Membrane Functionalization with Hyperbranched Polymers
Abstract
:1. Introduction
2. Results and Discussion
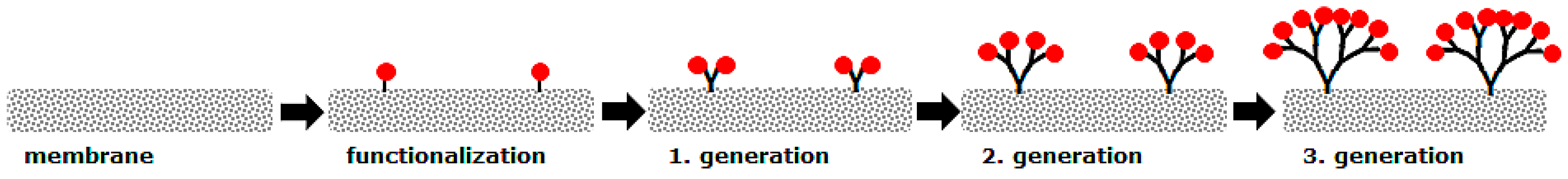
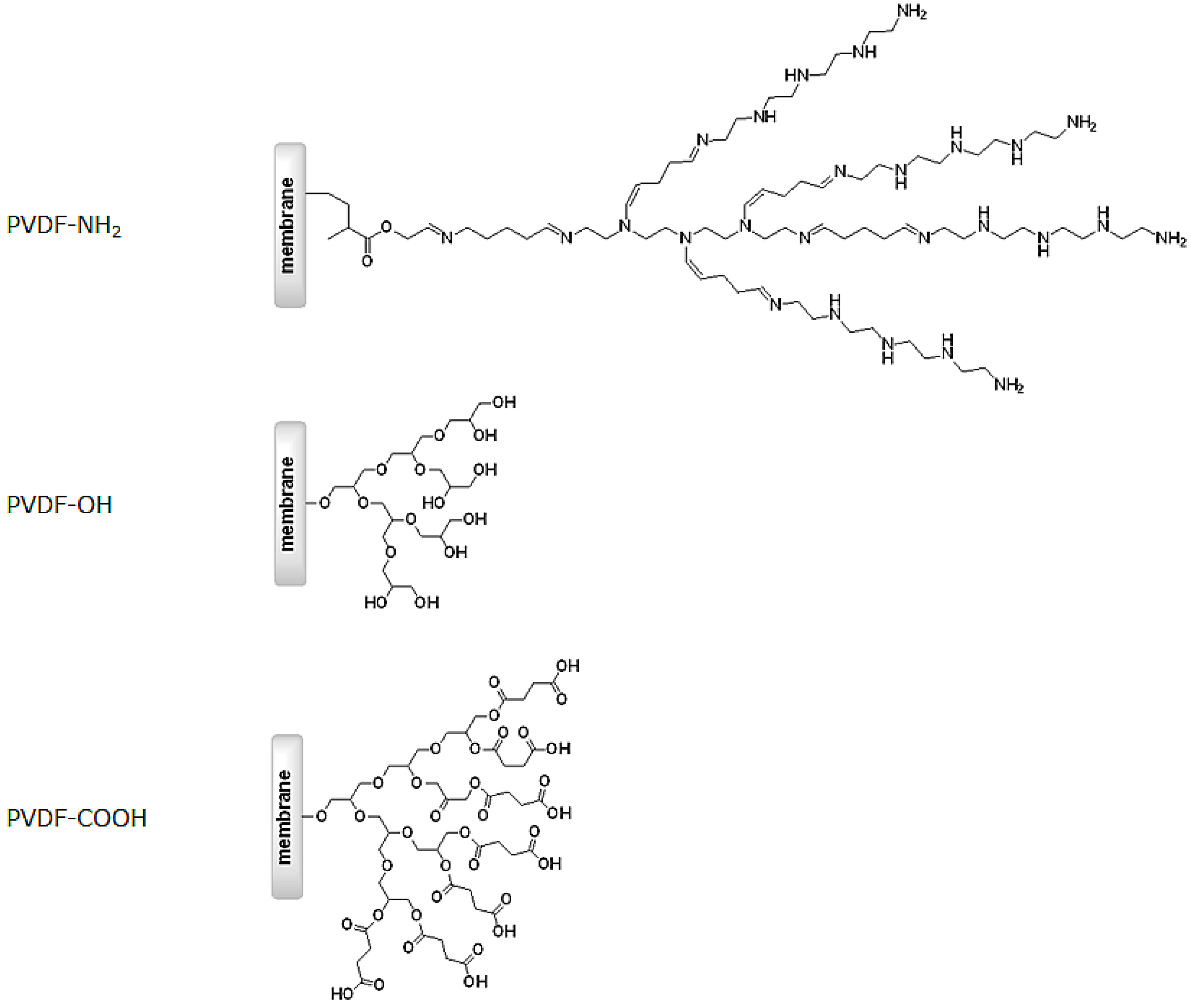
2.1. Membrane Surface Functionalization
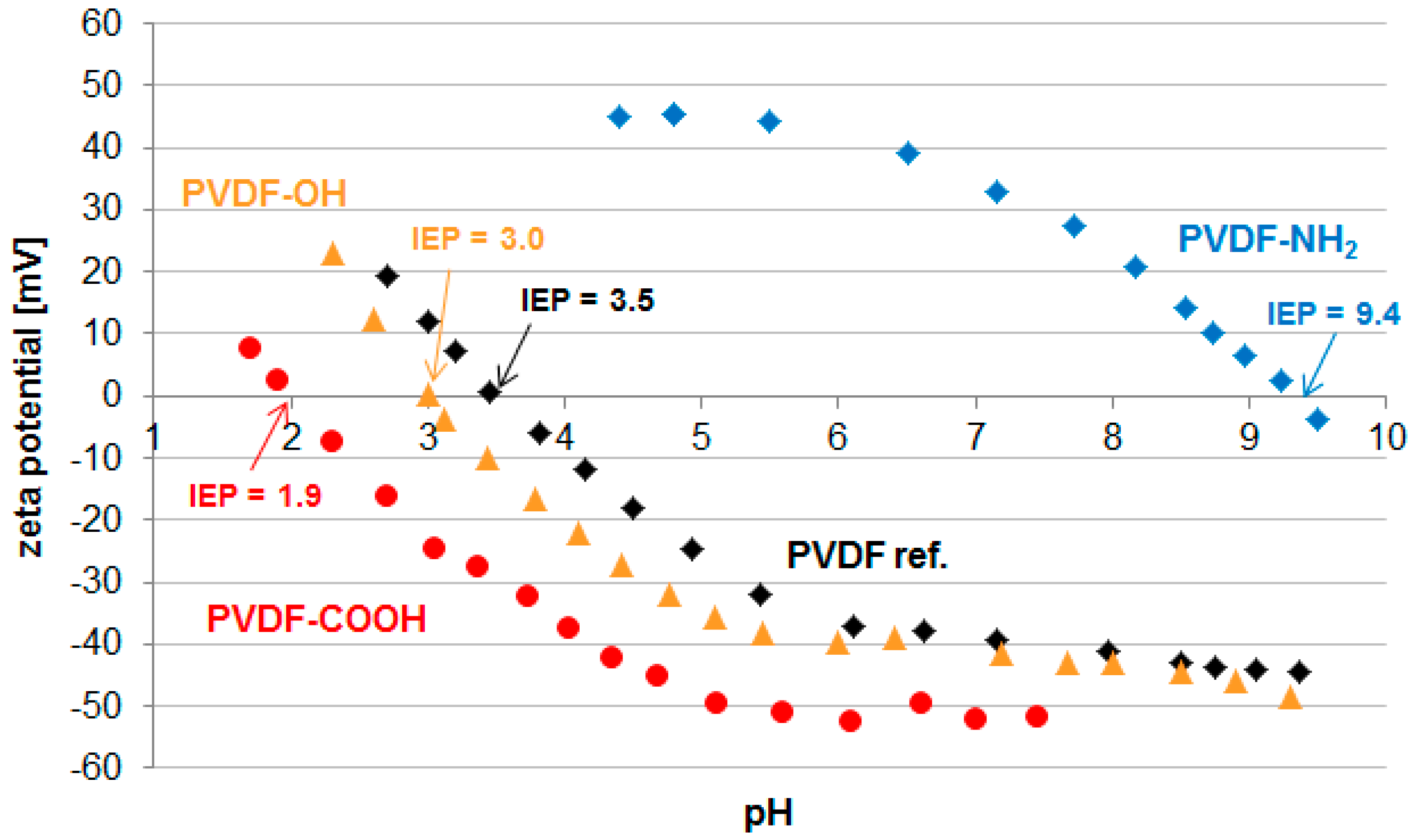
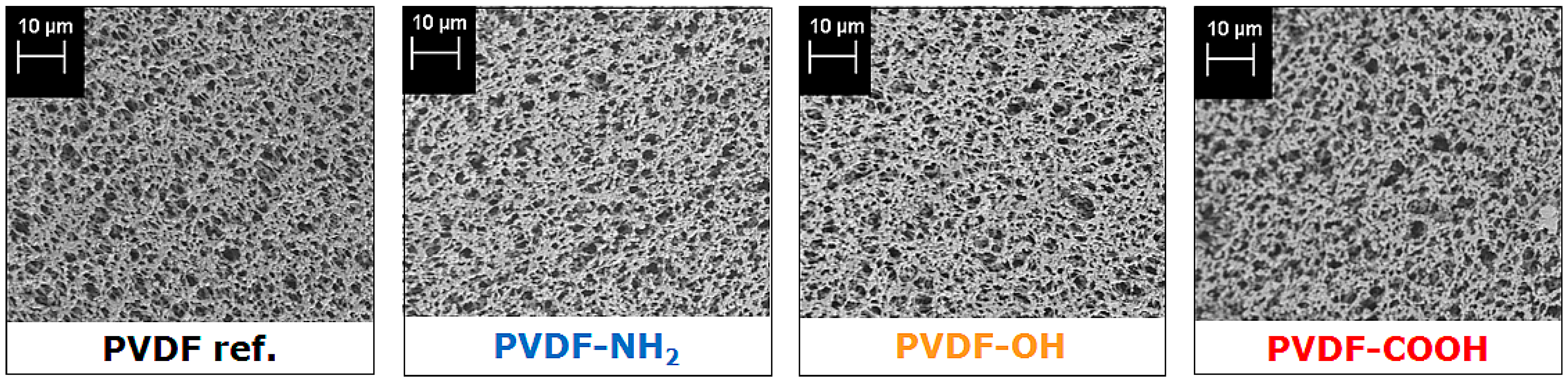
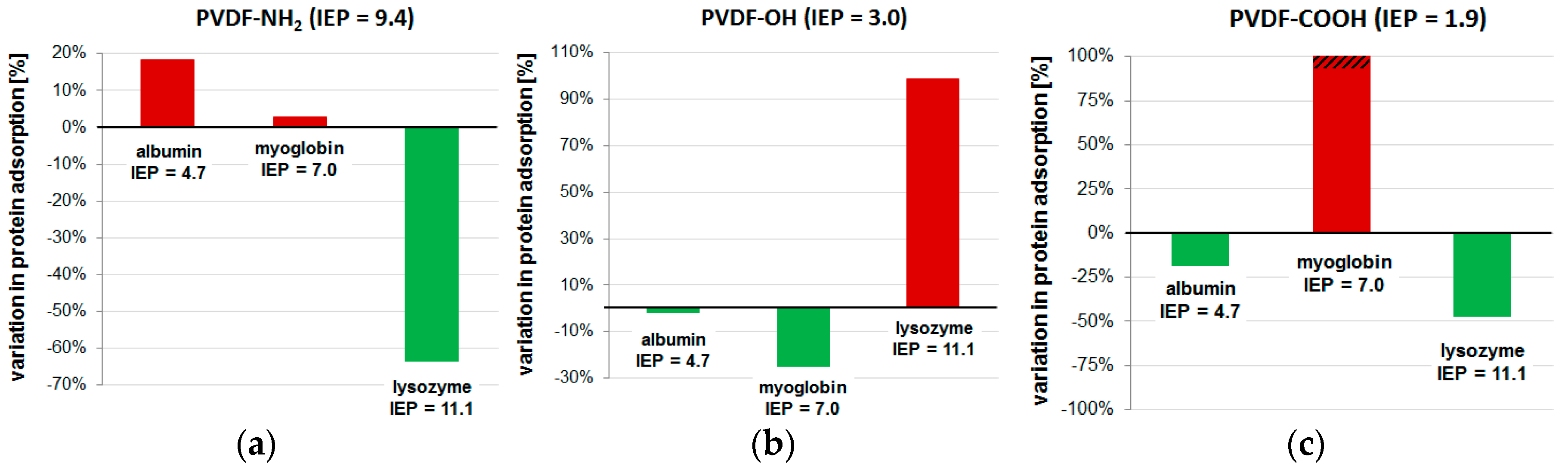
2.2. Membrane Properties
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Membrane Surface Functionalization with Hyperbranched Polymers
3.3. Membrane Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marchand-Brynaert, J. Polymer Membranes; CRC Press: Boca Raton, FL, USA, 2012; pp. 4854–4873. [Google Scholar]
- Nunes, S.P.; Peinemann, K.-V. Membrane Technology in the Chemical Industry, 2nd ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2006. [Google Scholar]
- Hilal, N.; Ogunbiyi, O.O.; Miles, N.J.; Nigmatullin, R. Methods employed for control of fouling in mf and uf membranes: A comprehensive review. Sep. Sci. Technol. 2005, 40, 1957–2005. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef]
- Fang, Y.; Xu, Z.-K.; Wu, J.; Hoek, E.M.V.; Tarabara, V.V. Surface modification of membranes. In Encyclopedia of Membrane Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Kochkodan, V.; Johnson, D.J.; Hilal, N. Polymeric membranes: Surface modification for minimizing (bio)colloidal fouling. Adv. Colloid Interface Sci. 2014, 206, 116–140. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Zhang, X.; Wu, G.; Ren, L.; Wang, Y. Comparative degradation study of surface-modified polyacrylamide/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) membranes. Polym. Sci. Ser. B 2015, 57, 538–546. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Wang, J.; Wang, S. Improving the water flux and bio-fouling resistance of reverse osmosis (ro) membrane through surface modification by zwitterionic polymer. J. Membr. Sci. 2015, 493, 188–199. [Google Scholar] [CrossRef]
- Nazri, N.; Lau, W.; Ismail, A. Improving water permeability and anti-fouling property of polyacrylonitrile-based hollow fiber ultrafiltration membranes by surface modification with polyacrylonitrile-g-poly(vinyl alcohol) graft copolymer. Korean J. Chem. Eng. 2015, 25, 1853–1863. [Google Scholar] [CrossRef]
- Ren, P.-F.; Fang, Y.; Wan, L.-S.; Ye, X.-Y.; Xu, Z.-K. Surface modification of polypropylene microfiltration membrane by grafting poly(sulfobetaine methacrylate) and poly(ethylene glycol): Oxidative stability and antifouling capability. J. Membr. Sci. 2015, 492, 249–256. [Google Scholar] [CrossRef]
- Li, F.; Ye, J.; Yang, L.; Deng, C.; Tian, Q.; Yang, B. Surface modification of ultrafiltration membranes by grafting glycine-functionalized pva based on polydopamine coatings. Appl.Surf. Sci. 2015, 345, 301–309. [Google Scholar] [CrossRef]
- Cheng, Q.; Zheng, Y.; Yu, S.; Zhu, H.; Peng, X.; Liu, J.; Liu, J.; Liu, M.; Gao, C. Surface modification of a commercial thin-film composite polyamide reverse osmosis membrane through graft polymerization of n-isopropylacrylamide followed by acrylic acid. J. Membr. Sci. 2013, 447, 236–245. [Google Scholar] [CrossRef]
- Chung, Y.T.; Ng, L.Y.; Mohammad, A.W. Sulfonated-polysulfone membrane surface modification by employing methacrylic acid through uv-grafting: Optimization through response surface methodology approach. J. Ind. Eng. Chem. 2014, 20, 1549–1557. [Google Scholar] [CrossRef]
- Schulze, A.; Marquardt, B.; Kaczmarek, S.; Schubert, R.; Prager, A.; Buchmeiser, M.R. Electron beam-based functionalization of poly(ethersulfone) membranes. Macromol. Rapid Commun. 2010, 31, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Marquardt, B.; Went, M.; Prager, A.; Buchmeiser, M.R. Electron beam-based functionalization of polymer membranes. Water Sci. Technol. 2012, 65, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Maitz, M.F.; Zimmermann, R.; Marquardt, B.; Fischer, M.; Werner, C.; Went, M.; Thomas, I. Permanent surface modification by electron-beam-induced grafting of hydrophilic polymers to pvdf membranes. RSC Adv. 2013, 3, 22518–22526. [Google Scholar] [CrossRef]
- Starke, S.; Went, M.; Prager, A.; Schulze, A. A novel electron beam-based method for the immobilization of trypsin on poly(ethersulfone) and poly(vinylidene fluoride) membranes. React. Funct. Polym. 2013, 73, 698–702. [Google Scholar] [CrossRef]
- Jahangiri, E.; Reichelt, S.; Thomas, I.; Hausmann, K.; Schlosser, D.; Schulze, A. Electron beam-induced immobilization of laccase on porous supports for waste water treatment applications. Molecules 2014, 19, 11860–11882. [Google Scholar] [CrossRef] [PubMed]
- Muthumeenal, A.; Neelakandan, S.; Rana, D.; Matsuura, T.; Kanagaraj, P.; Nagendran, A. Sulfonated polyethersulfone (spes)–charged surface modifying macromolecules (csmms) blends as a cation selective membrane for fuel cells. Fuel Cells 2014, 14, 853–861. [Google Scholar] [CrossRef]
- Roy, A.; Dadhich, P.; Dhara, S.; De, S. In vitro cytocompatibility and blood compatibility of polysulfone blend, surface-modified polysulfone and polyacrylonitrile membranes for hemodialysis. RSC Adv. 2015, 5, 7023–7034. [Google Scholar] [CrossRef]
- Rana, D.; Narbaitz, R.M.; Garand-Sheridan, A.-M.; Westgate, A.; Matsuura, T.; Tabe, S.; Jasim, S.Y. Development of novel charged surface modifying macromolecule blended pes membranes to remove edcs and ppcps from drinking water sources. J. Mater. Chem. A 2014, 2, 10059–10072. [Google Scholar]
- Ouradi, A.; Nguyen, Q.T.; Benaboura, A. Polysulfone–an69 blend membranes and its surface modification by polyelectrolyte-layer deposit—preparation and characterization. J. Membr. Sci. 2014, 454, 20–35. [Google Scholar] [CrossRef]
- Mehrparvar, A.; Rahimpour, A.; Jahanshahi, M. Modified ultrafiltration membranes for humic acid removal. J. Taiwan Inst. Chem. Eng. 2014, 45, 275–282. [Google Scholar] [CrossRef]
- Mahlicli, F.; Altinkaya, S. Surface modification of polysulfone based hemodialysis membranes with layer by layer self assembly of polyethyleneimine/alginate-heparin: A simple polyelectrolyte blend approach for heparin immobilization. J. Mater. Sci Mater. Med. 2013, 24, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Kang, Y.; Tiraferri, A.; Giannelis, E.P.; Huang, X.; Elimelech, M. Highly hydrophilic polyvinylidene fluoride (pvdf) ultrafiltration membranes via postfabrication grafting of surface-tailored silica nanoparticles. ACS Appl. Mater. Interface 2013, 5, 6694–6703. [Google Scholar] [CrossRef] [PubMed]
- Tiraferri, A.; Kang, Y.; Giannelis, E.P.; Elimelech, M. Superhydrophilic thin-film composite forward osmosis membranes for organic fouling control: Fouling behavior and antifouling mechanisms. Environ. Sci. Tech. 2012, 46, 11135–11144. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-M.; Wang, L.-L.; Wang, Y.; Gu, J.-S.; Yu, H.-Y. Surface modification of polypropylene macroporous membrane by marrying raft polymerization with click chemistry. J. Membr. Sci. 2012, 421–422, 60–68. [Google Scholar] [CrossRef]
- Wei, X.-Z.; Liu, X.-F.; Zhu, B.-K.; Xu, Y.-Y. Membranes of crosslinked hyperbranch polymers and their pervaporation properties. Desalination 2009, 247, 647–656. [Google Scholar] [CrossRef]
- Fang, J.; Kita, H.; Okamoto, K.-I. Gas permeation properties of hyperbranched polyimide membranes. J. Membr. Sci. 2001, 182, 245–256. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamada, Y.; Tsujita, Y. Gas transport properties of 6fda-tapob hyperbranched polyimide membrane. Polymer 2004, 45, 7167–7171. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamada, Y. Physical and gas transport properties of novel hyperbranched polyimide–silica hybrid membranes. Polym. Bull. 2005, 53, 139–146. [Google Scholar] [CrossRef]
- Shao, L.; Chung, T.-S.; Goh, S.H.; Pramoda, K.P. Transport properties of cross-linked polyimide membranes induced by different generations of diaminobutane (dab) dendrimers. J. Membr. Sci. 2004, 238, 153–163. [Google Scholar] [CrossRef]
- Fail, C.A.; Evenson, S.A.; Ward, L.J.; Schofield, W.C.E.; Badyal, J.P.S. Controlled attachment of pamam dendrimers to solid surfaces. Langmuir 2002, 18, 264–268. [Google Scholar] [CrossRef]
- Bergbreiter, D.E.; Kippenberger, A.M. Hyperbranched surface graft polymerizations. Adv. Polym. Sci. 2006, 198, 1–49. [Google Scholar]
- Kojima, C.; Yoshimura, K.; Harada, A.; Sakanishi, Y.; Kono, K. Synthesis and characterization of hyperbranched poly(glycidol) modified with ph- and temperature-sensitive groups. Bioconjugate Chem. 2009, 20, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- Dongen, S.F.M.V.; Hoog, H.-P.M.D.; Peters, R.J.R.W.; Nallani, M.; Nolte, R.J.M.; Hest, J.C.M.V. Biohybrid polymer capsules. Chem. Rev. 2009, 109, 6212–6274. [Google Scholar] [CrossRef] [PubMed]
- Boulares-Pender, A.; Prager-Duschke, A.; Elsner, C.; Buchmeiser, M.R. Surface-functionalization of plasma-treated polystyrene by hyperbranched polymers and use in biological applications. J. Appl. Polym. Sci. 2009, 112, 2701–2709. [Google Scholar] [CrossRef]
- Boulares-Pender, A.; Prager, A.; Reichelt, S.; Elsner, C.; Buchmeiser, M.R. Functionalization of plasma-treated polymer surfaces with glycidol. J. Appl. Polym. Sci. 2011, 121, 2543–2550. [Google Scholar] [CrossRef]
- Meyers, S.R.; Juhn, F.S.; Griset, A.P.; Luman, N.R.; Grinstaff, M.W. Anionic amphiphilic dendrimers as antibacterial agents. J. Am. Chem. Soc. 2008, 130, 14444–14445. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Huck, W.T.S. Hyperbranched polyglycidol on si/sio2 surfaces via surface-initiated polymerization. Macromolecules 2003, 36, 5088–5093. [Google Scholar] [CrossRef]
- Zimmermann, R.; Dukhin, S.; Werner, C. Electrokinetic measurements reveal interfacial charge at polymer films caused by simple electrolyte ions. J. Phys. Chem. B 2001, 105, 8544–8549. [Google Scholar] [CrossRef]
- Zimmermann, R.; Freudenberg, U.; Schweiß, R.; Küttner, D.; Werner, C. Hydroxide and hydronium ion adsorption—A survey. Curr. Opin. Colloid Interface Sci. 2010, 15, 196–202. [Google Scholar] [CrossRef]
- Maroni, P.; Montes Ruiz-Cabello, F.J.; Cardoso, C.; Tiraferri, A. Adsorbed mass of polymers on self-assembled monolayers: Effect of surface chemistry and polymer charge. Langmuir 2015, 31, 6045–6054. [Google Scholar] [CrossRef] [PubMed]
- Arkhangelsky, E.; Levitsky, I.; Gitis, V. Electrostatic repulsion as a mechanism in fouling of ultrafiltration membranes. Water Sci. Technol. 2008, 58, 1955–1961. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, R.; Klenert, P.; Prager, L. Low-energy electron accelerators for industrial radiation processing. Radiat. Phys. Chem. 1993, 42, 525–529. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fukimotot, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Zhao, W.; Su, Y.; Li, C.; Shi, Q.; Ning, X.; Jiang, Z. Fabrication of antifouling polyethersulfone ultrafiltration membranes using pluronic f127 as both surface modifier and pore-forming agent. J. Membr. Sci. 2008, 318, 405–412. [Google Scholar] [CrossRef]
- Ariza, M.J.; Benavente, J. Streaming potential along the surface of polysulfone membranes: A comparative study between two different experimental systems and determination of electrokinetic and adsorption parameters. J. Membr. Sci. 2001, 190, 119–132. [Google Scholar] [CrossRef]
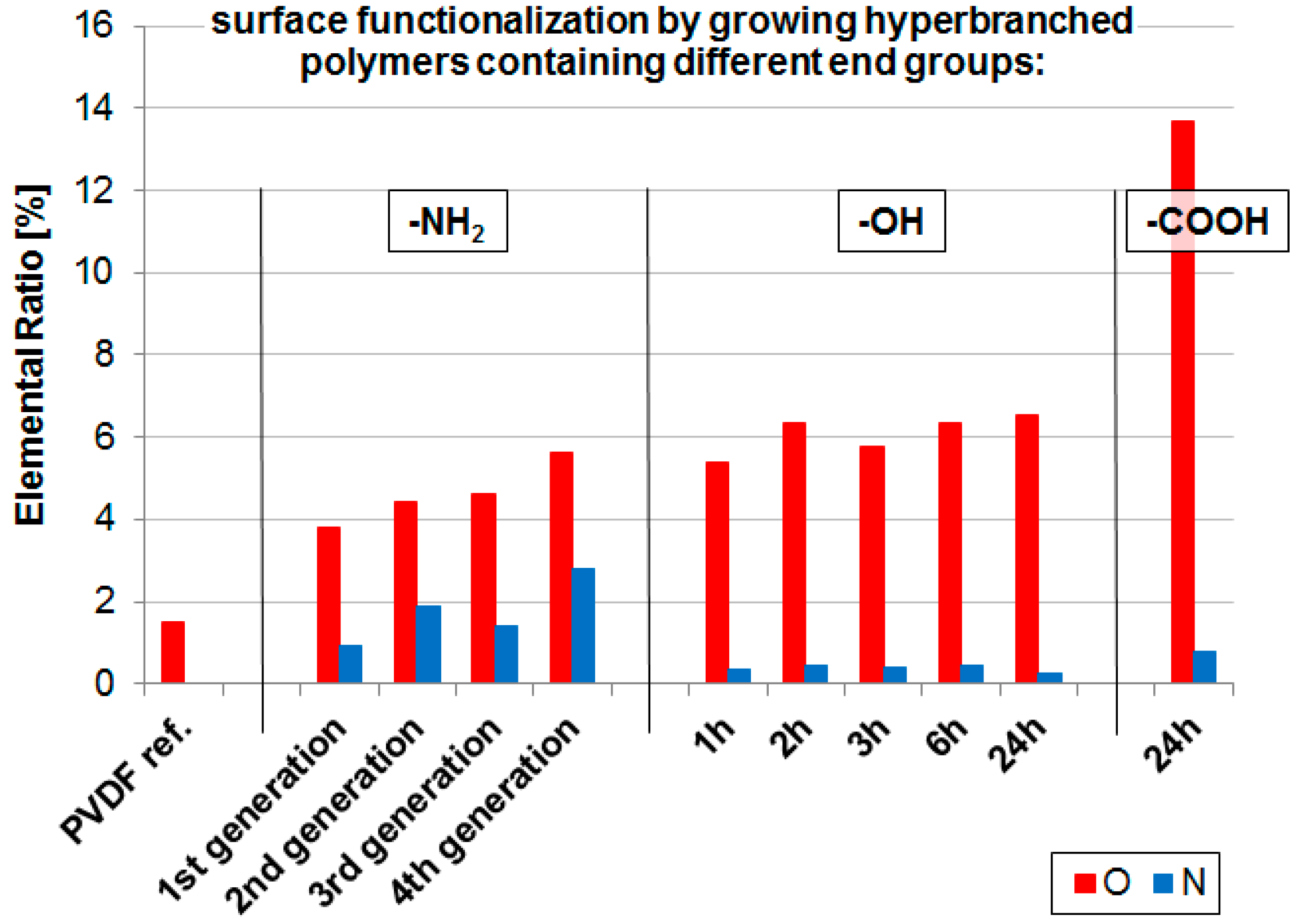
| Sample | Elemental Ratio [mol %] | C1s Deconvolution [eV; mol %] | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C 1s | F 1s | O 1s | N 1s | 284.7 C-C | 286.4 -CH2- | 287.2 C-O | 288.4 C-Ox | 290.9 -CF2- | |
| PVDF ref. | 51.5 | 47.0 | 1.5 | - | 5.7 | 23.6 | - | 1.0 | 21.2 |
| PVDF-NH2 1st | 52.5 | 42.8 | 3.8 | 0.9 | 6.1 | 24.3 | - | 3.7 | 19.3 |
| PVDF-NH2 2nd | 54.2 | 39.5 | 4.4 | 1.9 | 7.6 | 26.5 | - | 5.0 | 17.2 |
| PVDF-NH2 3rd | 53.4 | 40.6 | 4.6 | 1.4 | 4.1 | 25.4 | - | 3.3 | 19.8 |
| PVDF-NH2 4th | 56.3 | 35.3 | 5.6 | 2.8 | 4.9 | 26.6 | - | 4.1 | 18.6 |
| PVDF-OH 1 h | 49.4 | 44.9 | 5.4 | 0.4 | 0.6 | 23.5 | - | 4.5 | 21.0 |
| PVDF-OH 2 h | 49.7 | 43.6 | 6.4 | 0.5 | 1.6 | 24.1 | - | 5.0 | 19.1 |
| PVDF-OH 3 h | 50.5 | 43.4 | 5.8 | 0.4 | 1.6 | 23.8 | - | 5.7 | 19.5 |
| PVDF-OH 6 h | 50.7 | 42.6 | 6.4 | 0.5 | 2.0 | 24.8 | - | 4.7 | 19.3 |
| PVDF-OH 24 h | 51.7 | 41.6 | 6.6 | 0.3 | 2.9 | 25.7 | - | 4.6 | 18.6 |
| PVDF-COOH | 55.0 | 30.5 | 13.7 | 0.8 | 7.9 | 19.5 | 9.1 | 5.1 | 13.4 |
| Sample | Water Permeation Flux | Water Contact Angle | Protein Adsorption [µg/cm²] | ||
|---|---|---|---|---|---|
| [L/(h·m²·bar)] | [°] | Albumin IEP = 4.7 | Myoglobin IEP = 7.0 | Lysozyme IEP = 11.1 | |
| PVDF ref. | 27,605 | 119.8 ± 3.6 | 20.8 ± 1.0 | 21.4 ± 1.0 | 25.0 ± 2.1 |
| PVDF-NH2 | 24,431 | 82.8 ± 3.6 | 24.6 ± 6.7 | 22.0 ± 2.3 | 9.1 ± 2.5 |
| PVDF-OH | 22,814 | 116.4 ± 0.0 | 20.5 ± 0.8 | 16.1 ± 1.2 | 49.7 ± 1.9 |
| PVDF-COOH | 20,539 | 109.9 ± 0.0 | 16.9 ± 1.5 | 159.3 ± 16.2 | 13.2 ± 2.9 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulze, A.; Went, M.; Prager, A. Membrane Functionalization with Hyperbranched Polymers. Materials 2016, 9, 706. https://doi.org/10.3390/ma9080706
Schulze A, Went M, Prager A. Membrane Functionalization with Hyperbranched Polymers. Materials. 2016; 9(8):706. https://doi.org/10.3390/ma9080706
Chicago/Turabian StyleSchulze, Agnes, Marco Went, and Andrea Prager. 2016. "Membrane Functionalization with Hyperbranched Polymers" Materials 9, no. 8: 706. https://doi.org/10.3390/ma9080706






