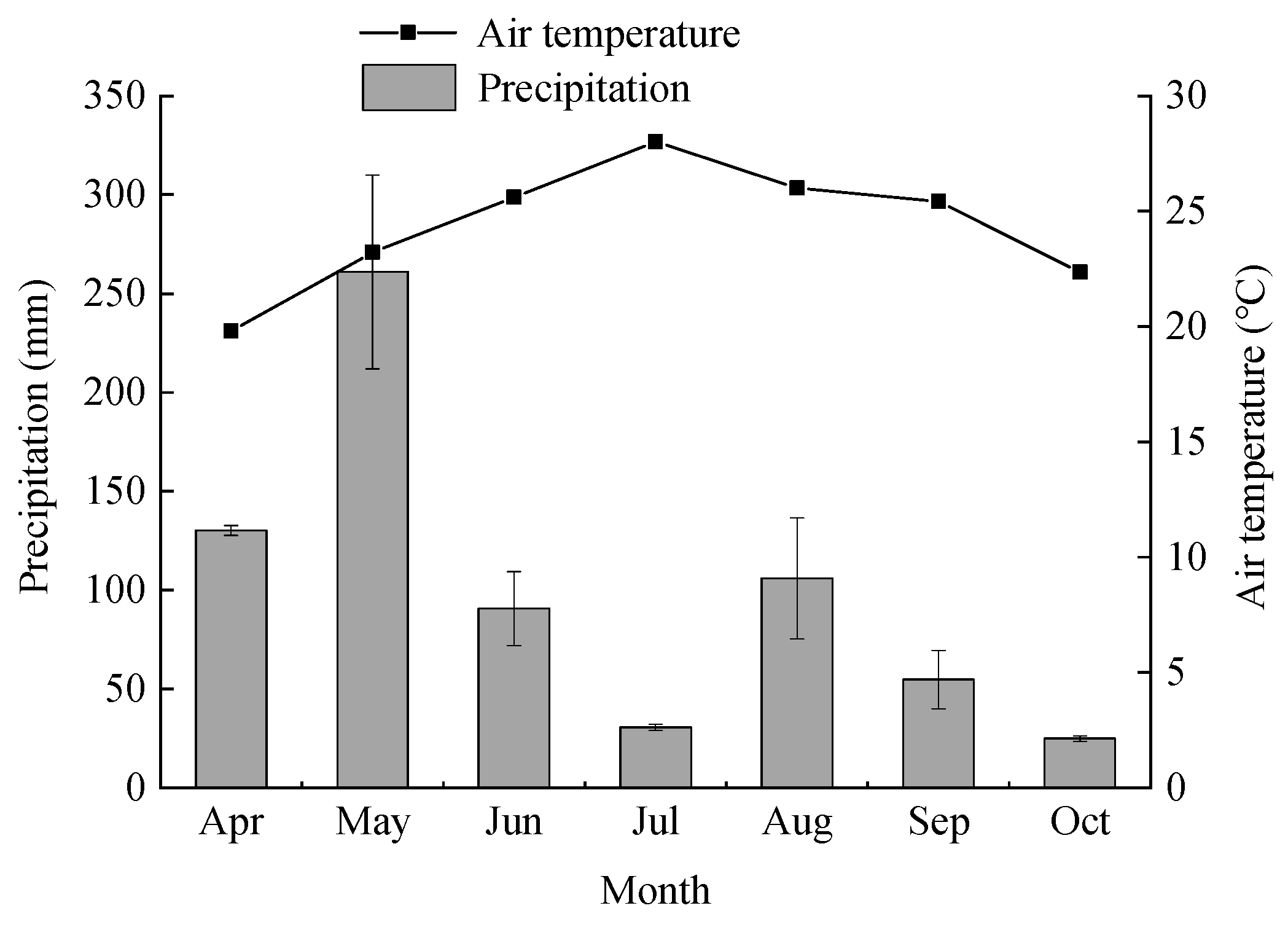
2.1. Study Site
2.2. Sampling and Chemical Analysis
2.3. Calculation and Analysis
3. Results
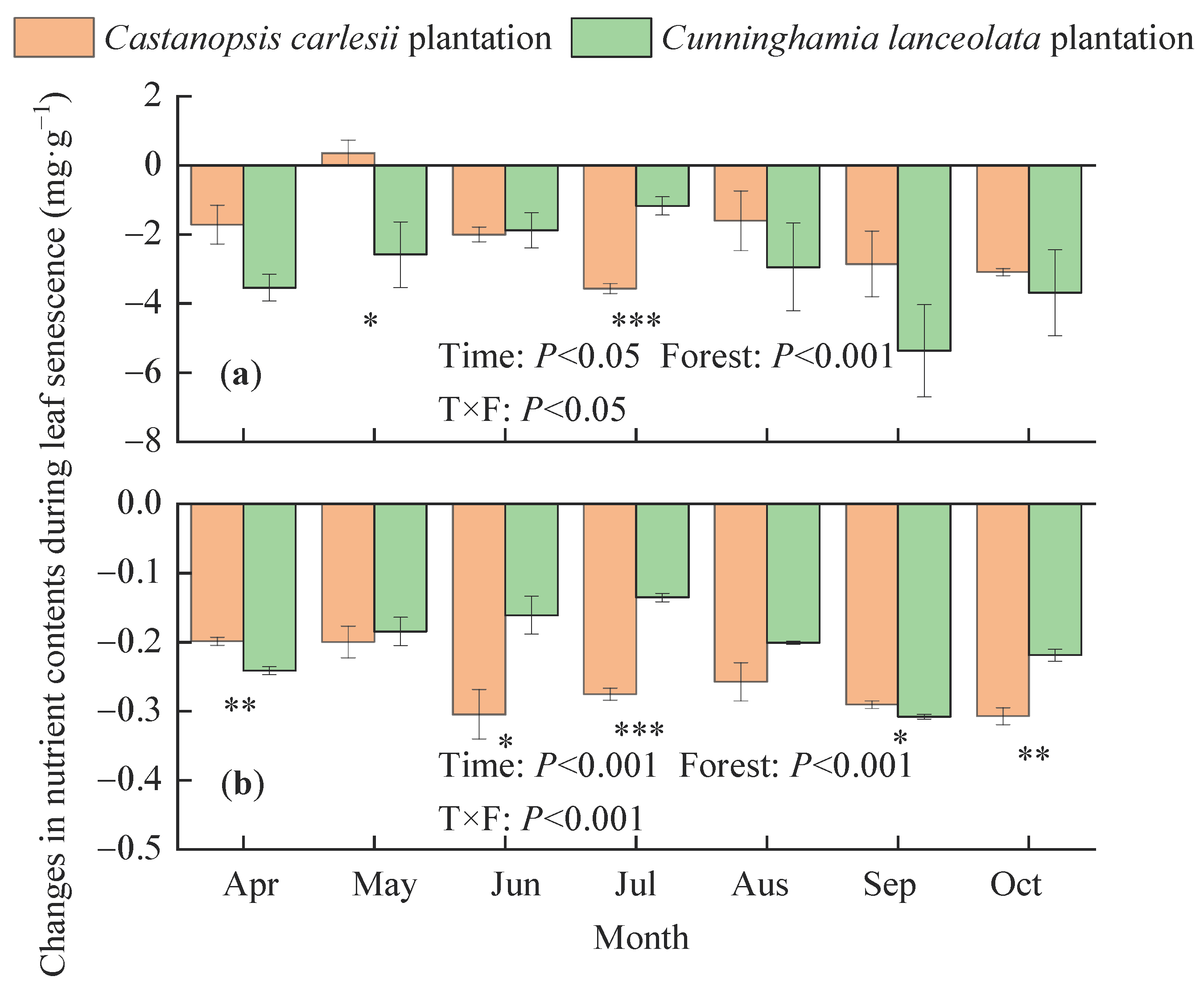
3.1. Carbon, Nitrogen, and Phosphorus Contents in Mature and Senescent Leaves
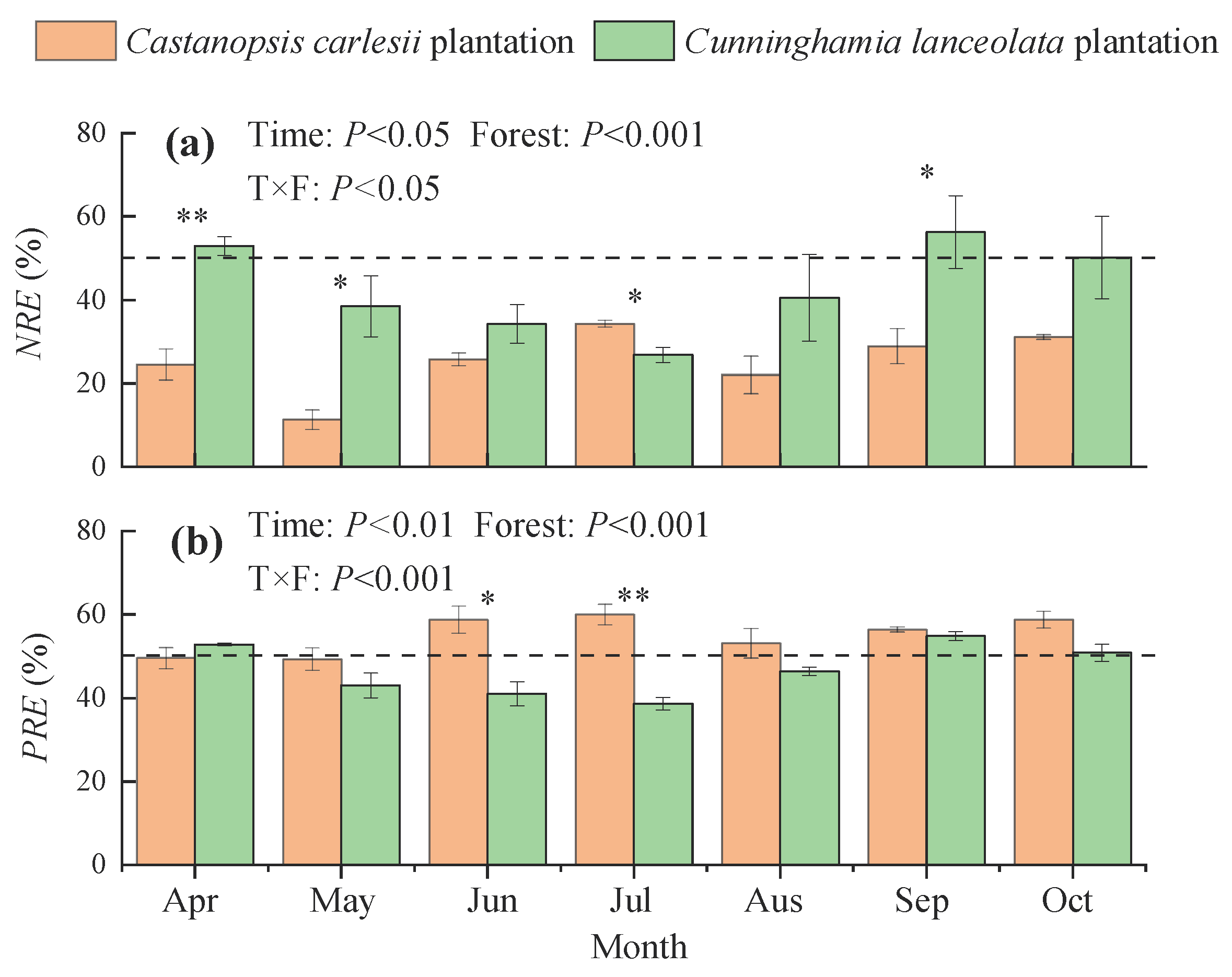
3.2. Nitrogen and Phosphorus Resorption Efficiencies in the Leaves of Castanopsis carlesii and Cunninghamia lanceolata Plantations
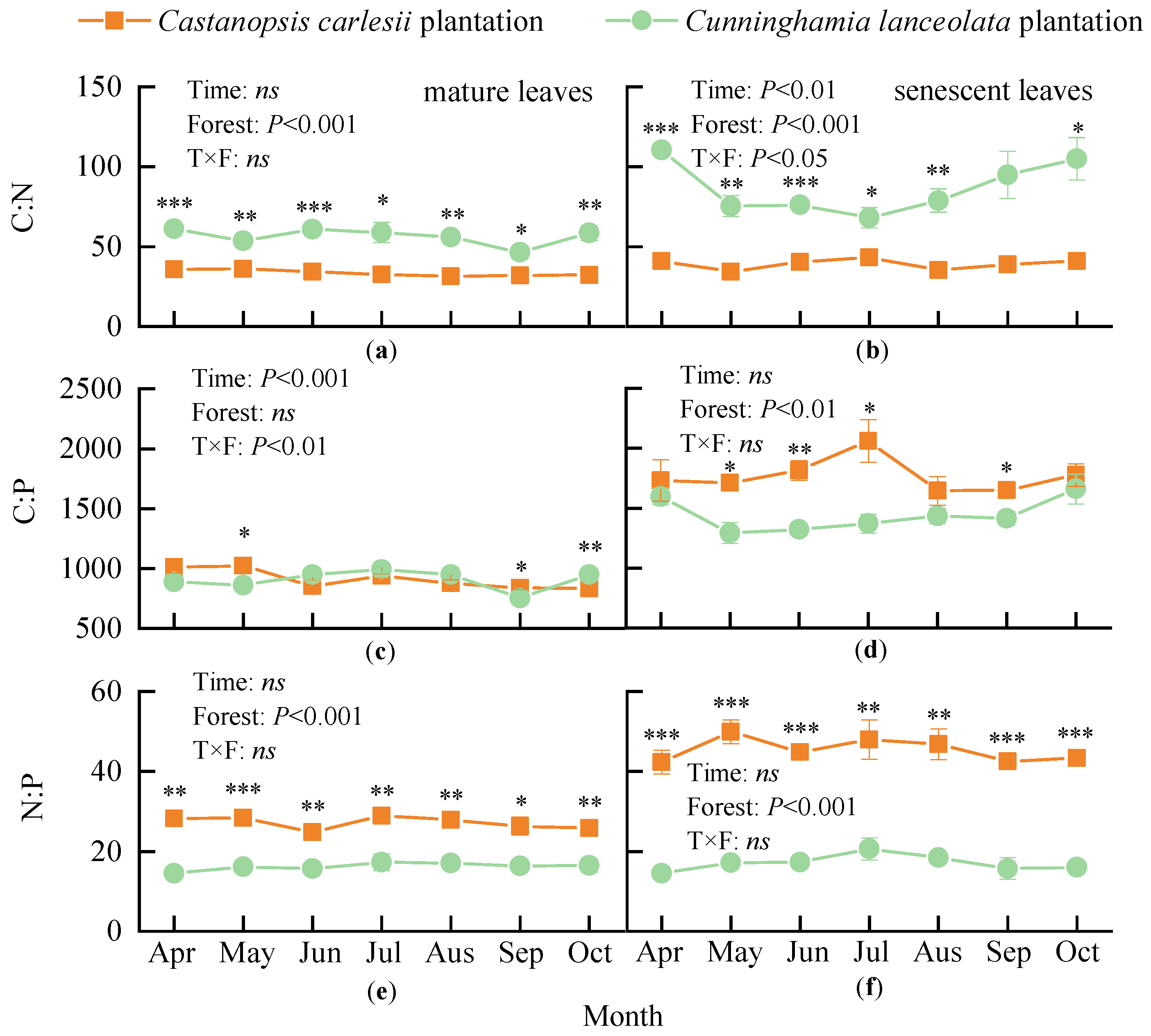
3.3. Carbon, Nitrogen, and Phosphorus Stoichiometric Ratios in Mature and Senescent Leaves
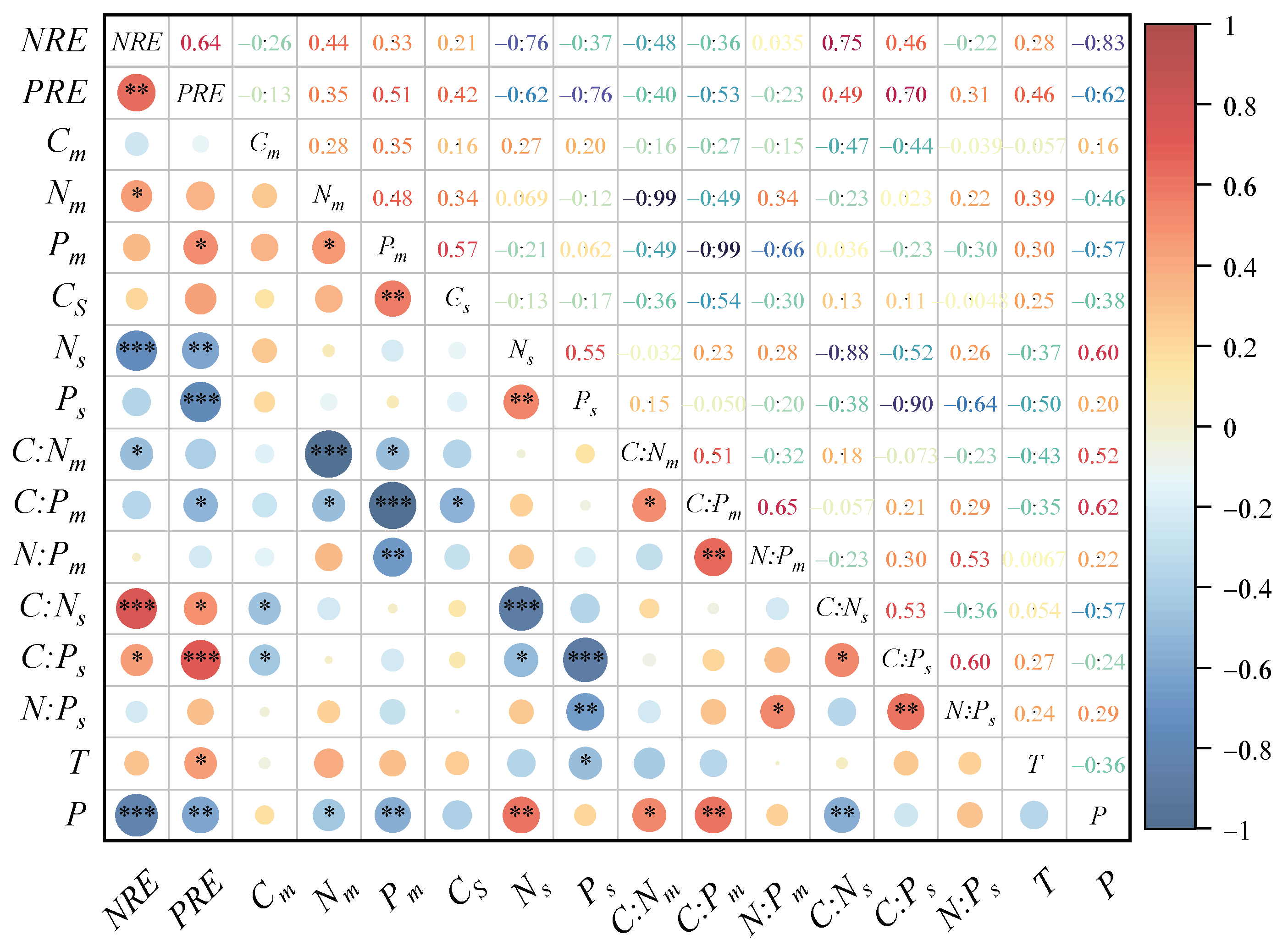
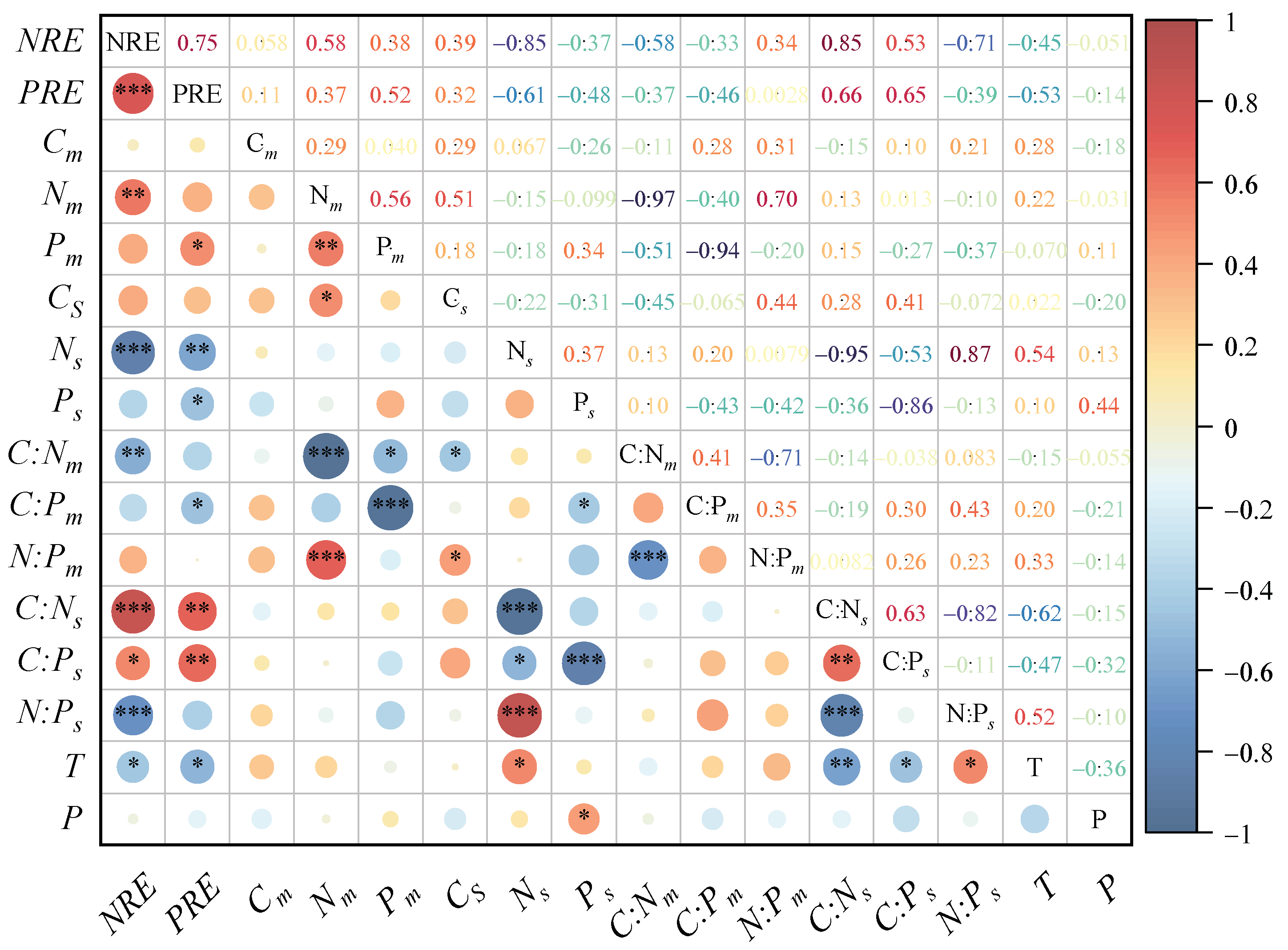
3.4. Correlation of Nutrient Contents, Resorption Efficiencies, and Stoichiometric Ratios with Climatic Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aerts, R. Nutrient Resorption from Senescing Leaves of Perennials: Are There General Patterns? J. Ecol. 1996, 84, 597. [Google Scholar] [CrossRef]
- Chen, H.; Reed, S.C.; Lü, X.; Xiao, K.; Wang, K.; Li, D. Global Resorption Efficiencies of Trace Elements in Leaves of Terrestrial Plants. Funct. Ecol. 2021, 35, 1596–1602. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.Y.H. Foliar Nutrient Resorption Dynamics of Trembling Aspen and White Birch during Secondary Succession in the Boreal Forest of Central Canada. For. Ecol. Manag. 2022, 505, 119876. [Google Scholar] [CrossRef]
- You, C.; Wu, F.; Yang, W.; Xu, Z.; Tan, B.; Zhang, L.; Yue, K.; Ni, X.; Li, H.; Chang, C.; et al. Does Foliar Nutrient Resorption Regulate the Coupled Relationship between Nitrogen and Phosphorus in Plant Leaves in Response to Nitrogen Deposition? Sci. Total Environ. 2018, 645, 733–742. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Pietrasiak, N.; Short, T.H. Global Temporal Patterns in Plant Nutrient Resorption Plasticity. Glob. Ecol. Biogeogr. 2019, 28, 728–743. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, H.; Peng, D.; Liu, X.; Yang, F.; Li, Z.; Cheng, S. Thinning Drives C:N:P Stoichiometry and Nutrient Resorption in Larix Principis-Rupprechtii Plantations in North China. For. Ecol. Manag. 2020, 462, 117984. [Google Scholar] [CrossRef]
- Killingbeck, K.T. Nutrients in Senesced Leaves: Keys to the Search for Potential Resorption and Resorption Proficiency. Ecology 1996, 77, 1716–1727. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Guo, K.; Wang, S.; Yang, Y. Concentrations and Resorption Patterns of 13 Nutrients in Different Plant Functional Types in the Karst Region of South-Western China. Ann. Bot. 2014, 113, 873–885. [Google Scholar] [CrossRef]
- Van Heerwaarden, L.M.V.; Toet, S.; Aerts, R. Current Measures of Nutrient Resorption Efficiency Lead to a Substantial Underestimation of Real Resorption Efficiency: Facts and Solutions. Oikos 2003, 101, 664–669. [Google Scholar] [CrossRef]
- González-Zurdo, P.; Escudero, A.; Mediavilla, S. N Resorption Efficiency and Proficiency in Response to Winter Cold in Three Evergreen Species. Plant Soil 2015, 394, 87–98. [Google Scholar] [CrossRef]
- Tian, D.; Kattge, J.; Chen, Y.; Han, W.; Luo, Y.; He, J.; Hu, H.; Tang, Z.; Ma, S.; Yan, Z.; et al. A Global Database of Paired Leaf Nitrogen and Phosphorus Concentrations of Terrestrial Plants. Ecology 2019, 100, e02812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.B.; Zhang, J.L.; Slik, J.; Cao, K.F. Leaf Element Concentrations of Terrestrial Plants across China Are Influenced by Taxonomy and the Environment. Glob. Ecol. Biogeogr. 2012, 21, 809–818. [Google Scholar] [CrossRef]
- Kerkhoff, A.J.; Enquist, B.J.; Fagan, E.W.F. Plant Allometry, Stoichiometry and the Temperature-Dependence of Primary Productivity. Glob. Ecol. Biogeogr. 2010, 14, 585–598. [Google Scholar] [CrossRef]
- Wang, C.G.; Zheng, X.B.; Wang, A.Z.; Dai, G.H.; Zhu, B.K.; Zhao, Y.M.; Dong, S.J.; Zu, W.Z.; Wang, W.; Zheng, Y.G.; et al. Temperature and Precipitation Diversely Control Seasonal and Annual Dynamics of Litterfall in a Temperate Mixed Mature Forest, Revealed by Long-Term Data Analysis. J. Geophys. Res.-Biogeosci. 2021, 126, e2020JG006204. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, W.; Chen, H.; Deng, Y.; Chen, C.; Zeng, H. Effects of Forest Transition on Litterfall, Standing Litter and Related Nutrient Returns: Implications for Forest Management in Tropical China. Geoderma 2019, 333, 123–134. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H. Global-Scale Patterns of Nutrient Resorption Associated with Latitude, Temperature and Precipitation. Glob. Ecol. Biogeogr. 2009, 18, 11–18. [Google Scholar] [CrossRef]
- Oleksyn, J.; Reich, P.B.; Zytkowiak, R.; Karolewski, P.; Tjoelker, M.G. Nutrient Conservation Increases with Latitude of Origin in European Pinus sylvestris Populations. Oecologia 2003, 136, 220–235. [Google Scholar] [CrossRef]
- Vergutz, L.; Manzoni, S.; Porporato, A.; Novais, R.F.; Jackson, R.B. Global Resorption Efficiencies and Concentrations of Carbon and Nutrients in Leaves of Terrestrial Plants. Ecol. Monogr. 2012, 82, 205–220. [Google Scholar] [CrossRef]
- Chapin, F.S.; Matson, P.A.; Vitousek, P.M. Principles of Terrestrial Ecosystem Ecology; Springer: New York, NY, USA, 2011; pp. 38–40. [Google Scholar]
- Tian, D.; Yan, Z.; Ma, S.; Ding, Y.; Luo, Y.; Chen, Y.; Du, E.; Han, W.; Kovacs, E.D.; Shen, H.; et al. Family-Level Leaf Nitrogen and Phosphorus Stoichiometry of Global Terrestrial Plants. Sci. China Life Sci. 2019, 62, 1047–1057. [Google Scholar] [CrossRef]
- Yan, T.; Lü, X.; Zhu, J.; Yang, K.; Yu, L.; Gao, T. Changes in Nitrogen and Phosphorus Cycling Suggest a Transition to Phosphorus Limitation with the Stand Development of Larch Plantations. Plant Soil 2018, 422, 385–396. [Google Scholar] [CrossRef]
- Jiang, D.; Geng, Q.; Li, Q.; Luo, Y.; Vogel, J.; Shi, Z.; Ruan, H.; Xu, X. Nitrogen and Phosphorus Resorption in Planted Forests Worldwide. Forests 2019, 10, 201. [Google Scholar] [CrossRef]
- Tang, L.; Han, W.; Chen, Y.; Fang, J. Resorption Proficiency and Efficiency of Leaf Nutrients in Woody Plants in Eastern China. J. Plant Ecol. 2013, 6, 408–417. [Google Scholar] [CrossRef]
- Pang, D.; Wang, G.; Li, G.; Sun, Y.; Liu, Y.; Zhou, J. Ecological Stoichiometric Characteristics of Two Typical Plantations in the Karst Ecosystem of Southwestern China. Forests 2018, 9, 56. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Denno, R.F.; Dobberfuhl, D.R.; Folarin, A.; Huberty, A.; Interlandi, S.; Kilham, S.S.; McCauley, E.; Schulz, K.L.; et al. Nutritional Constraints in Terrestrial and Freshwater Food Webs. Nature 2000, 408, 4. [Google Scholar] [CrossRef]
- Hu, M.; Ma, Z.; Chen, H.Y.H. Intensive Plantations Decouple Fine Root C:N:P in Subtropical Forests. For. Ecol. Manag. 2022, 505, 119901. [Google Scholar] [CrossRef]
- Su, H.; Cui, J.; Adamowski, J.F.; Zhang, X.; Biswas, A.; Cao, J. Using Leaf Ecological Stoichiometry to Direct the Management of Ligularia virgaurea on the Northeast Qinghai-Tibetan Plateau. Front. Environ. Sci. 2022, 9, 805405. [Google Scholar] [CrossRef]
- Güsewell, S. N: P Ratios in Terrestrial Plants: Variation and Functional Significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H. Decoupling of Nitrogen and Phosphorus in Terrestrial Plants Associated with Global Changes. Nat. Clim. Change 2015, 5, 465–469. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, B.; Luan, P.; Gu, B.; Wan, Q.; Huang, X.; Liao, W.; Liu, J. PI3K/AKT/MTOR/P70S6K Pathway Is Involved in A β 25-35-Induced Autophagy. BioMed Res. Int. 2015, 2015, 161020. [Google Scholar] [CrossRef]
- Yang, D.; Mao, H.; Jin, G. Divergent Responses of Foliar N:P Stoichiometry During Different Seasons to Nitrogen Deposition in an Old-Growth Temperate Forest, Northeast China. Forests 2019, 10, 257. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Liu, W.; Xu, M.; Deng, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Response of Forest Growth to C:N:P Stoichiometry in Plants and Soils during Robinia pseudoacacia Afforestation on the Loess Plateau, China. Geoderma 2019, 337, 280–289. [Google Scholar] [CrossRef]
- Ren, H.; Kang, J.; Yuan, Z.; Xu, Z.; Han, G. Responses of Nutrient Resorption to Warming and Nitrogen Fertilization in Contrasting Wet and Dry Years in a Desert Grassland. Plant Soil 2018, 432, 65–73. [Google Scholar] [CrossRef]
- Zhao, F.; Li, D.; Jiao, F.; Yao, J.; Du, H. The Latitudinal Patterns of Leaf and Soil C:N:P Stoichiometry in the Loess Plateau of China. Front. Plant Sci. 2019, 10, 85. [Google Scholar]
- Yu, G.; Chen, Z.; Piao, S.; Peng, C.; Ciais, P.; Wang, Q.; Li, X.; Zhu, X. High Carbon Dioxide Uptake by Subtropical Forest Ecosystems in the East Asian Monsoon Region. Proc. Natl. Acad. Sci. USA 2014, 111, 4910–4915. [Google Scholar] [CrossRef]
- Lin, R.; Zhang, J.; Li, S.; Yao, X.; Wang, X.; Chen, G. Overyielding of Pinus massoniana Mixed Forest and Its Influencing Factors. J. Subtropic. Resour. Environ. 2022, 17, 43–50. [Google Scholar]
- Ni, X.; Lin, C.; Chen, G.; Xie, J.; Yang, Z.; Liu, X.; Xiong, D.; Xu, C.; Yue, K.; Wu, F.; et al. Decline in Nutrient Inputs from Litterfall Following Forest Plantation in Subtropical China. For. Ecol. Manag. 2021, 496, 119445. [Google Scholar] [CrossRef]
- Lü, M.; Xie, J.; Wang, C.; Guo, J.; Wang, M.; Liu, X.; Chen, Y.; Chen, G.; Yang, Y. Forest Conversion Stimulated Deep Soil C Losses and Decreased C Recalcitrance through Priming Effect in Subtropical China. Biol. Fertil. Soils 2015, 51, 857–867. [Google Scholar] [CrossRef]
- Lin, K.; Lyu, M.; Jiang, M.; Chen, Y.; Li, Y.; Chen, G.; Xie, J.; Yang, Y. Improved Allometric Equations for Estimating Biomass of the Three Castanopsis carlesii H. Forest Types in Subtropical China. New For. 2017, 48, 115–135. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Yang, Z.; Xu, C.; Xie, J.; Chen, G.; Lin, C.; Guo, J.; Liu, X.; Xiong, D.; et al. Large Ecosystem Service Benefits of Assisted Natural Regeneration. J. Geophys. Res. Biogeosci. 2018, 123, 676–687. [Google Scholar] [CrossRef]
- Gao, Y.; Bao, Y.; Hu, W.; Li, H.; Si, Y. Soil DOM Quantity and Spectral Characteristics of Three Typical Forest Soils in Subtropical China. J. Subtropic. Resour. Environ. 2018, 13, 26–35. [Google Scholar]
- Wright, I.J.; Westoby, M. Nutrient Concentration, Resorption and Lifespan: Leaf Traits of Australian Sclerophyll Species. Funct. Ecol. 2003, 17, 10–19. [Google Scholar] [CrossRef]
- Zhou, L.; Li, S.; Jia, Y.; Heal, K.; He, Z.; Wu, P.; Ma, X. Spatiotemporal Distribution of Canopy Litter and Nutrient Resorption in a Chronosequence of Different Development Stages of Cunninghamia lanceolata in Southeast China. Sci. Total Environ. 2021, 762, 143153. [Google Scholar] [CrossRef] [PubMed]
- Bo, F.; Zhang, Y.; Chen, H.Y.H.; Wang, P.; Ren, X.; Guo, J. The C:N:P Stoichiometry of Planted and Natural Larix principis-rupprechtii Stands along Altitudinal Gradients on the Loess Plateau, China. Forests 2020, 11, 363. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.X.; Pan, G.X.; Li, L.Q.; Hu, Z.L.; Wang, X.Z. Leaf N/P ratio and nutrient reuse between dominant species and stands: Predicting phosphorus deficiencies in Karst ecosystems, southwestern China. Environ. Earth Sci. 2011, 64, 299–309. [Google Scholar] [CrossRef]
- Hofmeister, J.; Mihaljevic, M.; Hosek, J.; Sadlo, J. Eutrophication of deciduous forests in the Bohemian Karst (Czech Republic): The role of nitrogen and phosphorus. For. Ecol. Manag. 2002, 169, 213–230. [Google Scholar] [CrossRef]
- Zhang, Y.; Ni, X.; Yang, J.; Tan, S.; Liao, S.; Wu, F. Nitrogen and phosphorus resorption and stoichiometric characteristics of different tree species in a mid-subtropical common-garden, China. Chin. J. Appl. Ecol. 2021, 32, 1154–1162. [Google Scholar]
- Zhao, Z.; Wu, F.; Yang, Y.; Ni, X.; Xu, C.; Xiong, D.; Liao, S.; Yuan, J.; Tan, S.; Yue, K. Dynamics of Phosphorus Along with Stemflow and Throughfall in Middle Subtropical Cunninghamia lanceolata Plantations and Castanopsis carlesii Secondary Forests. J. Soil Water Conserv. 2021, 35, 129–134. [Google Scholar]
- Fu, Y.H.; Piao, S.; Delpierre, N.; Hao, F.; Hanninen, H.; Liu, Y.; Sun, W.; Janssens, I.A.; Campioli, M. Larger Temperature Response of Autumn Leaf Senescence than Spring Leaf-out Phenology. Glob. Change Biol. 2018, 24, 2159–2168. [Google Scholar] [CrossRef]
- Lawrence, B.T.; Melgar, J.C. Variable Fall Climate Influences Nutrient Resorption and Reserve Storage in Young Peach Trees. Front. Plant. Sci. 2018, 9, 1819. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, R.; Lin, J.; Zheng, J.; Hanninen, H.; Wu, J. High Autumn Temperatures Increase the Depth of Bud Dormancy in the Subtropical Torreya grandis and Carya illinoinensis and Delay Leaf Senescence in the Deciduous Carya. Trees-Struct. Funct. 2022, 36, 1053–1065. [Google Scholar] [CrossRef]
- Gerdol, R.; Marchesini, R.; Iacumin, P. Bedrock Geology Interacts with Altitude in Affecting Leaf Growth and Foliar Nutrient Status of Mountain Vascular Plants. J. Plant Ecol. 2017, 10, 839–850. [Google Scholar] [CrossRef]
- Jardine, K.J.; Chambers, J.Q.; Holm, J.; Jardine, A.B.; Fontes, C.G.; Zorzanelli, R.F.; Meyers, K.T.; de Souza, V.F.; Garcia, S.; Gimenez, B.O.; et al. Green Leaf Volatile Emissions during High Temperature and Drought Stress in a Central Amazon Rainforest. Plants 2015, 4, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Yan, Z.; Niklas, K.J.; Han, W.; Kattge, J.; Reich, P.B.; Luo, Y.; Chen, Y.; Tang, Z.; Hu, H.; et al. Global Leaf Nitrogen and Phosphorus Stoichiometry and Their Scaling Exponent. Natl. Sci. Rev. 2018, 5, 728–739. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Press Princeton University Press: Princeton, NJ, USA, 2002; pp. 55–58. [Google Scholar]
- Matzek, V.; Vitousek, P.M. N:P Stoichiometry and Protein: RNA Ratios in Vascular Plants: An Evaluation of the Growth-Rate Hypothesis. Ecol. Lett. 2010, 12, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Han, W.X.; Fang, J.Y.; Reich, P.B.; Woodward, F.I.; Wang, Z.H. Biogeography and Variability of Eleven Mineral Elements in Plant Leaves across Gradients of Climate, Soil and Plant Functional Type in China. Ecol. Lett. 2011, 14, 788–796. [Google Scholar] [CrossRef]
- Schreeg, L.A.; Santiago, L.S.; Wright, S.J.; Turner, B.L. Stem, Root, and Older Leaf N:P Ratios Are More Responsive Indicators of Soil Nutrient Availability than New Foliage. Ecology 2014, 95, 2062–2206. [Google Scholar] [CrossRef] [Green Version]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Plant. Physiological Ecology, 2nd ed.; Springer: New York, NY, USA, 2008; pp. 47–52. [Google Scholar]

| Parameters | Castanopsis carlesii Plantation | Cunninghamia lanceolata Plantation |
|---|---|---|
| Tree age (year) | 10 | 10 |
| Slope (°) | 32 | 33 |
| Stand density (plant·ha−1) | 2400 | 2860 |
| Average tree height (m) | 8.77 ± 0.17 | 11.30 ± 0.37 |
| Average breast diameter (cm) | 9.93 ± 0.62 | 14.80 ± 0.28 |
| Soil C (mg·g−1) | 23.83 ± 0.56 | 27.02 ± 0.27 |
| Soil N (mg·g−1) | 1.54 ± 0.03 | 1.79 ± 0.04 |
| Soil P (mg·g−1) | 0.19 ± 0.00 | 0.19 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yang, J.; Wei, X.; Ni, X.; Wu, F. Monthly Dynamical Patterns of Nitrogen and Phosphorus Resorption Efficiencies and C:N:P Stoichiometric Ratios in Castanopsis carlesii (Hemsl.) Hayata and Cunninghamia lanceolata (Lamb.) Hook. Plantations. Forests 2022, 13, 1458. https://doi.org/10.3390/f13091458
Zhang Y, Yang J, Wei X, Ni X, Wu F. Monthly Dynamical Patterns of Nitrogen and Phosphorus Resorption Efficiencies and C:N:P Stoichiometric Ratios in Castanopsis carlesii (Hemsl.) Hayata and Cunninghamia lanceolata (Lamb.) Hook. Plantations. Forests. 2022; 13(9):1458. https://doi.org/10.3390/f13091458
Chicago/Turabian StyleZhang, Yaoyi, Jing Yang, Xinyu Wei, Xiangyin Ni, and Fuzhong Wu. 2022. "Monthly Dynamical Patterns of Nitrogen and Phosphorus Resorption Efficiencies and C:N:P Stoichiometric Ratios in Castanopsis carlesii (Hemsl.) Hayata and Cunninghamia lanceolata (Lamb.) Hook. Plantations" Forests 13, no. 9: 1458. https://doi.org/10.3390/f13091458





