Electrochemical Hydrogel Lithography of Calcium-Alginate Hydrogels for Cell Culture
Abstract
:1. Introduction
2. Experimental Section
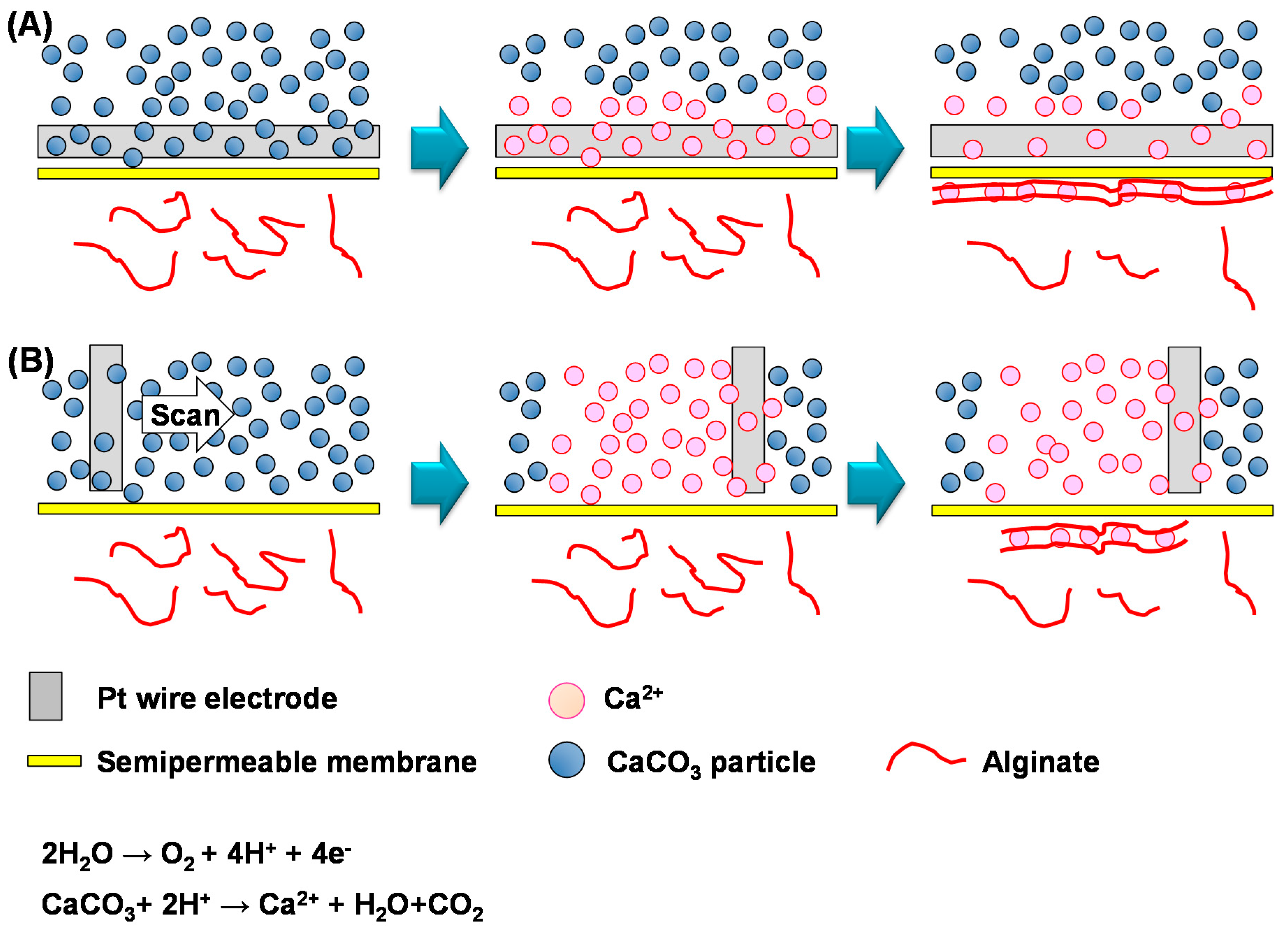
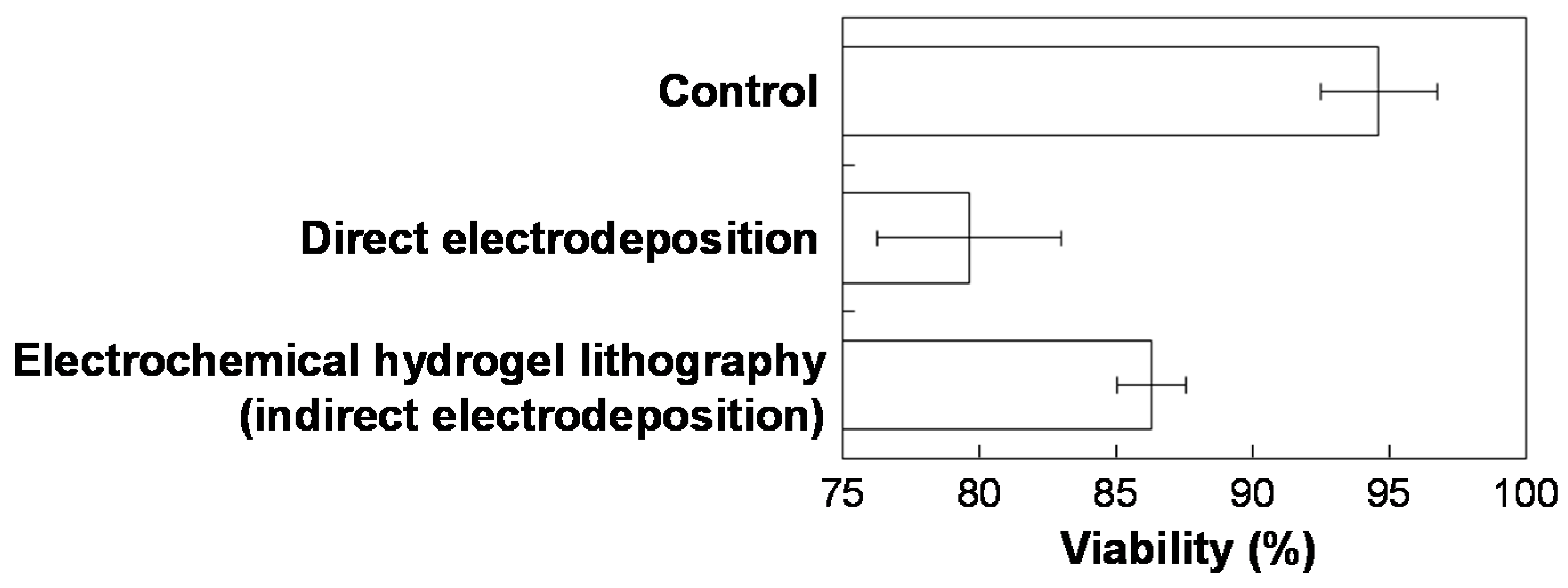
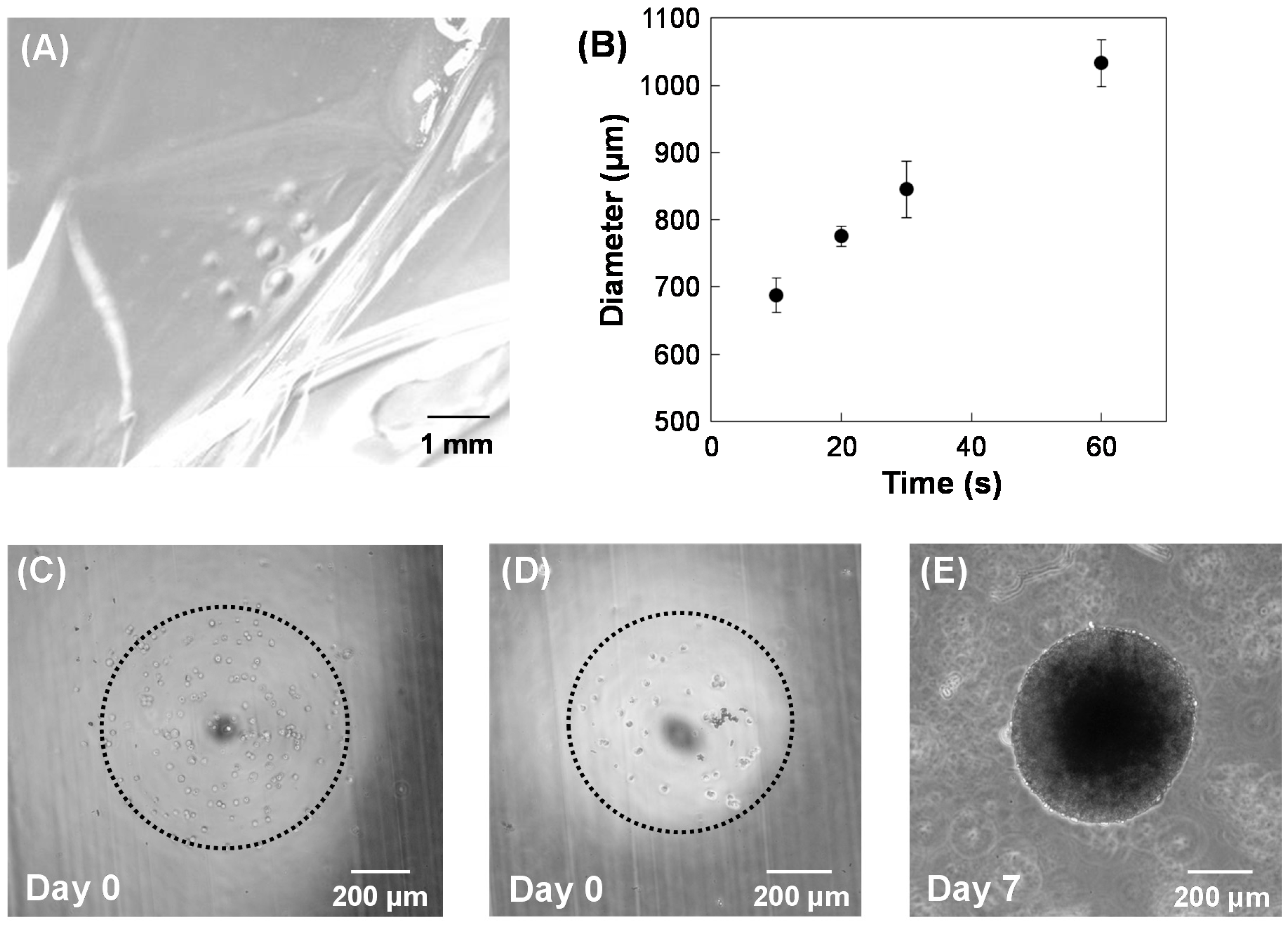
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shimizu, T.; Yamato, M.; Kikuchi, A.; Okano, T. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials 2003, 24, 2309–2316. [Google Scholar] [CrossRef]
- Ito, A.; Hayashida, M.; Honda, H.; Hata, K.; Kagami, H.; Ueda, M.; Kobayashi, T. Construction and harvest of multilayered keratinocyte sheets using magnetite nanoparticles and magnetic force. Tissue Eng. 2004, 10, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Ino, K.; Ito, A.; Honda, H. Cell patterning using magnetite nanoparticles and magnetic force. Biotechnol. Bioeng. 2007, 97, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, J.; Kameoka, Y.; Suzuki, H. Spatio-temporal detachment of single cells using microarrayed transparent electrodes. Biomaterials 2011, 32, 6663–6669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakegawa, T.; Mochizuki, N.; Sadr, N.; Suzuki, H.; Fukuda, J. Cell-adhesive and cell-repulsive zwitterionic oligopeptides for micropatterning and rapid electrochemical detachment of cells. Tissue Eng. Part A 2013, 19, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Utoh, R.; Ohashi, K.; Tatsumi, K.; Yamato, M.; Okano, T.; Seki, M. Controlled formation of heterotypic hepatic micro-organoids in anisotropic hydrogel microfibers for long-term preservation of liver-specific functions. Biomaterials 2012, 33, 8304–8315. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sakai, S.; Taya, M. Impact of the composition of alginate and gelatin derivatives in bioconjugated hydrogels on the fabrication of cell sheets and spherical tissues with living cell sheaths. Acta Biomater. 2013, 9, 6616–6623. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Cheng, Y.; Kim, E.; Rubloff, G.W.; Bentley, W.E.; Payne, G.F. Coupling electrodeposition with layer-by-layer assembly to address proteins within microfluidic channels. Adv. Mater. 2011, 23, 5817–5821. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Luo, X.; Tsao, C.Y.; Wu, H.C.; Betz, J.; Payne, G.F.; Bentley, W.E.; Rubloff, G.W. Biocompatible multi-address 3D cell assembly in microfluidic devices using spatially programmable gel formation. Lab Chip 2011, 11, 2316–2318. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.W.; Tsao, C.Y.; Yang, X.H.; Liu, Y.; Dykstra, P.; Rubloff, G.W.; Ghodssi, R.; Bentley, W.E.; Payne, G.F. Electroaddressing of cell populations by co-deposition with calcium alginate hydrogels. Adv. Funct. Mater. 2009, 19, 2074–2080. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, X.L.; Betz, J.; Payne, G.F.; Bentley, W.E.; Rubloff, G.W. Mechanism of anodic electrodeposition of calcium alginate. Soft Matter 2011, 7, 5677–5684. [Google Scholar] [CrossRef]
- Ozawa, F.; Ino, K.; Takahashi, Y.; Shiku, H.; Matsue, T. Electrodeposition of alginate gels for construction of vascular-like structures. J. Biosci. Bioeng. 2013, 115, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, F.; Ino, K.; Arai, T.; Ramón-Azcón, J.; Takahashi, Y.; Shiku, H.; Matsue, T. Alginate gel microwell arrays using electrodeposition for three-dimensional cell culture. Lab Chip 2013, 13, 3128–3135. [Google Scholar] [CrossRef] [PubMed]
- Kaji, H.; Tsukidate, K.; Matsue, T.; Nishizawa, M. In situ control of cellular growth and migration on substrates using microelectrodes. J. Am. Chem. Soc. 2004, 126, 15026–15027. [Google Scholar] [CrossRef] [PubMed]
- Shiku, H.; Uchida, I.; Matsue, T. Microfabrication of alkylsilanized glass substrate by electrogenerated hydroxyl radical using scanning electrochemical microscopy. Langmuir 1997, 13, 7239–7244. [Google Scholar] [CrossRef]
- Shiku, H.; Takeda, T.; Yamada, H.; Matsue, T.; Uchida, I. Microfabrication and characterization of diaphorase-patterned surfaces by scanning electrochemical microscopy. Anal. Chem. 1995, 67, 312–317. [Google Scholar] [CrossRef]
- Ramón-Azcón, J.; Ahadian, S.; Obregón, R.; Camci-Unal, G.; Ostrovidov, S.; Hosseini, V.; Kaji, H.; Ino, K.; Shiku, H.; Khademhosseini, A.; et al. Gelatin methacrylate as a promising hydrogel for 3D microscale organization and proliferation of dielectrophoretically patterned cells. Lab Chip 2012, 12, 2959–2969. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.Y.; Camci-Unal, G.; Huang, T.W.; Liao, R.; Chen, T.J.; Paul, A.; Tseng, F.G.; Khademhosseini, A. Gradient static-strain stimulation in a microfluidic chip for 3D cellular alignment. Lab Chip 2014, 14, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.; Ino, K.; Inoue, K.Y.; Arai, T.; Nishijo, T.; Suda, A.; Kunikata, R.; Shiku, H.; Matsue, T. LSI-based amperometric sensor for real-time monitoring of embryoid bodies. Biosens. Bioelectron. 2013, 48, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.Y.; Matsudaira, M.; Kubo, R.; Nakano, M.; Yoshida, S.; Matsuzaki, S.; Suda, A.; Kunikata, R.; Kimura, T.; Tsurumi, R.; et al. LSI-based amperometric sensor for bio-imaging and multi-point biosensing. Lab Chip 2012, 12, 3481–3490. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Hwang, S.; Kwak, J. A hydrogel pen for electrochemical reaction and its applications for 3D printing. Nanoscale 2015, 7, 994–1001. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozawa, F.; Ino, K.; Shiku, H.; Matsue, T. Electrochemical Hydrogel Lithography of Calcium-Alginate Hydrogels for Cell Culture. Materials 2016, 9, 744. https://doi.org/10.3390/ma9090744
Ozawa F, Ino K, Shiku H, Matsue T. Electrochemical Hydrogel Lithography of Calcium-Alginate Hydrogels for Cell Culture. Materials. 2016; 9(9):744. https://doi.org/10.3390/ma9090744
Chicago/Turabian StyleOzawa, Fumisato, Kosuke Ino, Hitoshi Shiku, and Tomokazu Matsue. 2016. "Electrochemical Hydrogel Lithography of Calcium-Alginate Hydrogels for Cell Culture" Materials 9, no. 9: 744. https://doi.org/10.3390/ma9090744





