Soil Functional Responses to Natural Ecosystem Restoration of a Pine Forest Peucedano-Pinetum after a Fire
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Meteorological Conditions
2.3. Soil Description and Soil Sampling
2.4. SWR and SMC Measurements
2.5. Estimation of the Diversity of Microorganism Communities in the Soil
2.6. Statistical Analysis
3. Results and Discussion
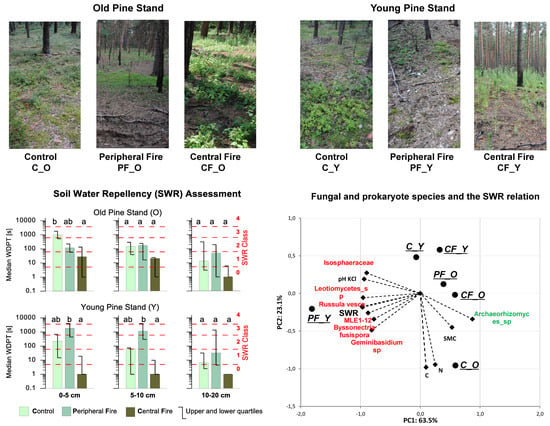
3.1. Actual Soil Water Repellency
3.2. Soil Moisture Content
3.3. SWR and SMC Relation
3.4. Dominant Eukaryotic and Prokaryotic Species in the Control and Recovery Soil Sites
3.5. Fungal and Prokaryote Species and the SWR Relation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Breshears, D.D.; Cobb, N.S.; Rich, P.M.; Price, K.P.; Allen, C.D.; Balice, R.G.; Romme, W.H.; Kastens, J.H.; Floyd, M.L.; Belnap, J.; et al. Regional Vegetation Die-Off in Response to Global-Change-Type Drought. Proc. Natl. Acad. Sci. 2005, 102, 15144–15148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neary, D.G.; Ice, G.G.; Jackson, C.R. Linkages between forest soils and water quality and quantity. For. Ecol. Manag. 2009, 258, 2269–2281. [Google Scholar] [CrossRef]
- GCOS. Implementation plan for the global observing system for climate in support of the UNFCCC (2010 Update), GCOS-138. Geneva, Switzerland: Secretariat of the World Meteorological Organization. Available online: https://eprints.soton.ac.uk/162953/ (accessed on 28 February 2020).
- Goebel, M.O.; Bachmann, J.; Reichstein, M.; Janssens, I.A.; Guggenberger, G. Soil water repellency and its implications for organic matter decomposition—is there a link to extreme climatic events? Glob. Chang. Biol. 2011, 17, 2640–2656. [Google Scholar] [CrossRef]
- Robinson, D.A.; Hopmans, J.W.; Filipovic, V.; van der Ploeg, M.; Lebron, I.; Jones, S.B.; Reinsch, S.; Jarvis, N.; Tuller, M. Global environmental changes impact soil hydraulic functions through biophysical feedbacks. Glob. Chang. Biol. 2019, 25, 1895–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutanto, S.J.; Vitolo, C.; Di Napoli, C.; D’Andrea, M.; van Lanen, H.A. Heatwaves, droughts, and fires: Exploring compound and cascading dry hazards at the pan-European scale. Environ. Int. 2020, 134, 105276. [Google Scholar] [CrossRef]
- Querner, P.; Bruckner, A.; Weigand, E.; Prötsch, M. Short- and long-term effects of fire on the Collembola communities of a sub-alpine dwarf pine ecosystem in the Austrian Alps. Eco. Mont J. Prot. Mt. Areas Res. 2010, 2, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Gongalsky, K.B.; Malmström, A.; Zaytsev, A.S.; Shakhab, S.V.; Persson, T.; Bentsson, I. Do burned areas recover from inside? An experiment with soil fauna in a heterogenous landscape. Appl. Soil Ecol. 2012, 59, 73–86. [Google Scholar] [CrossRef]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef]
- Malmström, A. The importance of measuring fire severity-Evidence from microarthropod studies. For. Ecol. Manag. 2010, 260, 62–70. [Google Scholar] [CrossRef]
- Malmström, A.; Persson, T.; Ahlström, K. Effects of fire intensity on survival and recovery of soil microarthropods after a clearcut burn. Can. J. For. Res. 2008, 38, 2465–2475. [Google Scholar] [CrossRef]
- García-Orenes, F.; Arcenegui, V.; Chrenkova, K.; Mataix-Solera, J.; Molto, J.; Jara-Navarro, A.B.; Torres, M.P. Effects of salvage logging on soil properties and vegetation recovery in a fire-affected Mediterranean forest: A two year monitoring research. Sci. Total Environ. 2017, 586, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Mataix-Solera, J.; Guerrero, C.; García-Orenes, F.; Bárcenas, G.M.; Torres, M.P.; Barcenas, M. Forest fire effects on soil microbiology. Fire Eff. Soils Restor. Strateg. 2009, 5, 133–175. [Google Scholar]
- DeBano, L.F.; Neary, D.G.; Ffolliott, P.F. Fire Effects on Ecosystems; John Wiley & Sons: Hoboken, NJ, USA, 1998. [Google Scholar]
- Hart, S.C.; DeLuca, T.H.; Newman, G.S.; MacKenzie, M.D.; Boyle, S.I. Post-fire vegetative dynamics as drivers of microbial community structure and function in forest soils. For. Ecol. Manag. 2005, 220, 166–184. [Google Scholar] [CrossRef]
- Shakesby, R.A.; Doerr, S.H. Wildfire as a hydrological and geomorphological agent. Earth-Sci. Rev. 2006, 74, 269–307. [Google Scholar] [CrossRef]
- Doerr, S.H.; Shakesby, R.A.; Walsh, R. Soil water repellency: Its causes, characteristics and hydro-geomorphological significance. Earth-Sci. Rev. 2000, 51, 33–65. [Google Scholar] [CrossRef]
- Bodí, M.B.; Muñoz-Santa, I.; Armero, C.; Doerr, S.H.; Mataix-Solera, J.; Cerdà, A. Spatial and temporal variations of water repellency and probability of its occurrence in calcareous Mediterranean rangeland soils affected by fires. Catena 2013, 108, 14–25. [Google Scholar] [CrossRef]
- León, J.; Echeverría, M.T.; Badía, D.; Martí, C.; Álvarez, C.J. Effectiveness of wood chips cover at reducing erosion in two contrasted burnt soils. Zeitschrift für Geomorphologie 2013, 57, 27–37. [Google Scholar]
- King, P.M. Comparison of methods for measuring severity of water repellency of sandy soils and assessment of some factors that affect its measurement. Aust. J. Soil Res. 1981, 21, 2356–2364. [Google Scholar]
- Fox, D.; Berolo, W.; Carrega, P.; Darboux, F. Mapping erosion risk and selecting sites for simple erosion control measures after a forest fire in Mediterranean France. Earth Surf. Process. Landf. 2006, 31, 606–621. [Google Scholar] [CrossRef]
- Granged, A.J.; Jordán, A.; Zavala, L.M.; Bárcenas, G. Fire-induced changes in soil water repellency increased fingered flow and runoff rates following the 2004 Huelva wildfire. Hydrol. Process. 2011, 25, 1614–1629. [Google Scholar] [CrossRef]
- Keesstra, S.; Wittenberg, L.; Maroulis, J.; Sambalino, F.; Malkinson, D.; Cerdà, A.; Pereira, P. The influence of fire history, plant species and post-fire management on soil water repellency in a Mediterranean catchment: The Mount Carmel range, Israel. Catena 2017, 149, 857–866. [Google Scholar] [CrossRef]
- Marcos, E.; Fernández-García, V.; Fernández-Manso, A.; Quintano, C.; Valbuena, L.; Tárrega, R.; Luis-Calabuig, E.; Calvo, L. Evaluation of composite burn index and land surface temperature for assessing soil burn severity in mediterranean fire-prone pine ecosystems. Forests 2018, 9, 494. [Google Scholar] [CrossRef] [Green Version]
- Buczko, U.; Bens, O.; Hüttl, R.F. Variability of soil water repellency in sandy forest soils with different stand structure under Scots pine (Pinus sylvestris) and beech (Fagus sylvatica). Geoderma 2005, 126, 317–336. [Google Scholar] [CrossRef]
- Rodríguez-Alleres, M.; Varela, M.E.; Benito, E. Natural severity of water repellency in pine forest soils from NW Spain and influence of wildfire severity on its persistence. Geoderma 2012, 191, 125–131. [Google Scholar]
- Hewelke, E. Influence of Abandoning Agricultural Land Use on Hydrophysical Properties of Sandy Soil. Water 2019, 11, 525. [Google Scholar] [CrossRef] [Green Version]
- Hallett, P.D. A brief overview of the causes, impacts and amelioration of soil water repellency—A review. Soil Water Res. 2008, 3, 521–528. [Google Scholar] [CrossRef] [Green Version]
- Hallett, P.D.; Ritz, K.; Wheatley, R.E. Microbial derived water repellency in golf course soil. Int. Turfgrass Soc. Res. J. 2001, 9, 518–524. [Google Scholar]
- Hallett, P.D.; Gordon, D.C.; Bengough, A.G. Plant influence on rhizosphere hydraulic properties: Direct measurements using a miniaturized infiltrometer. New Phytol. 2003, 157, 597–603. [Google Scholar] [CrossRef]
- Czarnes, S.; Hallett, P.D.; Bengough, A.G.; Young, I.M. Root-and microbial-derived mucilages affect soil structure and water transport. Eur. J. Soil Sci. 2000, 51, 435–443. [Google Scholar] [CrossRef]
- Jordán, A.; Zavala, L.M.; Granged, A.J.; Gordillo-Rivero, Á.J.; García-Moreno, J.; Pereira, P.; Bárcenas-Moreno, G.; de Celis, R.; Jiménez-Compán, E.; Alanís, N. Wettability of ash conditions splash erosion and runoff rates in the post-fire. Sci. Total Environ. 2016, 572, 1261–1268. [Google Scholar] [CrossRef]
- Hewelke, E.; Szatyłowicz, J.; Hewelke, P.; Gnatowski, T.; Aghalarov, R. The impact of diesel oil pollution on the hydrophobicity and CO2 efflux of forest soils. Water Air Soil Pollut. 2018, 229, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francos, M.; Úbeda, X.; & Pereira, P. Impact of torrential rainfall and salvage logging on post-wildfire soil properties in NE Iberian Peninsula. Catena 2019, 177, 210–218. [Google Scholar] [CrossRef]
- Wallach, R.; Jortzick, C. Unstable finger-like flow in water-repellent soils during wetting and redistribution–The case of a point water source. J. Hydrol. 2008, 351, 26–41. [Google Scholar] [CrossRef]
- Rye, C.F.; Smettem, K.R.J. The effect of water repellent soil surface layers on preferential flow and bare soil evaporation. Geoderma 2017, 289, 142–149. [Google Scholar] [CrossRef]
- Hewelke, E.; Szatyłowicz, J.; Gnatowski, T.; Oleszczuk, R. Effects of soil water repellency on moisture patterns in a degraded sapric histosol. Land Degrad. Dev. 2016, 27, 955–964. [Google Scholar] [CrossRef]
- Dekker, L.W.; Ritsema, C.J. How water moves in a water repellent sandy soil: 1. Potential and actual water repellency. Water Resour. Res. 1994, 30, 2507–2517. [Google Scholar] [CrossRef]
- Li, Y.; Yao, N.; Tang, D.; Chau, H.W.; Feng, H. Soil water repellency decreases summer maize growth. Agric. For. Meteorol. 2019, 266, 1–11. [Google Scholar] [CrossRef]
- Robinson, D.A.; Lebron, I.; Ryel, R.J.; Jones, S.B. Soil water repellency: A method of soil moisture sequestration in pinyon–juniper woodland. Soil Sci. Soc. Am. J. 2010, 74, 624–634. [Google Scholar] [CrossRef]
- Zeppenfeld, T.; Balkenhol, N.; Kóvacs, K.; Carminati, A. Rhizosphere hydrophobicity: A positive trait in the competition for water. PLoS ONE 2017, 12, e0182188. [Google Scholar] [CrossRef] [Green Version]
- Urbanek, E.; Doerr, S.H. CO2 efflux from soils with seasonal water repellency. Biogeosciences 2017, 14, 4781–4794. [Google Scholar] [CrossRef]
- Sánchez-García, C.; Oliveira, B.R.; Keizer, J.J.; Doerr, S.H.; Urbanek, E. Water repellency reduces soil CO2 efflux upon rewetting. Sci. Total Environ. 2020, 708, 135014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewelke, E.; Gozdowski, D. Hydrophysical Properties of Sandy Clay Contaminated by Petroleum Hydrocarbon. Environ. Sci. Pollut. Res. 2020, 1, 10. [Google Scholar] [CrossRef] [Green Version]
- Keesstra, S.; Nunes, J.P.; Saco, P.; Parsons, T.; Poeppl, R.; Masselink, R.; Cerdà, A. The way forward: Can connectivity be useful to design better measuring and modelling schemes for water and sediment dynamics? Sci. Total Environ. 2018, 644, 1557–1572. [Google Scholar] [CrossRef] [PubMed]
- Robinne, F.N.; Burns, J.; Kant, P.; Flannigan, M.; Kleine, M.; de Groot, B.; Wotton, D.M. Global Fire Challenges in a Warming World; IUFRO: Vienna, Austria, 2018. [Google Scholar]
- Piniewski, M.; Gottschalk, L.; Krasovskaia, I.; Chormański, J.A. GIS-based model for testing effects of restoration measures in wetlands: A case study in the Kampinos National Park, Poland. Ecol. Eng. 2012, 44, 25–35. [Google Scholar] [CrossRef]
- Okruszko, T.; Mioduszewski, W.; Kucharski, L. Conservation and Restoration of Wetlands of Kampinos National Park; Wydawnictwo SGGW: Warszawa, Poland, 2011. (in Polish) [Google Scholar]
- Szczygielski, M. The Frame of Protection of Forest Ecosystems for the Period 01.01.2002–31.12.2021; Office of Forest Management and Forest Surveying: Warsaw, Poland, 2002. (in Polish)
- Boczoń, A.; Kowalska, A.; Dudzińnska, M.; Wróbel, M. Drought in Polish Forests in 2015. Pol. J. Environ. Stud. 2016, 5, 1857–1862. [Google Scholar]
- European Commission. Forest Fires in Europe, Middle East and North Africa 2015; Joint Research Centre: Ispra, Italy, 2016; p. 117. ISBN 978-92-79-62958-7. [Google Scholar]
- Olszewski, A.; Wierzbicki, A.; Degórska, A.; Ferchmin, M.; Gudowicz, J.; Lenartowicz, M.; Otręba, A. Report from the Realization of a Research-Measurement Programme—Integrated Monitoring of the Natural Environment at the Kampinos Base Station in 2017; Kampinoski Park Narodowy: Granica, Poland, 2018; p. 175. (in Polish) [Google Scholar]
- Hewelke, E.; Oktaba, L.; Gozdowski, D.; Kondras, M.; Olejniczak, I.; Górska, E.B. Intensity and persistence of soil water repellency in pine forest soil in a temperate continental climate under drought conditions. Water 2018, 10, 1121. [Google Scholar] [CrossRef] [Green Version]
- International Union of Soil Sciences (IUSS). World Reference Base for Soil Resources 2014, International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Food and Agriculture Organizaiton of the United Nations (FAO): Rome, Italy, 2015; p. 192. ISBN 978-92-5-108369-7. [Google Scholar]
- Keeley, J.E. Fire intensity, fire severity and burn severity: A brief review and suggested usage. Int. J. Wildland Fire 2009, 18, 116–126. [Google Scholar] [CrossRef]
- Zaniewski, P.T.; Otreba, A. Response of vegetation to the surface fire in the pine forest Peucedano-Pinetum, W. Mat. (1962) 1973 in the Kampinoski National Park. Sylwan 2017, 161, 991–1001. (in Polish). [Google Scholar]
- Papierowska, E.; Matysiak, W.; Szatyłowicz, J.; Debaene, G.; Urbanek, E.; Kalisz, B.; Łachacz, A. Compatibility of methods used for soil water repellency determination for organic and organo-mineral soils. Geoderma 2018, 314, 221–231. [Google Scholar] [CrossRef]
- Dekker, L.W.; Jungerius, P.D. Water repellency in the dunes with special reference to The Netherlands. Catena Suppl. 1990, 18, 173–183. [Google Scholar]
- Olejniczak, I.; Gorska, E.B.; Kondras, M.; Oktaba, L.; Gozdowski, D.; Jankiewicz, U.; Predecka, A.; Dobrzynski, J.; Otreba, A.; Tyburski, L.; et al. Fire-a Factor Forming the Numbers of Microorganisms and Mesofauna in Forest Soils. Rocznik Ochrona Srodowiska 2017, 19, 511–526. (in Polish). [Google Scholar]
- Olejniczak, I.; Górska, E.B.; Prędecka, A.; Hewelke, E.; Gozdowski, D.; Korc, M.; Panek, E.; Tybursk, Ł.; Skawińska, M.; Oktaba, I.; et al. Selected Biological Properties of the Soil in a Burnt-Out Area under Old Pine Trees Three Years after an Fire. Rocznik Ochrona Środowiska 2019, 21, 1279–1293. [Google Scholar]
- Górska, E.B.; Olejniczak, I.; Gozdowski, D.; Panek, E.; Kondras, M.; Oktaba, L.; Prędecka, A.; Biedugnis, S.; Boniecki, P.; Tyburski, Ł.; et al. A Long-Term Reaction of Microorganisms and Mezofauna to Fires Forest Soils of Anthropogenic Origin. Rocznik Ochrona Środowiska 2018, 20, 1776–1792. (in Polish). [Google Scholar]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Aronesty, E. Ea-utils: Command-line tools for processing biological sequencing data. Available online: https://expressionanalysis.github.io/ea-utils/ (accessed on 28 February 2020).
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Pena, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Method. 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [Green Version]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [Green Version]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.; Bahram, M.; Douglas, B. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Methé, B. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef] [Green Version]
- Leighton-Boyce, G.; Doerr, S.H.; Shakesby, R.A.; Walsh, R.P.D.; Ferreira, A.J.D.; Boulet, A.K.; Coelho, C.O.A. Temporal dynamics of water repellency and soil moisture in eucalypt plantations, Portugal. Aus. J. Soil Res. 2005, 43, 269–280. [Google Scholar] [CrossRef]
- Dekker, L.W.; Doerr, S.H.; Oostindie, K.; Ziogas, A.K.; Ritsema, C.J. Water repellency and critical soil water content in a dune sand. Soil Sci. Soc. Am. J. 2001, 65, 1667–1674. [Google Scholar] [CrossRef]
- Zhiguang, H.; Xin, S.; Mengsha, L. Effects of forest age on soil fungal community in a northern temperate ecosystem. Indian J. Microbiol. 2016, 56, 328–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koide, R.T.; Xu, B.; Sharda, J.; Lekberg, Y.; Ostiguy, N. Evidence of species interactions within an ectomycorrhizal fungal community. New Phytol 2005, 165, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Kipfer, T.; Moser, B.; Egli, S.; Wohlgemuth, T.; Ghazoul, J. Ectomycorrhiza succession patterns in Pinus sylvestris forests after stand-replacing fire in the Central Alps. Oecologia 2011, 167, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.-Y.; Park, M.S.; Lim, Y.W. The Influence of Microfungi on the Mycelial Growth of Ectomycorrhizal Fungus Tricholoma matsutake. Microorganisms 2019, 7, 169. [Google Scholar] [CrossRef] [Green Version]
- Kluber, L.A.; Smith, J.E.; Myrold, D.D. Distinctive fungal and bacterial communities are associated with mats formed by ectomycorrhizal fungi. Soil Biol. Biochem. 2011, 43, 1042–1050. [Google Scholar] [CrossRef]
- Agerer, R. Exploration types of ectomycorrhizae. Mycorrhiza 2001, 11, 107–114. [Google Scholar] [CrossRef]
- Mäkipää, R.; Rajala, T.; Schigel, D.; Rinne, K.T.; Pennanen, T.; Abrego, N.; Ovaskainen, O. Interactions between soil-and dead wood-inhabiting fungal communities during the decay of Norway spruce logs. ISME J. 2017, 11, 1964. [Google Scholar]
- Adejumo, T.O.; Awosanya, O.B. Proximate and mineral composition of four edible mushroom species from South Western Nigeria. Afr. J. Biotechnol. 2005, 4, 10. [Google Scholar]
- Unestam, T.; Sun, Y.P. Extramatrical structures of hydrophobic and hydrophilic ectomycorrhizal fungi. Mycorrhiza 1995, 5, 301–311. [Google Scholar] [CrossRef]
- Fujimura, K.E.; Smith, J.E.; Horton, T.R.; Weber, N.S.; Spatafora, J.W. Pezizalean mycorrhizas and sporocarps in ponderosa pine (Pinus ponderosa) after prescribed fires in eastern Oregon, USA. Mycorrhiza 2005, 15, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.D.; Nickerson, N.L.; Seifert, K.A. Basidioascus and Geminibasidium: A new lineage of heat-resistant and xerotolerant basidiomycetes. Mycologia 2013, 105, 1231–1250. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, D.K. Endophytes: Biology and Biotechnology; Springer International Publishing: Berlin, Germany, 2017. [Google Scholar]
- Di Rienzi, S.C.; Sharon, I.; Wrighton, K.C.; Koren, O.; Hug, L.A.; Thomas, B.C.; Goodrich, J.K.; Bell, J.T.; Spector, T.D.; Banfield, J.F.; et al. The human gut and groundwater harbor non-photosynthetic bacteria belonging to a new candidate phylum sibling to Cyanobacteria. Microbiol. Infect. Dis. 2013, 1, 10. [Google Scholar]
- Porter, T.M.; Schadt, C.W.; Rizvi, L.; Martin, A.P.; Schmidt, S.K.; Scott-Denton, L.; Moncalvo, J.M. Widespread occurrence and phylogenetic placement of a soil clone group adds a prominent new branch to the fungal tree of life. Mol. Phylogenetics Evol. 2008, 46, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Rosling, A.; Cox, F.; Cruz-Martinez, K.; Ihrmark, K.; Grelet, G.A.; Lindahl, B.D.; Menkis, A.; James, T.Y. Archaeorhizomycetes: Unearthing an ancient class of ubiquitous soil fungi. Science 2011, 333, 876–879. [Google Scholar] [CrossRef] [PubMed]
- Rosling, A.; Timling, I.; Taylor, D.L. Archaeorhizomycetes: Patterns of distribution and abundance in soil. In Genomics of Soil-and Plant-Associated Fungi; Springer: Berlin, Heidelberg, Germany, 2013; pp. 333–349. [Google Scholar]
- Menkis, A.; Urbina, H.; James, T.Y.; Rosling, A. Archaeorhizomyces borealis sp.nov. and a sequencebased classification of related soil fungal species. Fungal Biol. 2014, 118, 943–955. [Google Scholar] [CrossRef]
- Choi, J.; Détry, N.; Kim, K.T.; Asiegbu, F.O.; Valkonen, J.P.; Lee, Y.H. fPoxDB: Fungal peroxidase database for comparative genomics. BMC Microbiol. 2014, 14, 117. [Google Scholar] [CrossRef] [Green Version]
- Iovino, M.; Pekárová, P.; Hallett, P.D.; Pekár, J.; Lichner, Ľ.; Mataix-Solera, J.; Alagna, V.; Walsh, R.; Raffan, A.; Schach, K.; et al. Extent and persistence of soil water repellency induced by pines in different geographic regions. J. Hydrol. Hydromech. 2018, 66, 360–368. [Google Scholar] [CrossRef] [Green Version]
- Lilleskov, E.A.; Bruns, T.D.; Dawson, T.E.; Camacho, F.J. Water sources and controls on water-loss rates of epigeous ectomycorrhizal fungal sporocarps during summer drought. New Phytol. 2009, 182, 483–494. [Google Scholar] [CrossRef]





| Ecosystem | Site | TOC (%) | N (%) | C/N | pH H2O | pH KCl |
|---|---|---|---|---|---|---|
| Old pine stand | Control | 1.31 a (0.34) | 0.061 a (0.026) | 22.98 a (5.38) | 2.87 a (0.14) | 3.43 a (0.09) |
| Peripheral Fire | 1.26 a (0.20) | 0.057 a (0.002) | 22.17 a (3.34) | 2.76 a (0.06) | 3.43 a (0.07) | |
| Central Fire | 1.10 a (0.22) | 0.058 a (0.003) | 18.94 a (2.73) | 2.85 a (0.01) | 3.48 a (0.16) | |
| Young pine stand | Control | 1.13 b (0.13) | 0.056 a (0.015) | 21.84 a (8.80) | 2.94 a (0.01) | 3.62 a (0.21) |
| Peripheral Fire | 1.11 ab (0.12) | 0.059 a (0.013) | 19.16 a (1.10) | 2.97 a (0.10) | 3.44 a (0.07) | |
| Central Fire | 0.77a (0.05) | 0.050 a (0.005) | 15.50 a (2.64) | 2.78 a (0.23) | 3.20 a (0.15) |
| Characteristic | F | p |
|---|---|---|
| Site | 5.85 | 0.005 |
| Depth | 21.64 | <0.001 |
| Age of pine stand | 0.11 | 0.742 |
| Site | Depth (cm) | Correlation Coefficient |
|---|---|---|
| Control | 0–5 | 0.029 |
| 5–10 | −0.229- | |
| 10–20 | −0.741 | |
| Peripheral Fire | 0–5 | −0.714 |
| 5–10 | −0.500 | |
| 10–20 | −0.683 | |
| Central Fire | 0–5 | −0.759 |
| 5–10 | −0.880 | |
| 10–20 | −0.655 | |
| Old pine stand | 0–5 | −0.140 |
| 5–10 | −0.339 | |
| 10–20 | −0.694 | |
| Young pine stand | 0–5 | −0.550 |
| 5–10 | −0.673 | |
| 10–20 | −0.781 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hewelke, E.; Górska, E.B.; Gozdowski, D.; Korc, M.; Olejniczak, I.; Prędecka, A. Soil Functional Responses to Natural Ecosystem Restoration of a Pine Forest Peucedano-Pinetum after a Fire. Forests 2020, 11, 286. https://doi.org/10.3390/f11030286
Hewelke E, Górska EB, Gozdowski D, Korc M, Olejniczak I, Prędecka A. Soil Functional Responses to Natural Ecosystem Restoration of a Pine Forest Peucedano-Pinetum after a Fire. Forests. 2020; 11(3):286. https://doi.org/10.3390/f11030286
Chicago/Turabian StyleHewelke, Edyta, Ewa Beata Górska, Dariusz Gozdowski, Marian Korc, Izabella Olejniczak, and Anna Prędecka. 2020. "Soil Functional Responses to Natural Ecosystem Restoration of a Pine Forest Peucedano-Pinetum after a Fire" Forests 11, no. 3: 286. https://doi.org/10.3390/f11030286