Soil Physicochemical Properties and the Rhizosphere Soil Fungal Community in a Mulberry (Morus alba L.)/Alfalfa (Medicago sativa L.) Intercropping System
Abstract
:1. Introduction
2. Results
2.1. Physicochemical Properties of the Rhizosphere Soil of the Mulberry and Alfalfa Intercropping System
2.2. Fungal Shannon-Wiener Curve
2.3. Fungal Community Richness and Diversity
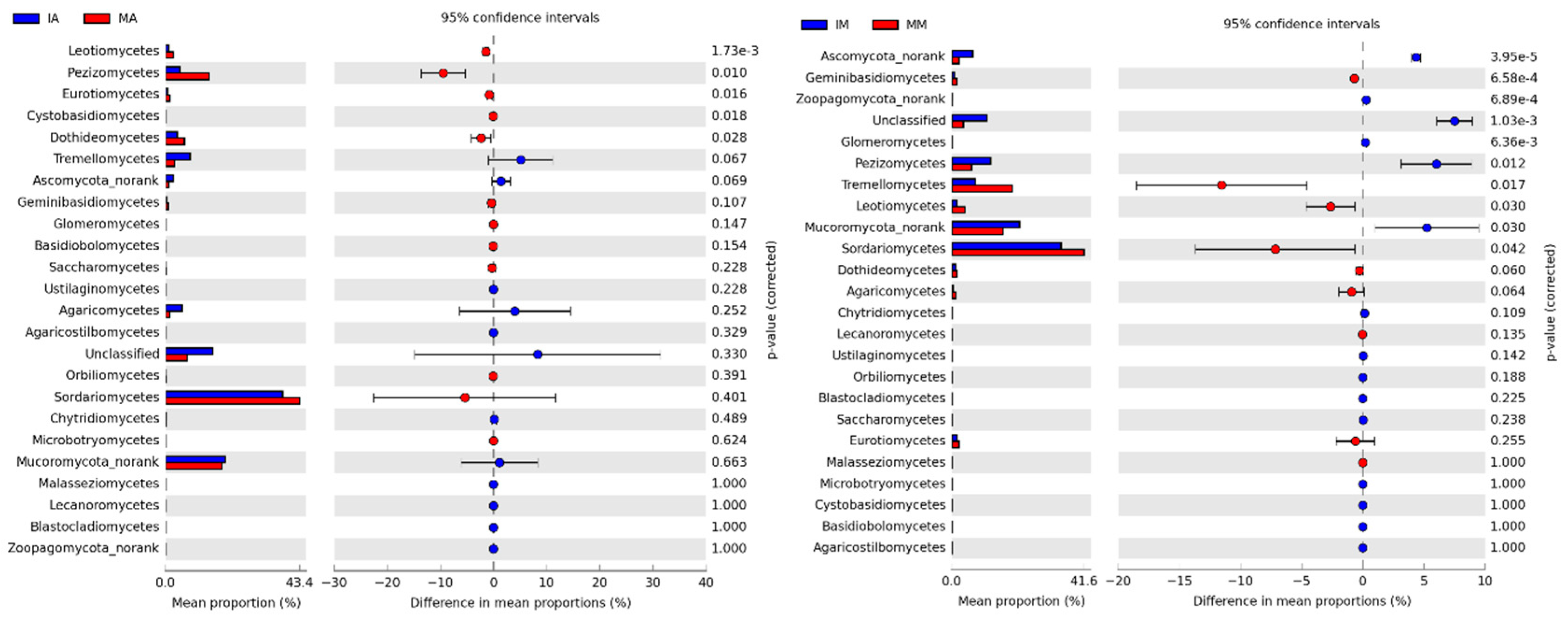
2.4. Taxonomic Structure of Fungal Communities among Different Treatments
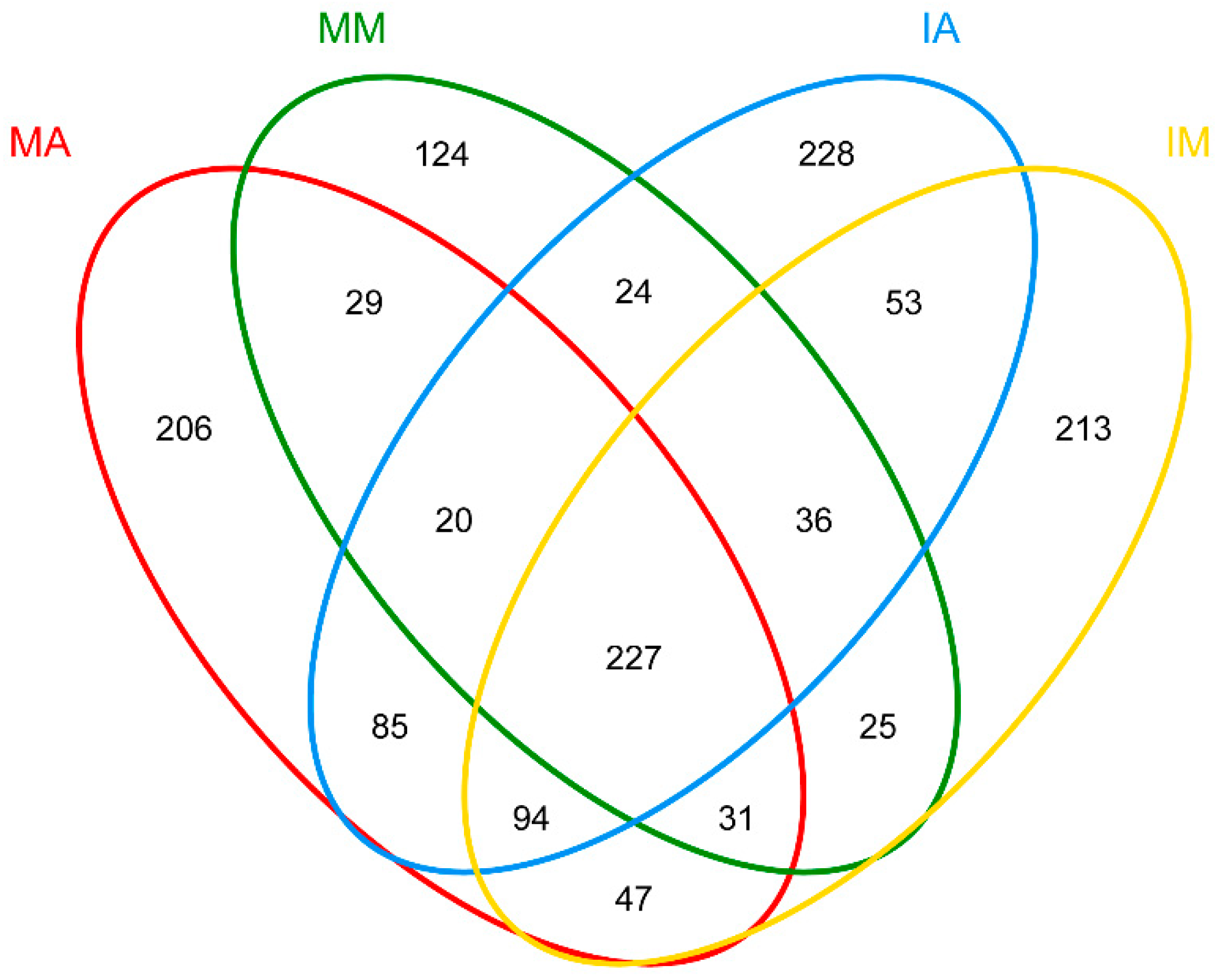
2.5. Shared Fungal OTUs
2.6. Relationship of the Soil Physicochemical Properties, Fungal Phyla, and Fungal Communities
3. Discussion
3.1. Soil Physicochemical Properties
3.2. Fungal Community Diversity and Structure in the Intercropping System
3.3. Correlation between Soil Physicochemical Properties and the Dominant Fungal Phyla
3.4. Effects of Soil Properties and Planting Pattern on the Fungal Community Structure
4. Materials and Methods
4.1. Site Description and Soil Sampling
4.2. Soil DNA Extraction, PCR Amplification and MiSeq Sequencing
4.3. Data Analysis
4.3.1. Sequence Optimization and Data Statistics/Processing the Sequencing Data
4.3.2. OTU Cluster
4.3.3. Shannon-Wiener Curves, Alpha Diversity, and Beta Diversity
4.3.4. Statistical and Bioinformatics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Meier, C.L.; Bowman, W.D. Links between plant litter chemistry, species diversity, and below-ground ecosystem function. Proc. Natl. Acad. Sci. USA 2008, 105, 19780–19785. [Google Scholar] [CrossRef] [Green Version]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; van der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Bais, H.P.; Park, S.-W.; Weir, T.L.; Callaway, R.M.; Vivanco, J.M. How plants communicate using the underground information superhighway. Trends Plant Sci. 2004, 9, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Birouste, M.; Kazakou, E.; Blanchard, A.; Roumet, C. Plant traits and decomposition: Are the relationships for roots comparable to those for leaves? Ann. Bot. 2011, 109, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Guo-Chun, D.; Piceno, Y.M.; Holger, H.; Nicole, W.; Dohrmann, A.B.; Angel, C.; Andersen, G.L.; Thelma, C.; Tebbe, C.C.; Kornelia, S. Changes of soil bacterial diversity as a consequence of agricultural land use in a semi-arid ecosystem. PLoS ONE 2013, 8, e59497. [Google Scholar]
- Bissett, A.; Richardson, A.E.; Baker, G.; Thrall, P.H. Long-term land use effects on soil microbial community structure and function. Appl. Soil Ecol. 2011, 51, 66–78. [Google Scholar] [CrossRef]
- Bainard, L.D.; Koch, A.M.; Gordon, A.M.; Newmaster, S.G.; Thevathasan, N.V.; Klironomos, J.N. Influence of trees on the spatial structure of arbuscular mycorrhizal communities in a temperate tree-based intercropping system. Agric. Ecosyst. Environ. 2011, 144, 13–20. [Google Scholar] [CrossRef]
- Nair, P.R. An Introduction to Agroforestry; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1993. [Google Scholar]
- Thevathasan, N.; Gordon, A.; Simpson, J.; Reynolds, P.; Price, G.; Zhang, P. Biophysical and ecological interactions in a temperate tree-based intercropping system. J. Crop Improv. 2004, 12, 339–363. [Google Scholar] [CrossRef]
- Kimura, E.; Fransen, S.C.; Collins, H.P.; Stanton, B.J.; Himes, A.; Smith, J.; Guy, S.O.; Johnston, W.J. Effect of intercropping hybrid poplar and switchgrass on biomass yield, forage quality, and land use efficiency for bioenergy production. Biomass Bioenerg. 2018, 111, 31–38. [Google Scholar] [CrossRef]
- Trenbath, B.R. Multispecies cropping systems in India: Predictions of their productivity, stability, resilience and ecological sustainability. Agrofor. Syst. 1999, 45, 81–107. [Google Scholar] [CrossRef]
- Hauggaard-Nielsen, H.; Ambus, P.; Jensen, E.S. The comparison of nitrogen use and leaching in sole cropped versus intercropped pea and barley. Nutr. Cycl. Agroecosyst. 2003, 65, 289–300. [Google Scholar] [CrossRef]
- Bainard, L.; Koch, A.; Gordon, A.; Klironomos, J. Growth response of crops to soil microbial communities from conventional monocropping and tree-based intercropping systems. Plant Soil 2013, 363, 345–356. [Google Scholar] [CrossRef]
- Chifflot, V.; Rivest, D.; Olivier, A.; Cogliastro, A.; Khasa, D. Molecular analysis of arbuscular mycorrhizal community structure and spores distribution in tree-based intercropping and forest systems. Agric. Ecosyst. Environ. 2009, 131, 32–39. [Google Scholar] [CrossRef]
- Lacombe, S.; Bradley, R.L.; Hamel, C.; Beaulieu, C. Do tree-based intercropping systems increase the diversity and stability of soil microbial communities? Agric. Ecosyst. Environ. 2009, 131, 25–31. [Google Scholar] [CrossRef]
- Zhang, M.M.; Wang, N.; Hu, Y.B.; Sun, G.Y. Changes in soil physicochemical properties and soil bacterial community in mulberry (Morus alba L.)/alfalfa (Medicago sativa L.) intercropping system. Microbiol. Open 2018, 7, e00555. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Heerink, N.; Jin, S.; Berentsen, P.; Zhang, L.; van der Werf, W. Intercropping and agroforestry in China–Current state and trends. Agric. Ecosyst. Environ. 2017, 244, 52–61. [Google Scholar] [CrossRef]
- Gou, F.; Yin, W.; Hong, Y.; van der Werf, W.; Chai, Q.; Heerink, N.; van Ittersum, M.K. On yield gaps and yield gains in intercropping: Opportunities for increasing grain production in northwest China. Agric. Syst. 2017, 151, 96–105. [Google Scholar] [CrossRef]
- Paul, E.A. Soil Microbiology, Ecology and Biochemistry; Academic Press: London, UK, 2014. [Google Scholar]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef]
- Kuramae, E.E.; Yergeau, E.; Wong, L.C.; Pijl, A.S.; van Veen, J.A.; Kowalchuk, G.A. Soil characteristics more strongly influence soil bacterial communities than land-use type. FEMS Microbial. Ecol. 2012, 79, 12–24. [Google Scholar] [CrossRef]
- Kaur, B.; Gupta, S.; Singh, G. Soil carbon, microbial activity and nitrogen availability in agroforestry systems on moderately alkaline soils in northern India. Appl. Soil Ecol. 2000, 15, 283–294. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Augé, R.M. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Duddridge, J.; Malibari, A.; Read, D. Structure and function of mycorrhizal rhizomorphs with special reference to their role in water transport. Nature 1980, 287, 834–836. [Google Scholar] [CrossRef]
- Johri, A.K.; Oelmüller, R.; Dua, M.; Yadav, V.; Kumar, M.; Tuteja, N.; Varma, A.; Bonfante, P.; Persson, B.L.; Stroud, R.M. Fungal association and utilization of phosphate by plants: Success, limitations, and future prospects. Front. Microbial. 2015, 6, 984. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ran, W.; Zhang, R.; Sun, S.; Xu, G. Facilitated legume nodulation, phosphate uptake and nitrogen transfer by arbuscular inoculation in an upland rice and mung bean intercropping system. Plant Soil 2009, 315, 285–296. [Google Scholar] [CrossRef]
- Rains, K.C.; Bledsoe, C.S. Rapid uptake of 15N-ammonium and glycine-13C, 15N by arbuscular and ericoid mycorrhizal plants native to a Northern California coastal pygmy forest. Soil Biol. Biochem. 2007, 39, 1078–1086. [Google Scholar] [CrossRef]
- Palm, C.A. Contribution of agroforestry trees to nutrient requirements of intercropped plants. Agrofor. Syst. 1995, 30, 105–124. [Google Scholar] [CrossRef]
- Garcia, K.; Zimmermann, S.D. The role of mycorrhizal associations in plant potassium nutrition. Front. Plant Sci. 2014, 5, 337. [Google Scholar] [CrossRef]
- Rachid, C.T.; Balieiro, F.C.; Fonseca, E.S.; Peixoto, R.S.; Chaer, G.M.; Tiedje, J.M.; Rosado, A.S. Intercropped silviculture systems, a key to achieving soil fungal community management in Eucalyptus plantations. PLoS ONE 2015, 10, e0118515. [Google Scholar] [CrossRef]
- Treseder, K.K.; Allen, E.B.; Egerton-Warburton, L.M.; Hart, M.M.; Klironomos, J.N.; Maherali, H.; Tedersoo, L. Arbuscular mycorrhizal fungi as mediators of ecosystem responses to nitrogen deposition: A trait-based predictive framework. J. Ecol. 2018, 106, 480–489. [Google Scholar] [CrossRef] [Green Version]
- Anderson, I.C.; Campbell, C.D.; Prosser, J.I. Potential bias of fungal 18S rDNA and internal transcribed spacer polymerase chain reaction primers for estimating fungal biodiversity in soil. Environ. Microbiol. 2003, 5, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.W.; Kim, B.K.; Kim, C.; Jung, H.S.; Kim, B.-S.; Lee, J.-H.; Chun, J. Assessment of soil fungal communities using pyrosequencing. J. Microbiol. 2010, 48, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Ranjard, L.; Poly, F.; Lata, J.-C.; Mougel, C.; Thioulouse, J.; Nazaret, S. Characterization of bacterial and fungal soil communities by automated ribosomal intergenic spacer analysis fingerprints: Biological and methodological variability. Appl. Environ. Microbial. 2001, 67, 4479–4487. [Google Scholar] [CrossRef]
- Jose, S.; Gillespie, A.; Pallardy, S. Interspecific interactions in temperate agroforestry. In New Vistas in Agroforestry; Springer: Berlin, Germany, 2004; pp. 237–255. [Google Scholar]
- Harley, J.L.; Smith, S.E. Mycorrhizal symbiosis. Q. Rev. Biol. 2008, 3, 273–281. [Google Scholar]
- Sepp, S.K.; Jairus, T.; Vasar, M.; Zobel, M.; Öpik, M. Effects of land use on arbuscular mycorrhizal fungal communities in Estonia. Mycorrhiza 2018, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Burrows, R.L.; Pfleger, F.L. Arbuscular mycorrhizal fungi respond to increasing plant diversity. Can. J. Bot. 2002, 80, 120–130. [Google Scholar] [CrossRef]
- Leal, P.L.; Stürmer, S.L.; Siqueira, J.O. Occurrence and diversity of arbuscular mycorrhizal fungi in trap cultures from soils under different land use systems in the Amazon, Brazil. Braz. J. Microbiol. 2009, 40, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Wal, A.; Geydan, T.D.; Kuyper, T.W.; Boer, W. A thready affair: Linking fungal diversity and community dynamics to terrestrial decomposition processes. FEMS Microbiol. Rev. 2013, 37, 477–494. [Google Scholar]
- Floudas, D.; Binder, M.; Riley, R.; Barry, K.; Blanchette, R.A.; Henrissat, B.; Martínez, A.T.; Otillar, R.; Spatafora, J.W.; Yadav, J.S.; et al. The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 2012, 336, 1715–1719. [Google Scholar] [CrossRef]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A.; et al. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef] [Green Version]
- Buee, M.; Reich, M.; Murat, C.; Morin, E.; Nilsson, R.H.; Uroz, S.; Martin, F. 454 Pyrosequencing analyses of forest soils reveal an unexpectedly high fungal diversity. New Phytol. 2009, 184, 449–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, S.E.; Legge, R.; Weber, K.A.; Brodie, E.L.; Goldfarb, K.C.; Benson, A.K.; Tan, S. Bacterial community structure of contrasting soils underlying Bornean rain forests: Inferences from microarray and next-generation sequencing methods. Soil Biol. Biochem. 2012, 55, 48–59. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Núñez, J.A.D.; Serrano, J.S.; Barreal, J.A.R.; de Omeñaca González, J.A.S. The influence of mycorrhization with Tuber melanosporum in the afforestation of a Mediterranean site with Quercus ilex and Quercus faginea. For. Ecol. Manag. 2006, 231, 226–233. [Google Scholar] [CrossRef]
- Wallander, H.; Mahmood, S.; Hagerberg, D.; Johansson, L.; Pallon, J. Elemental composition of ectomycorrhizal mycelia identified by PCR–RFLP analysis and grown in contact with apatite or wood ash in forest soil. FEMS Microbial. Ecol. 2003, 44, 57–65. [Google Scholar] [CrossRef]
- Wallander, H.K.; Pallon, J. Temporal changes in the elemental composition of Rhizopogon rhizomorphs during colonization of patches with fresh organic matter or acid-washed sand. Mycologia 2005, 97, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Lambilliotte, R.; Cooke, R.; Samson, D.; Fizames, C.; Gaymard, F.; Plassard, C.; Tatry, M.V.; Berger, C.; Laudié, M.; Legeai, F. Large-scale identification of genes in the fungus Hebeloma cylindrosporum paves the way to molecular analyses of ectomycorrhizal symbiosis. New Phytol. 2004, 164, 505–513. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Guo, W.Q.; Shi, D.C. Seed germination, seedling survival, and physiological response of sunflowers under saline and alkaline conditions. Photosynthetica 2010, 48, 278–286. [Google Scholar] [CrossRef]
- Borcard, D.; Legendre, P.; Drapeau, P. Partialling out the spatial component of ecological variation. Ecology 1992, 73, 1045–1055. [Google Scholar] [CrossRef]
- H., H.C. Study on the efficient planting pattern of mulberry and grain intercropping. Mod. Agric. Sci. Technol. 2011, 21, 68–69. [Google Scholar]
- Nazih, N.; Finlay-Moore, O.; Hartel, P.G.; Fuhrmann, J.J. Whole soil fatty acid methyl ester (FAME) profiles of early soybean rhizosphere as affected by temperature and matric water potential. Soil Biol. Biochem. 2001, 33, 693–696. [Google Scholar] [CrossRef]
- Schinner, F.; Öhlinger, R.; Kandeler, D.E.; Margesin, R. Methods in Soil Biology; Springer: Berlin, Germany, 1995. [Google Scholar]
- Lu, R.K. Analytical Methods of Soil and Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Consortium, F.B. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
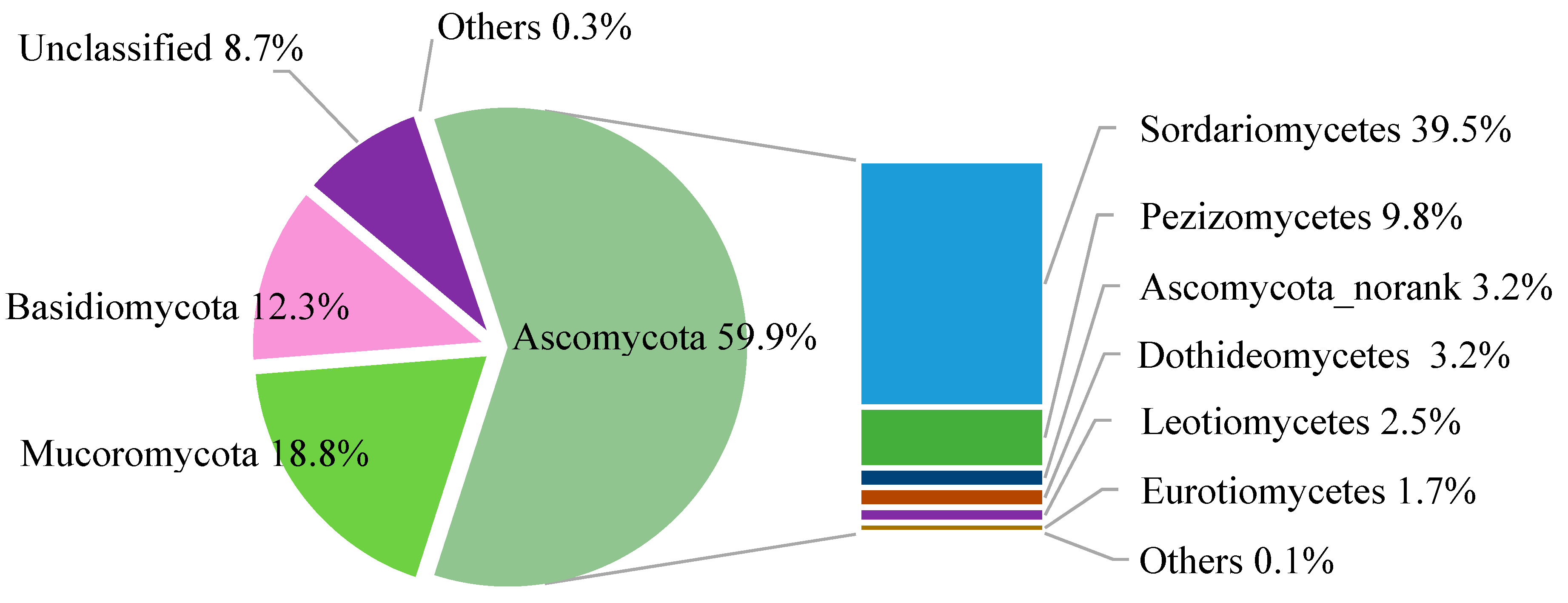




| Sample | TN g/kg | AHN mg/kg | TP g/kg | AK mg/kg | TC g/kg |
|---|---|---|---|---|---|
| MM | 0.91 ± 0.00 c | 148.88 ± 6.96 ab | 0.41 ± 0.06 a | 5.02 ± 0.7 c | 2.23 ± 0.00 a |
| IM | 0.54 ± 0.00 b | 123.07 ± 10.88 a | 0.46 ± 0.02 a | 3.35 ± 0.00 b | 2.22 ± 0.00 a |
| MA | 0.79 ± 0.04 a | 111.37 ± 3.37 a | 0.42 ± 0.00 a | 2.99 ± 0.07 a | 2.16 ± 0.00 a |
| IA | 0.93 ± 0.06 c | 227.34 ± 87.84 b | 0.46 ± 0.01 a | 6.26 ± 0.21 d | 2.52 ± 0.00 b |
| Results of two-way ANOVA | |||||
| Int | 29.15 ** | 2.73 | 7.29 | 144.90 * | 11.49 ** |
| Crv | 94.29 ** | 3.23 | 0.029 | 710.75 ** | 9.00 ** |
| Sample | Cluster Distance (0.03) | ||||
|---|---|---|---|---|---|
| OTUs | Chao 1 | Coverage | Shannon | Simpson | |
| MM-1 | 321 | 416 | 1.00 | 4.08 | 0.040 |
| MM-2 | 382 | 473 | 1.00 | 4.10 | 0.048 |
| MM-3 | 480 | 873 | 0.99 | 4.20 | 0.041 |
| IM-1 | 605 | 788 | 0.99 | 4.34 | 0.037 |
| IM-2 | 503 | 600 | 1.00 | 4.34 | 0.033 |
| IM-3 | 705 | 1006 | 0.99 | 4.51 | 0.028 |
| MA-1 | 486 | 544 | 1.00 | 4.38 | 0.028 |
| MA-2 | 522 | 695 | 0.99 | 4.42 | 0.026 |
| MA-3 | 657 | 898 | 0.99 | 4.50 | 0.025 |
| IA-1 | 321 | 416 | 1.00 | 4.08 | 0.040 |
| IA-2 | 382 | 473 | 1.00 | 4.10 | 0.048 |
| IA-3 | 480 | 873 | 0.99 | 4.20 | 0.041 |
| Total | 5844 | -- | -- | -- | -- |
| Taxonomic Group | TN | AHN | TP | AK | TC | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | |
| Ascomycota | −0.295 | 0.353 | −0.150 | 0.643 | −0.391 | 0.208 | −0.726 | 0.008 | −0.550 | 0.064 |
| Basidiomycota | 0.580 | 0.048 | 0.170 | 0.598 | −0.318 | 0.314 | 0.669 | 0.017 | 0.159 | 0.622 |
| Blastocladiomycota | −0.660 | 0.019 | −0.222 | 0.489 | 0.434 | 0.158 | −0.336 | 0.286 | −0.194 | 0.545 |
| Chytridiomycota | 0.082 | 0.800 | −0.165 | 0.607 | 0.558 | 0.059 | 0.205 | 0.523 | 0.462 | 0.131 |
| Mucoromycota | −0.656 | 0.021 | 0.367 | 0.240 | 0.599 | 0.040 | −0.224 | 0.484 | 0.005 | 0.988 |
| Unclassified | 0.067 | 0.836 | −0.129 | 0.690 | 0.507 | 0.093 | 0.306 | 0.333 | 0.490 | 0.106 |
| Zoopagomycota | −0.925 | 0.000 | −0.307 | 0.331 | 0.401 | 0.197 | −0.588 | 0.044 | −0.397 | 0.201 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Wang, N.; Zhang, J.; Hu, Y.; Cai, D.; Guo, J.; Wu, D.; Sun, G. Soil Physicochemical Properties and the Rhizosphere Soil Fungal Community in a Mulberry (Morus alba L.)/Alfalfa (Medicago sativa L.) Intercropping System. Forests 2019, 10, 167. https://doi.org/10.3390/f10020167
Zhang M, Wang N, Zhang J, Hu Y, Cai D, Guo J, Wu D, Sun G. Soil Physicochemical Properties and the Rhizosphere Soil Fungal Community in a Mulberry (Morus alba L.)/Alfalfa (Medicago sativa L.) Intercropping System. Forests. 2019; 10(2):167. https://doi.org/10.3390/f10020167
Chicago/Turabian StyleZhang, Mengmeng, Ning Wang, Jingyun Zhang, Yanbo Hu, Dunjiang Cai, Jianhua Guo, Di Wu, and Guangyu Sun. 2019. "Soil Physicochemical Properties and the Rhizosphere Soil Fungal Community in a Mulberry (Morus alba L.)/Alfalfa (Medicago sativa L.) Intercropping System" Forests 10, no. 2: 167. https://doi.org/10.3390/f10020167





