Ectomycorrhizal Community on Norway Spruce Seedlings Following Bark Beetle Infestation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Seedlings and Ectomycorrhizae
2.3. DNA Extraction, PCR, and Sequencing
2.4. Data Processing and Analysis
2.5. Statistical Analysis
3. Results
3.1. Morphometric Characteristics of Norway Spruce Seedlings
3.2. ECM Community on Root System of Norway Spruce Seedlings
3.3. Exploration Types
3.4. Environmental Drivers of Fungal Community Composition and Exploration Type Proportion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martin, F.; Kohler, A.; Murat, C.; Veneault-Fourrey, C.; Hibbett, D.S. Unearthing the roots of ectomycorrhizal symbioses. Nat. Rev. Microbiol. 2016, 14, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Dickie, I.A.; Oleksyn, J.; Reich, P.B.; Karolewski, P.; Zytkowiak, R.; Jagodzinski, A.M.; Turzanska, E. Soil modification by different tree species influences the extent of seedling ectomycorrhizal infection. Mycorrhiza 2006, 16, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, L.M.; Nilsson, M.; Wardle, D.A.; Zackrisson, O. Context dependent effects of ectomycorrhizal species richness on tree seedling productivity. OIKOS 2001, 93, 353–364. [Google Scholar] [CrossRef]
- Gorzelak, M.A.; Asay, A.K.; Pickles, B.J.; Simard, S.W. Inter-plant communication through mycorrhizal networks mediates complexadaptive behaviour in plant communities. AoB Plants 2015, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Van der Heiden, M.G.A.; Horton, T.R. Socialism in soil? The importance of mycorrhizal fungal networks for facilitation in natural ecosystems. J. Ecol. 2009, 97, 1139–1150. [Google Scholar] [CrossRef]
- Seidl, R.; Müller, J.; Hothorn, T.; Bässler, C.; Heurich, M.; Kautz, M. Small beetle, large-scale drivers: How regional and landscape factors affect outbreaks of the European spruce bark beetle. J. Appl. Ecol. 2016, 53, 530–540. [Google Scholar] [CrossRef]
- Papaik, M.J.; Canham, C.D. Species resistance and community response to wind disturbance regimes in northern temperate forests. J. Ecol. 2006, 94, 1011–1026. [Google Scholar] [CrossRef]
- Fischer, A.; Marshall, P.; Camp, A. Disturbances in deciduous forest ecosystems of the northern hemisphere: Their effects on both recent and future forest development. Biodivers. Conserv. 2013, 22, 1863–1893. [Google Scholar] [CrossRef]
- Gömöryová, E.; Střelcová, K.; Škvarenina, J.; Bebej, J.; Gömöry, D. The impact of windthrow and fire disturbances on selected soil properties in the Tatra national park. Soil Water Res. 2008, 3, S74–S80. [Google Scholar] [CrossRef]
- Treu, R.; Karst, J.; Randall, M.; Pec, G.J.; Cigan, P.W.; Simard, S.W.; Cooke, J.E.; Erbilgin, N.; Cahill, J.F., Jr. Decline of ectomycorrhizal fungi following a mountain pine beetle epidemic. Ecology 2014, 95, 1096–1103. [Google Scholar] [CrossRef]
- Štursová, M.; Šnajdr, J.; Cajthaml, T.; Bárta, J.; Šantrůčková, H.; Baldrian, P. When the forest dies: The response of forest soil fungi to a bark beetle-induced tree dieback. ISME J. 2014, 8, 1920. [Google Scholar] [CrossRef] [PubMed]
- Avolio, M.L.; Tuininga, A.R.; Lewis, J.D.; Marchese, M. Ectomycorrhizal responses to organic and inorganic nitrogen sources when associating with two host species. Mycol. Res. 2009, 113, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Niklaus, P.A.; Zimmermann, S.; Schmutz, S.; Kremer, J.; Abarenkov, K.; Lüscher, P.; Widmer, F.; Frey, B. Resistance and resilience of the forest soil microbiome to logging-associated compaction. ISME J. 2014, 8, 226–244. [Google Scholar] [CrossRef] [PubMed]
- Vašutová, M.; Edwars-Jonášová, M.; Veselá, P.; Effenberková, L.; Fleischer, P.; Cudlín, P. Management regime is the most important factor influencing ectomycorrhizal species community in Norway spruce forests after windthrow. Mycorrhiza 2018, 28, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Gehring, C.; Bennett, A. Mycorrhizal-fungal-plant-insect interactions: The importance of a community approach. Environ. Entomol. 2009, 38, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, R.; Rhoades, C.C.; Elder, K.; Negron, J. Changes in transpiration and foliage growth in lodgepole pine trees following mountain pine beetle attack and mechanical girdling. For. Ecol. Manag. 2013, 289, 312–317. [Google Scholar] [CrossRef]
- Saikkonen, K.; Ahonen-Jonnarth, U.; Markkola, A.M.; Helander, M.; Tuomi, J.; Roitto, M.; Ranta, H. Defoliation and mycorrhizal symbiosis: A functional balance between carbon sources and below-ground sinks. Ecol. Lett. 1999, 2, 19–26. [Google Scholar] [CrossRef]
- Kuikka, K.; Härmä, E.; Markkola, A.; Rautio, P.; Roitto, M.; Saikkonen, K.; Ahonen-Jonnarth, U.; Finlay, R.; Tuomi, J. Severe defoliation of scots pine reduces reproductive investment by ectomycorrhizal symbionts. Ecology 2003, 84, 2051–2061. [Google Scholar] [CrossRef]
- Saravesi, K.; Markkola, A.; Rautio, P.; Roitto, M.; Tuomi, J. Defoliation causes parallel temporal responses in a host tree and its fungal symbionts. Oecologia 2008, 156, 117–123. [Google Scholar] [CrossRef]
- Veselá, P.; Vašutová, M.; Edwards-Jonášová, M.; Cudlín, P. Soil fungal community in Norway spruce forests under bark beetle attack. Forests 2019, 10, 109. [Google Scholar] [CrossRef]
- Karst, J.; Erbilgin, N.; Pec, G.J.; Cigan, P.W.; Najar, A.; Simard, S.W.; Cahill Jr, J.F. Ectomycorrhizal fungi mediate indirect effect of a bark beetle outbreak on secondary chemistry and establishment of pine seedlings. New Phytol. 2015, 208, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, P.; Homolová, Z. Tatra Mts. as the object for long-term ecological research of natural disturbances. Životné Prostredie 2016, 50, 40–43. [Google Scholar]
- Šebeň, V.; Konopka, B.; Bošela, M.; Pajtík, J. Contrasting development of declining and living larch-spruce stands after a disturbance event: A case study from the High Tatra Mts. For. J. 2015, 61, 157–166. [Google Scholar] [CrossRef]
- Fleischer, P. Windfall research and monitoring in the High Tatra Mts, objectives, principles, methods and current status. Contrib. Geophys. Geod. 2008, 38, 233–248. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes: Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, N., Gelfand, D., Sninsky, J., White, T., Eds.; Academic Press Inc.: New York, NY, USA, 1990. [Google Scholar]
- Nikolcheva, L.G.; Bärlocher, F. Taxon-specific fungal primers reveal unexpectedly high diversity during leaf decomposition in a stream. Mycol. Prog. 2004, 3, 41–49. [Google Scholar] [CrossRef]
- Abarenkov, K.; Henrik Nilsson, R.; Larsson, K.H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Høiland, K.; Kjøller, R.; Larsson, E.; Pennanen, T.; et al. The UNITE database for molecular identification of fungi-recent updates and future perspectives. New Phytol. 2010, 186, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2007, 41, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Agerer, R. Fungal relationships and structural identity of their ectomycorrhizae. Mycol. Prog. 2006, 5, 67–107. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; The R Foundation for Statistical Computing: Vienna, Austria, 2019. Available online: http://www.R-project.org/ (accessed on 3 March 2019).
- Ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Peter, M.; Ayer, F.; Cudlín, P.; Egli, S. Belowground ectomycorrhizal communities in three Norway spruce stands with different degrees of decline in the Czech Republic. Mycorrhiza 2008, 18, 157–169. [Google Scholar] [CrossRef] [Green Version]
- Visser, S. Ectomycorrhizal fungal succession in jack pine stands following wildfire. New Phytol 1995, 129, 389–401. [Google Scholar] [CrossRef]
- Wu, Y.T.; Wubet, T.; Trogisch, S.; Both, S.; Scholten, T.; Bruelheide, H.; Buscot, F. Forest age and plant species composition determine the soil fungal community composition in a Chinese subtropical forest. PLoS ONE 2013, 8, e66829. [Google Scholar] [CrossRef] [PubMed]
- Beule, L.; Grüning, M.M.; Karlovsky, P.; L-M-Arnold, A. Changes of Scots pine phyllosphere and soil fungal communities during outbreaks of defoliating insects. Forests 2017, 8, 316. [Google Scholar] [CrossRef]
- Pec, G.J.; Karst, J.; Taylor, D.L.; Cigan, P.W.; Erbilgin, N.; Cooke, J.E.; Simard, S.W.; Cahill, J.F. Change in soil fungal community structure driven by a decline in ectomycorrhizal fungi following a mountain pine beetle (Dendroctonus ponderosae) outbreak. New Phytol. 2017, 213, 864c873. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Köljalg, U.; Hallenberg, N.; Larsson, K. Fine scale distribution of ectomycorrhizal fungi and roots across substrate layers including coarse woody debris in a mixed forest. New Phytol. 2003, 159, 153–165. [Google Scholar] [CrossRef]
- Otgonsuren, B.; Lee, M. Pinus sylvestris can form ectomycorrhiza with Phialocephala fortinii. Taiwan J. For. Sci. 2012, 27, 265–281. [Google Scholar]
- Grelet, G.; Johnson, D.; Vralstad, T.; Alexander, I.J.; Anderson, I.C. New insights into the mycorrhizal Rhizoscyphus ericae aggregate: Spatial structure and co-colonization of ectomycorrhizal and ericoid roots. New Phytol. 2010, 188, 210–222. [Google Scholar] [CrossRef]
- Sietiö, O.; Tuomivirta, T.; Santalahti, M.; Kiheri, H.; Timonen, S.; Sun, H.; Fritze, H.; Heinonsalo, J. Ericoid plant species and Pinus sylvestris shape fungal communities in their roots and surrounding soil. New Phytol. 2018, 218, 738–751. [Google Scholar] [CrossRef]
- Robakowski, P.; Wyka, T.; Samardakiewicz, S.; Kierzkowski, D. Growth, photosynthesis, and needle structure of silver fir (Abies alba Mill.) seedlings under different canopies. For. Ecol. Manag. 2004, 201, 211–227. [Google Scholar] [CrossRef]
- Teste, F.; Simard, S. Mycorrhizal networks and distance from mature trees alter patterns of competition and facilitation in dry Douglas-fir forests. Oecologia 2008, 158, 193–2013. [Google Scholar] [CrossRef]
- Haas, J.C.; Street, N.R.; Sjödin, A.; Lee, N.M.; Högberg, M.N.; Näsholm, T.; Hurry, V. Microbial community response to growing season and plant nutrient optimisation in a boreal Norway spruce forest. Soil Biol. Biochem. 2018, 125, 197–209. [Google Scholar] [CrossRef]
- Pena, R. Nitrogen acquisition in ectomycorrhizal symbiosis. In Molecular Mycorrhizal Symbiosis; Martin, F., Ed.; John Wiley & Sons: New York, NY, USA, 2016; pp. 179–196. [Google Scholar]
- Bakker, M.R.; Augusto, L.; Achat, D.L. Fine root distribution of trees and understory in mature stands of maritime pine (Pinus pinaster) on dry and humid sites. Plant. Soil 2006, 286, 37–51. [Google Scholar] [CrossRef]
- Guignabert, A.; Delerue, F.; Gonzalez, M.; Augusto, L.; Bakker, M.R. Effects of management practices and topography on ectomycorrhizal fungi of maritime pine during seedling recruitment. Forests 2018, 9, 245. [Google Scholar] [CrossRef]
- Rosinger, C.; Sandén, H.; Matthews, B.; Mayer, M.; Godbold, D.L. Patterns in ectomycorrhizal diversity, community composition, and exploration types in European Beech, Pine, and Spruce forests. Forests 2018, 9, 445. [Google Scholar] [CrossRef]
- Bending, G.D.; Read, D.J. Lignin and soluble phenolic degradation by ectomycorrhizal and ericoid mycorrhizal fungi. Mycol. Res. 1997, 101, 1348–1354. [Google Scholar] [CrossRef]
- Gallet, C.; Pellissier, F. Phenolic compounds in natural solutions of a coniferous forest. J. Chem. Ecol. 1997, 23, 2401–2412. [Google Scholar] [CrossRef]
- Cote, J.F.; Thibault, J.R. Allelopathic potential of raspberry foliar leachates on growth of ectomycorrhizal fungi associated with black spruce. Am. J. Bot. 1988, 75, 966–970. [Google Scholar] [CrossRef]
- Javaid, A. Allelopathic interactions in mycorrhizal associations. Allelopathy J. 2007, 20, 29–42. [Google Scholar]



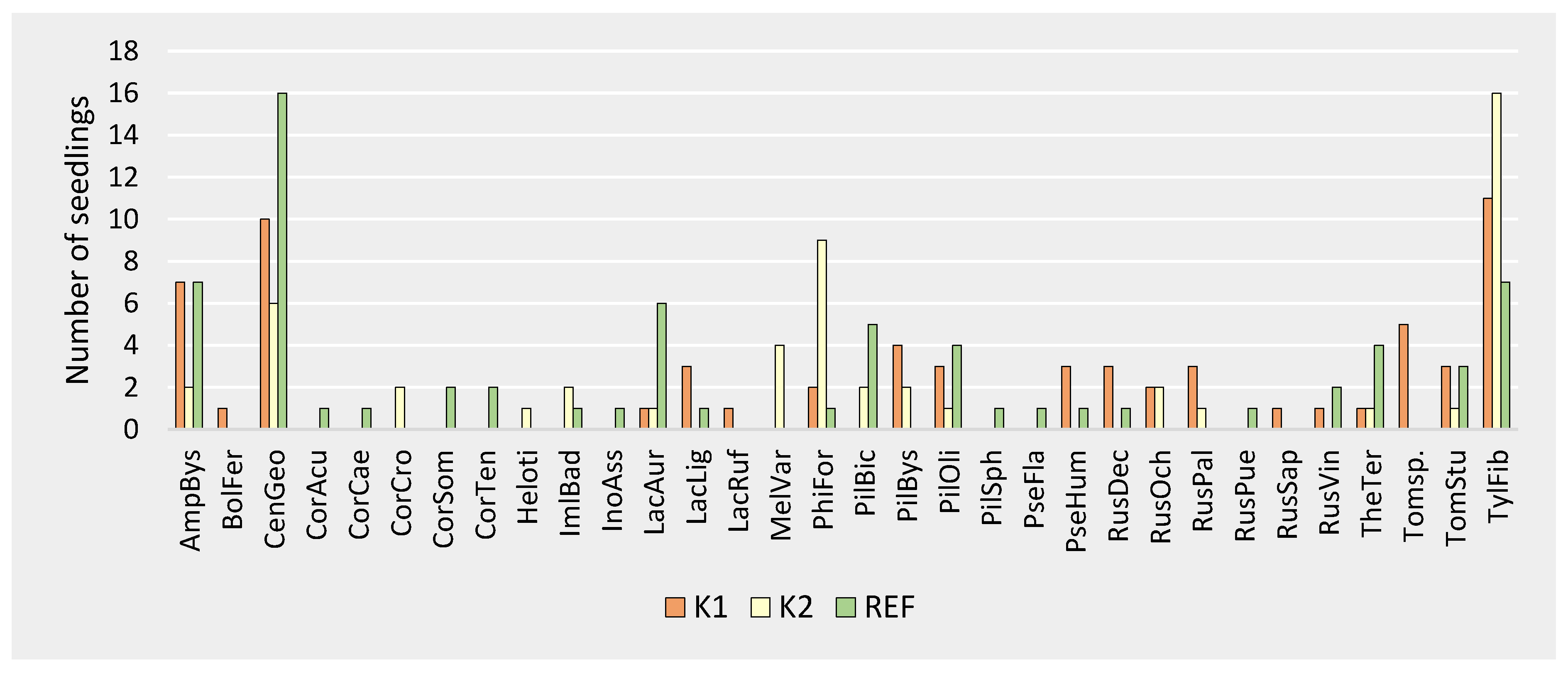
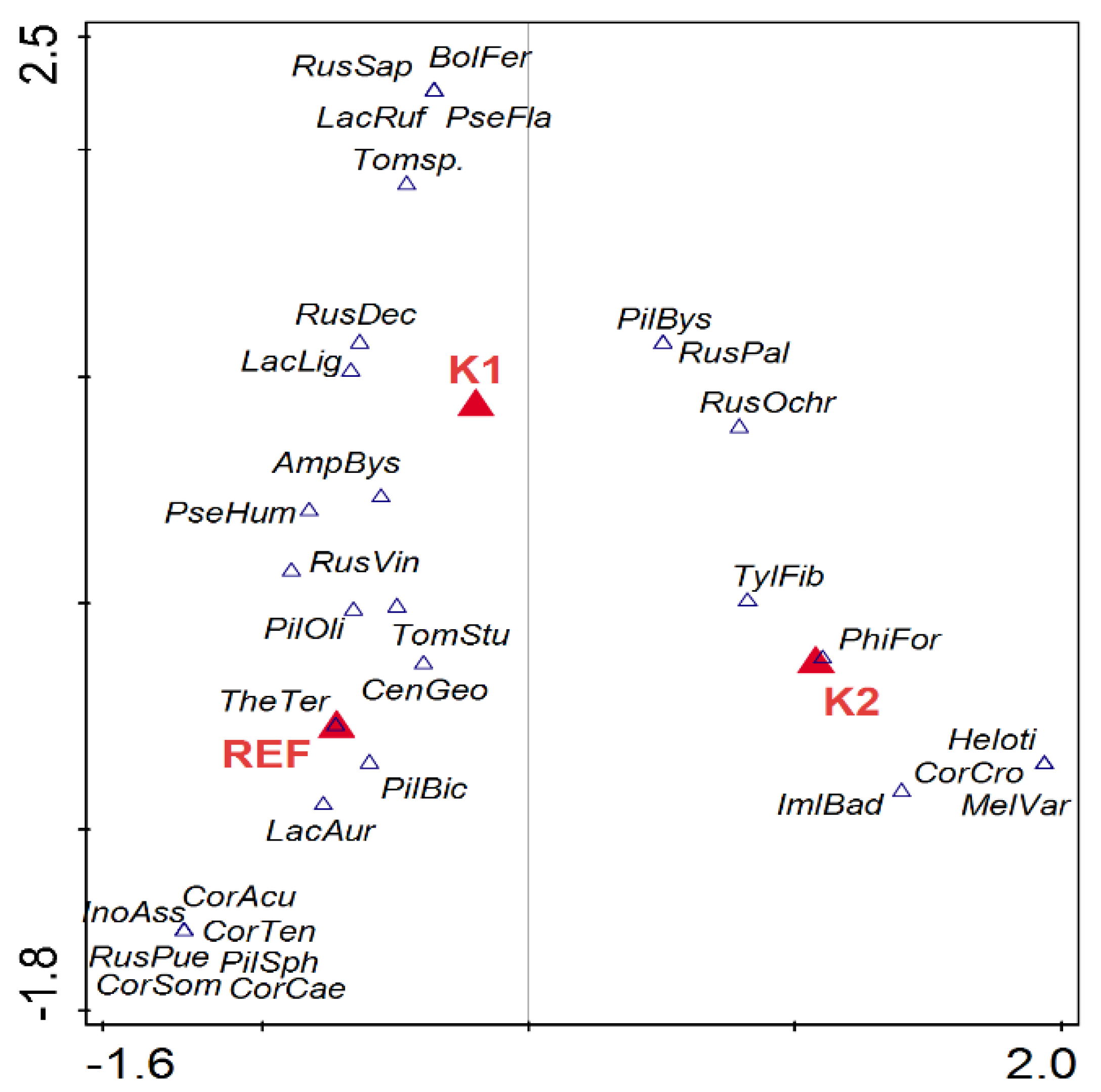
| K1 | K2 | REF | |
|---|---|---|---|
| tree cover | 43 ± 10.9 | 0 | 43 ± 9.2 |
| cover of dominant trees | Picea abies (L.) H. Karst. (26.5 ± 4.9), Larix decidua Mill. (15.0 ± 7.1), Abies alba Mill. (1.5 ± 1.4), Sorbus aucuparia L. (0.7 ± 0.5) | Larix decidua (1.0 ± 0.6), Sorbus aucuparia (3.8 ± 1.3) | Picea abies (36.0 ± 9.8), Larix decidua (7.0 ± 2.9) |
| cover of dominant herbs (3 dominants) * | Vaccinium myrtillus (37.7 ± 5.2), Avenella flexuosa (10.2 ± 2.1), Oxalis acetosella L. (4.7 ± 1.3) | Vaccinium myrtillus (38.1 ± 5.6), Calamagrostis villosa (10.4 ± 4.5), Epilobium angustifolium L. (8 ± 2.1) | Vaccinium myrtillus (32.6 ± 5), Avenella flexuosa (14.3 ± 3.3), Calamagrostis villosa (11.4 ± 2.5) |
| spruce seedling cover * | 0.5 ± 0.3 | 4.5 ± 3 | 2.6 ± 1.3 |
| larch seedling cover * | 1 ± 1 | 0.2 ± 0.1 | 0.8 ± 0.6 |
| rowan seedling cover * | 0.3 ± 0.2 | 2.4 ± 0.8 | 0.1 ± 0.1 |
| CWD * | 2.5 ± 0.7 | 5.9 ± 1.1 | 0.5 ± 0.3 |
| Type of Analysis | Explanatory Variables | Explained Variability (%) | Pseudo-F | p |
|---|---|---|---|---|
| CCA | ECM composition | |||
| Site type (K1, K2, REF) | 6.7 | 2.0 | 0.001 | |
| RDA | Exploration types | |||
| Site type (K1, K2, REF) | 10.5 | 3.3 | 0.011 | |
| Vegetation cover: Epilobium angustifolium, Luzula luzuloides | 14.7 | 4.9 | 0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veselá, P.; Vašutová, M.; Hofmannová, K.; Edwards-Jonášová, M.; Cudlín, P. Ectomycorrhizal Community on Norway Spruce Seedlings Following Bark Beetle Infestation. Forests 2019, 10, 740. https://doi.org/10.3390/f10090740
Veselá P, Vašutová M, Hofmannová K, Edwards-Jonášová M, Cudlín P. Ectomycorrhizal Community on Norway Spruce Seedlings Following Bark Beetle Infestation. Forests. 2019; 10(9):740. https://doi.org/10.3390/f10090740
Chicago/Turabian StyleVeselá, Petra, Martina Vašutová, Karolína Hofmannová, Magda Edwards-Jonášová, and Pavel Cudlín. 2019. "Ectomycorrhizal Community on Norway Spruce Seedlings Following Bark Beetle Infestation" Forests 10, no. 9: 740. https://doi.org/10.3390/f10090740





