Extensive Characterization of Oxide-Coated Colloidal Gold Nanoparticles Synthesized by Laser Ablation in Liquid
Abstract
:1. Introduction
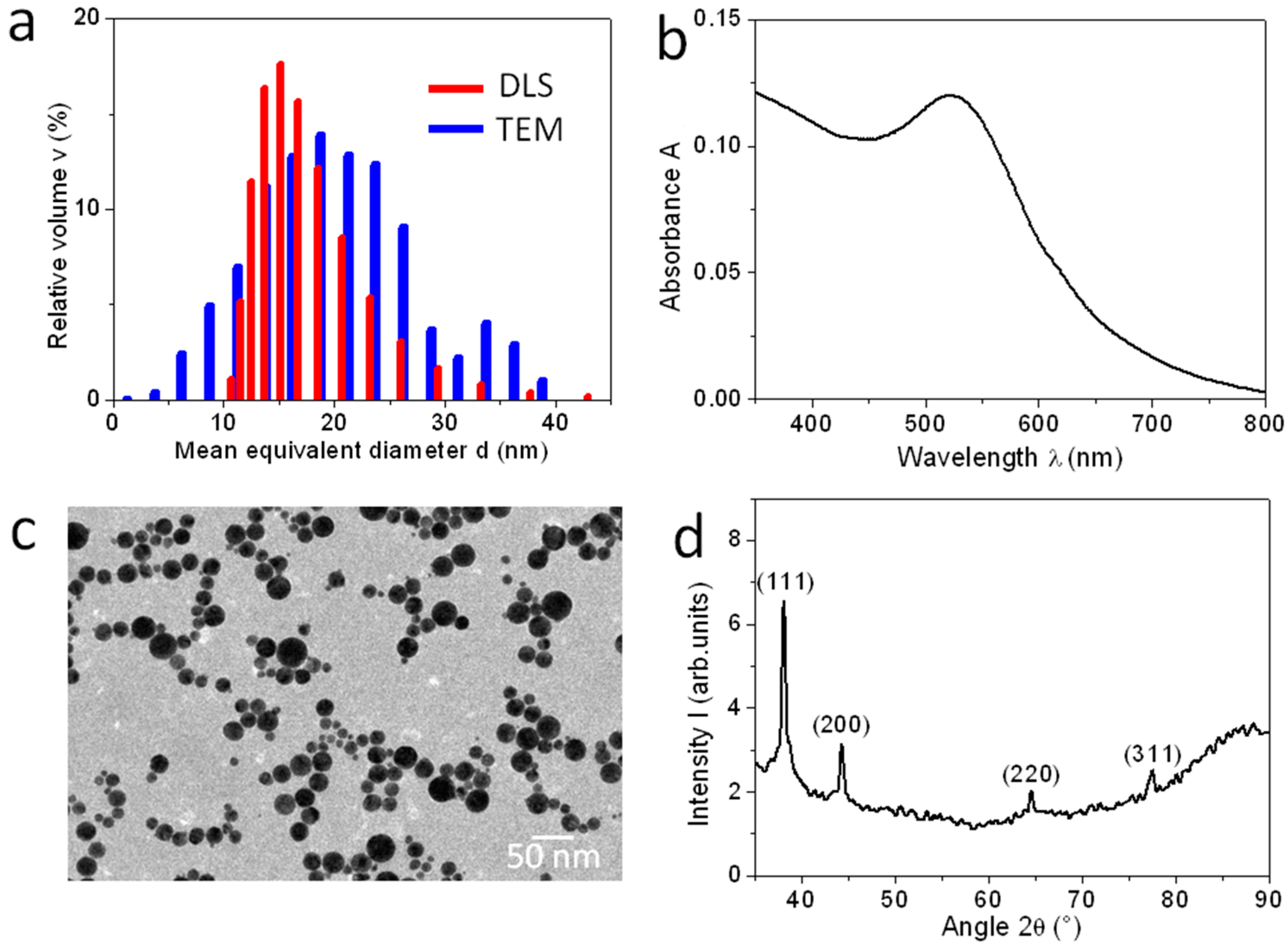
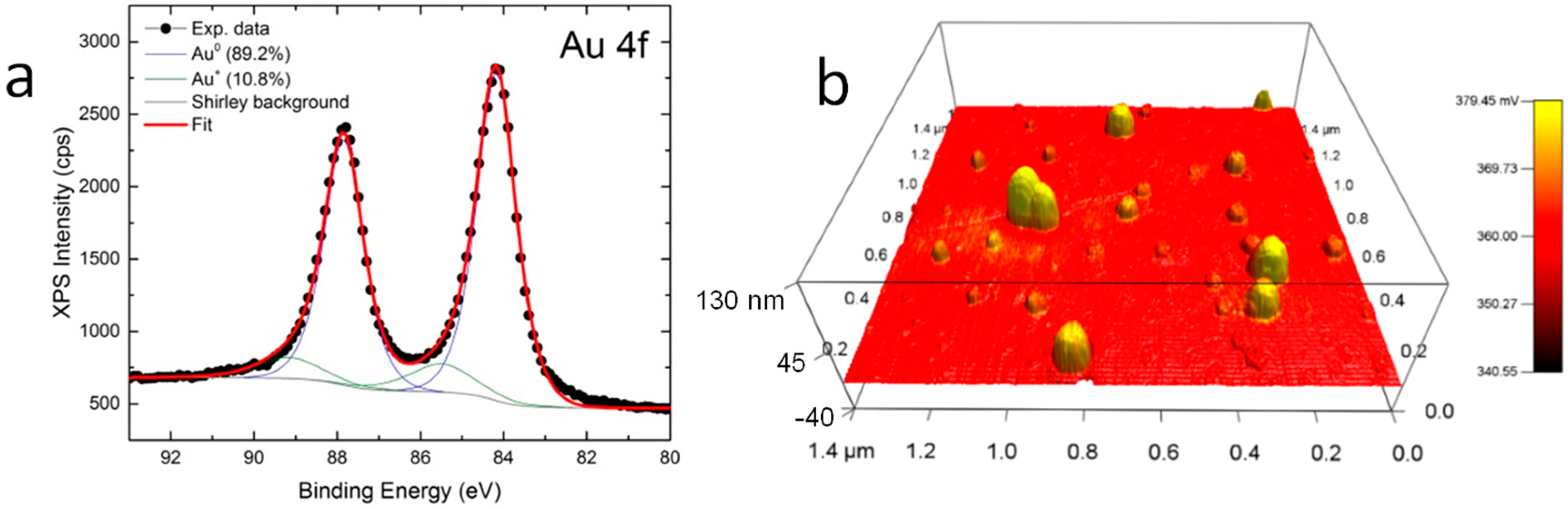
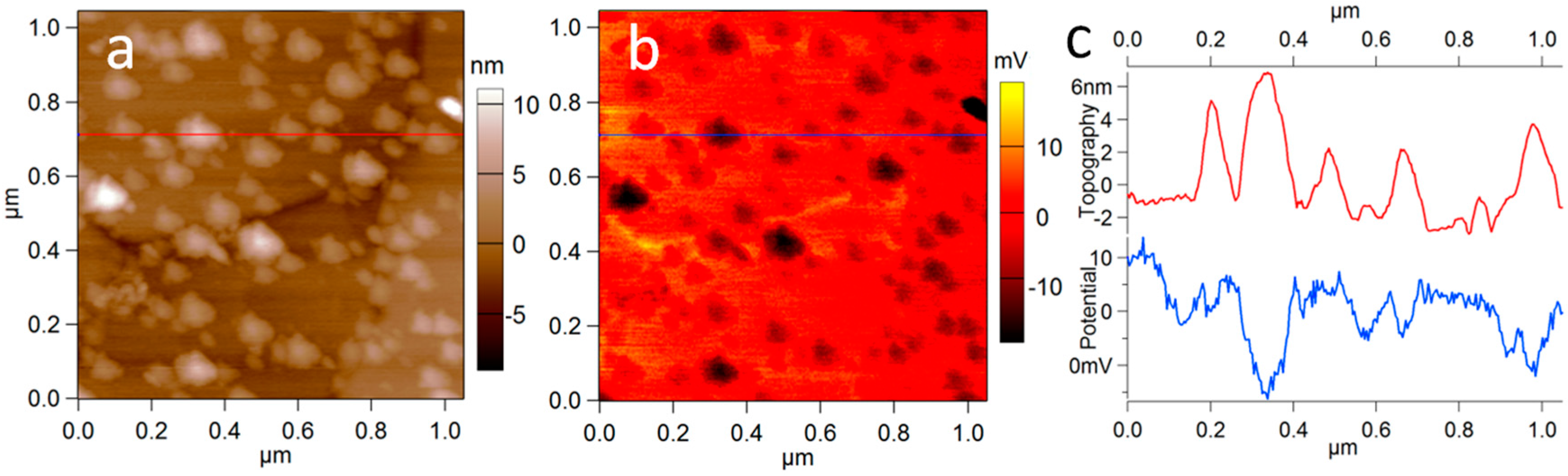
2. Results
3. Discussion
4. Materials and Methods
4.1. Au NPs Investigated
4.2. NPs Characterization Techniques
4.2.1. In Solution
4.2.2. In Dried Form
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| NPs | nanoparticles |
| SERS | surface-enhanced Raman scattering |
| LA | laser ablation |
| DLS | dynamic light scattering |
| UV-Vis | ultraviolet-visible |
| XPS | X-ray photoelectron spectroscopy |
| AFM | atomic force microscopy |
| SKPM | scanning Kelvin probe microscopy |
References
- Zhang, X. Gold Nanoparticles: Recent Advances in the Biomedical Applications. Cell. Biochem. Biophys. 2015, 72, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Cabuzu, D.; Cirja, A.; Puiu, R.; Grumezescu, A.M. Biomedical applications of gold nanoparticles. Curr. Top. Med. Chem. 2015, 15, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Dasog, M.; Leontowich, A.F.G.; Scott, R.W.J.; Paige, M.F. Fluorescently Labeled Gold Nanoparticles with Minimal Fluorescence Quenching. J. Phys. Chem. C 2010, 114, 17446–17454. [Google Scholar] [CrossRef]
- Khan, A.; Rashid, R.; Murtaza, G.; Zahra, A. Gold Nanoparticles: Synthesis and Applications in Drug Delivery. Trop. J. Pharm. Res. 2014, 13, 1169–1177. [Google Scholar]
- Dreaden, E.C.; Austin, L.A.; Mackey, M.A.; El-Sayed, M.A. Size matters: Gold nanoparticles in targeted cancer drug delivery. Ther. Deliv. 2012, 3, 457–478. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Bonnier, F.; Casey, A.; Shanahan, A.E.; Byrne, H.J. Surface enhanced Raman scattering with gold nanoparticles: Effect of particle shape. Anal. Methods 2014, 6, 9116–9123. [Google Scholar] [CrossRef]
- Intartaglia, R.; Beke, S.; Moretti, M.; De Angelis, F.; Diaspro, A. Fast and cost-effective fabrication of large-area plasmonic transparent biosensor array. Lab Chip 2015, 15, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid–Liquid system. J. Chem. Soc. Chem. Commun. 1994, 801–802. [Google Scholar] [CrossRef]
- Perrault, S.D.; Chan, W.C.W. Synthesis and Surface Modification of Highly Monodispersed, Spherical Gold Nanoparticles of 50–200 (vol 131, pg 17042, 2009). J. Am. Chem. Soc. 2010, 132, 11824. [Google Scholar] [CrossRef]
- Martin, M.N.; Basham, J.I.; Chando, P.; Eah, S.K. Charged gold nanoparticles in non-polar solvents: 10-min synthesis and 2D self-assembly. Langmuir 2010, 26, 7410–7417. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.R.G.; Lerouge, F.; Cepraga, C.; Micouin, G.; Favier, A.; Chateau, D.; Charreyre, M.T.; Lanoë, P.H.; Monnereau, C.; Chaput, F.; et al. Nanocarriers with ultrahigh chromophore loading for fluorescence bio-imaging and photodynamic therapy. Biomaterials 2013, 34, 8344–8351. [Google Scholar] [CrossRef] [PubMed]
- Fojtik, A.; Giersig, M.; Henglein, A. Formation of Nanometer-Size Silicon Particles in a Laser Induced Plasma in SiH4. Ber. Bunsenges. Phys. Chem. 1993, 97, 1493–1496. [Google Scholar] [CrossRef]
- Sibbald, M.S.; Chumanov, G.; Cotton, T.M. Reduction of Cytochrome c by Halide-Modified, Laser-Ablated Silver Colloids. J. Phys. Chem. C 1996, 100, 4672–4678. [Google Scholar] [CrossRef]
- Mafuné, F.; Kohno, J.; Takeda, Y. Formation of gold nanoparticles by laser ablation in aqueous solution of surfactant. J. Phys. Chem. 2001, 105, 5114–5120. [Google Scholar] [CrossRef]
- Tsuji, T.; Yahata, T.; Yasutomo, M.; Igawa, K.; Tsuji, M.; Ishikawa, Y.; Koshizaki, N. Preparation and investigation of the formation mechanism of submicron-sized spherical particles of gold using laser ablation and laser irradiation in liquids. Phys. Chem. Chem. Phys. 2013, 15, 3099–3107. [Google Scholar] [CrossRef] [PubMed]
- Rehbock, C.; Jakobi, J.; Gamrad, L.; van der Meer, S.; Tiedemann, D.; Taylor, U.; Kues, W.; Rath, D.; Barcikowski, S. Current state of laser synthesis of metal and alloy nanoparticles as ligand-free reference materials for nano-toxicological assays. Beilstein J. Nanotechnol. 2014, 5, 1523–1541. [Google Scholar] [CrossRef] [PubMed]
- Nichols, W.T.; Sasaki, T.; Koshizaki, N. Laser ablation of a platinum target in water. I. Ablation mechanisms. J. Appl. Phys. 2006, 100, 114911. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 3805–3821. [Google Scholar] [CrossRef] [PubMed]
- Farkas, B.; Rodio, M.; Romano, I.; Diaspro, A.; Intartaglia, R. Fabrication of hybrid nanocomposite scaffolds by incorporating ligand-free hydroxyapatite nanoparticles into biodegradable polymer scaffolds and release studies. Beilstein J. Nanotechnol. 2015, 6, 2217–2223. [Google Scholar] [CrossRef] [PubMed]
- Kőrösi, L.; Rodio, M.; Dömötör, D.; Kovács, T.; Papp, S.; Diaspro, A.; Intartaglia, R.; Beke, S. Ultrasmall, Ligand-Free Ag Nanoparticles with High Antibacterial Activity Prepared by Pulsed Laser Ablation in Liquid. J. Chem. 2016, 2016, 4143560. [Google Scholar] [CrossRef]
- Rodio, M.; Brescia, R.; Diaspro, A.; Intartaglia, R. Direct surface modification of ligand-free silicon quantum dots prepared by femtosecond laser ablation in deionized water. J. Colloid Interface Sci. 2016, 465, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Calzada, R.; Rodio, M.; Bagga, K.; Intartaglia, R.; Bianchini, P.; Chirvony, V.S.; Martinez-Pastor, J.P. Facile laser-assisted synthesis of inorganic nanoparticles covered by a carbon shell with tunable luminescence. RSC Adv. 2015, 5, 50604–50610. [Google Scholar] [CrossRef]
- Zhou, J.; Ralston, J.; Sedev, R.; Beattie, D.A. Functionalized gold nanoparticles: Synthesis, structure and colloid stability. J. Colloid Interface Sci. 2009, 331, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Ray, T.R.; Lettiere, B.; de Rutte, J.; Pennathur, S. Quantitative Characterization of the Colloidal Stability of Metallic Nanoparticles Using UV-vis Absorbance Spectroscopy. Langmuir 2015, 31, 3577–3586. [Google Scholar] [CrossRef] [PubMed]
- Gates, B. Determining the Stability of Nanoparticles in Solution and Implications for Using these Materials. Available online: http://www.workplace-safety-toolkit.ca/Portals/wcb/Gates_StabilityNanoparticles.pdf (accessed on 31 April 2012).
- Abdellatif, M.H.; Ghosh, S.; Liakos, I.; Scarpellini, A.; Marras, S.; Diaspro, A. Effect of nanoscale size and medium on metal work function in oleylamine-capped gold nanocrystals. J. Phys. Chem. Solids 2016, 89, 7–14. [Google Scholar] [CrossRef]
- Pignatelli, F.; Carzino, R.; Salerno, M.; Scotto, M.; Canale, C.; Distaso, M.; Rizzi, F.; Caputo, G.; Cozzoli, P.D.; Cingolani, R.; et al. Directional enhancement of refractive index and tunable wettability of polymeric coatings due to preferential dispersion of colloidal TiO2 nanorods towards their surface. Thin Solid Films 2010, 518, 4425–4431. [Google Scholar] [CrossRef]
- Patra, N.; Salerno, M.; Cozzoli, P.D.; Barone, A.C.; Ceseracciu, L.; Pignatelli, F.; Carzino, R.; Marini, L.; Athanassiou, A. Thermal and mechanical characterization of poly(methyl methacrylate) nanocomposites filled with TiO2 nanorods. Compos. Part B Eng. 2012, 43, 3114–3119. [Google Scholar] [CrossRef]
- Ţălu, Ş.; Patra, N.; Salerno, M. Micromorphological characterization of polymer-oxide nanocomposite thin films by atomic force microscopy and fractal geometry analysis. Prog. Org. Coat. 2015, 89, 50–56. [Google Scholar] [CrossRef]
- Sylvestre, J.; Poulin, S.; Kabashin, A.V.; Sacher, E.; Meunier, M.; Luong, J.H.T.; Montre, Ä.P.; De Postale, C. Surface Chemistry of Gold Nanoparticles Produced by Laser Ablation in Aqueous Media. J. Phys. Chem. B 2004, 108, 16864–16869. [Google Scholar] [CrossRef]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer Series in Materials Science; Springer-Verlag: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Krishnaraj, C.; Muthukumaran, P.; Ramachandran, R.; Balakumaran, M.D.; Kalaichelvan, P.T. Acalypha indica Linn: Biogenic synthesis of silver and gold nanoparticles and their cytotoxic effects against MDA-MB-231, human breast cancer cells. Biotechnol. Rep. 2014, 4, 42–49. [Google Scholar] [CrossRef]
- Baigent, C.L.; Müller, G. A colloidal gold prepared with ultrasonics. Experientia 1980, 36, 472–473. [Google Scholar] [CrossRef]
- Eliasson, C.; Lorén, A.; Engelbrektsson, J.; Josefson, M.; Abrahamsson, J.; Abrahamsson, K. Surface-enhanced Raman scattering imaging of single living lymphocytes with multivariate evaluation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.-M.; Thaxton, C.S.; Mirkin, C.A. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science 2003, 301, 1884–1886. [Google Scholar] [CrossRef] [PubMed]
- Intartaglia, R.; Das, G.; Bagga, K.; Gopalakrishnan, A.; Genovese, A.; Povia, M.; Di Fabrizio, E.; Cingolani, R.; Diaspro, A.; Brandi, F. Laser synthesis of ligand-free bimetallic nanoparticles for plasmonic applications. Phys. Chem. Chem. Phys. 2013, 15, 3075–3082. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Intartaglia, R.; Rodio, M.; Abdellatif, M.; Prato, M.; Salerno, M. Extensive Characterization of Oxide-Coated Colloidal Gold Nanoparticles Synthesized by Laser Ablation in Liquid. Materials 2016, 9, 775. https://doi.org/10.3390/ma9090775
Intartaglia R, Rodio M, Abdellatif M, Prato M, Salerno M. Extensive Characterization of Oxide-Coated Colloidal Gold Nanoparticles Synthesized by Laser Ablation in Liquid. Materials. 2016; 9(9):775. https://doi.org/10.3390/ma9090775
Chicago/Turabian StyleIntartaglia, Romuald, Marina Rodio, Mohamed Abdellatif, Mirko Prato, and Marco Salerno. 2016. "Extensive Characterization of Oxide-Coated Colloidal Gold Nanoparticles Synthesized by Laser Ablation in Liquid" Materials 9, no. 9: 775. https://doi.org/10.3390/ma9090775





