Nature of the Electrochemical Properties of Sulphur Substituted LiMn2O4 Spinel Cathode Material Studied by Electrochemical Impedance Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goodenough, J.B.; Park, K.S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Braun, P.V.; Cho, J.; Pikul, J.H.; King, W.P.; Zhang, H. High power rechargeable batteries. Curr. Opin. Solid State Mater. Sci. 2012, 16, 186–198. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable batteries. J. Power Sources 2011, 196, 6688–6694. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, M.S. Lithium Batteries and Cathode Materials. Chem. Rev. 2004, 104, 4271–4301. [Google Scholar] [CrossRef] [PubMed]
- Croguennec, L.; Rosa Palacin, M. Recent Achievements on Inorganic Electrode Materials for Lithium Ion Batteries. J. Am. Chem. Soc. 2015, 137, 3140–3156. [Google Scholar] [CrossRef] [PubMed]
- Myung, S.T.; Amine, K.; Sun, Y.K. Nanostructured cathode materials for rechargeable lithium batteries. J. Power Sources 2015, 283, 219–236. [Google Scholar] [CrossRef]
- Fergus, J.W. Recent developments in cathode materials for lithium ion batteries. J. Power Sources 2010, 195, 939–954. [Google Scholar] [CrossRef]
- Scrosati, B. Recent advances in lithium ion battery materials. Electrochim. Acta 2000, 45, 2461–2466. [Google Scholar] [CrossRef]
- Yang, L.; Takahashi, M.; Wang, B. A study on capacity fading of lithium-ion battery with manganese spinel positive electrode during cycling. Electrochim. Acta 2006, 51, 3228–3234. [Google Scholar] [CrossRef]
- Shin, Y.; Manthiram, A. Factors Influencing the Capacity Fade of Spinel Lithium Manganese Oxides. J. Electrochem. Soc. 2004, 151, A204–A208. [Google Scholar] [CrossRef]
- Raveendranath, K.; Ravi, J.; Tomy, R.M.; Jayalekshmi, S.; Mangalaraja, R.V.; Lee, S.T. Evidence of Jahn–Teller distortion in LixMn2O4 by thermal diffusivity measurements. Appl. Phys. A 2008, 90, 437–440. [Google Scholar] [CrossRef]
- Li, G.; Iijima, Y.; Kudo, Y.; Azuma, H. Structural changes of manganese spinel at elevated temperatures. Solid State Ion. 2002, 146, 55–63. [Google Scholar] [CrossRef]
- Goodenough, J.B. Jahn-Teller phenomena in solids. Annu. Rev. Mater. Sci. 1998, 28, 1–27. [Google Scholar] [CrossRef]
- Zhan, C.; Lu, J.; Kropf, A.J.; Wu, T.; Jansen, A.N.; Sun, Y.K.; Qiu, X.; Amine, K. Mn(II) deposition on anodes and its effects on capacity fade in spinel lithium manganate–carbon systems. Nat. Commun. 2013, 4, 2437–2354. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Wang, L.F.; Fang, B.J.; Lee, S.Y.; Guo, R.Z. Rotating ring–disk electrode measurements on Mn dissolution and capacity losses of spinel electrodes in various organic electrolytes. J. Power Sources 2006, 157, 515–521. [Google Scholar] [CrossRef]
- Aoshima, T.; Okahara, K.; Kiyohara, C.; Shizuka, K. Mechanisms of manganese spinels dissolution and capacity fade at high temperature. J. Power Sources 2001, 97–98, 377–380. [Google Scholar] [CrossRef]
- Wen, W.; Ju, B.; Wang, X.; Wu, C.; Shu, H.; Yang, X. Effects of magnesium and fluorine co-doping on the structural and electrochemical performance of the spinel LiMn2O4 cathode materials. Electrochim. Acta 2014, 147, 271–278. [Google Scholar] [CrossRef]
- Molenda, J.; Marzec, J.; Świerczek, K.; Ojczyk, W.; Ziemnicki, M.; Molenda, M.; Drozdek, M.; Dziembaj, R. The effect of 3d substitutions in the manganese sublattice on the charge transport mechanism and electrochemical properties of manganese spinel. Solid State Ion. 2004, 171, 215–227. [Google Scholar] [CrossRef]
- Vogler, C.; Butz, A.; Dittrich, H.; Arnold, G.; Wohlfahrt-Mehrens, M. Electrochemical and structural comparison of doped lithium manganese spinels. J. Power Sources 1999, 84, 243–247. [Google Scholar] [CrossRef]
- Shang, Y.; Lin, X.; Lu, X.; Huang, T.; Yu, A. Nano-TiO2(B) coated LiMn2O4 as cathode materials for lithium-ion batteries at elevated temperatures. Electrochim. Acta 2015, 156, 121–126. [Google Scholar] [CrossRef]
- Qing, C.; Bai, Y.; Yang, J.; Zhang, W. Enhanced cycling stability of LiMn2O4 cathode by amorphous FePO4 coating. Electrochim. Acta 2011, 56, 6612–6618. [Google Scholar] [CrossRef]
- Lee, C.W.; Kim, H.S.; Moon, S.I. Effects on surface modification of spinel LiMn2O4 material for lithium-ion batteries. Mater. Sci. Eng. B 2005, 123, 234–237. [Google Scholar] [CrossRef]
- Zhao, H.; Li, F.; Liu, X.; Xiong, W.; Chen, B.; Shao, H.; Que, D.; Zhang, Z.; Wu, Y. A simple, low-cost and eco-friendly approach to synthesize single-crystalline LiMn2O4 nanorods with high electrochemical performance for lithium-ion batteries. Electrochim. Acta 2015, 166, 124–133. [Google Scholar] [CrossRef]
- Xia, H.; Luo, Z.; Xie, J. Nanostructured LiMn2O4 and their composites as high-performance cathodes for lithium-ion batteries. Prog. Nat. Sci. Mater. Int. 2012, 22, 572–584. [Google Scholar] [CrossRef]
- Shaju, K.M.; Bruce, P.G. A Stoichiometric Nano-LiMn2O4 Spinel Electrode Exhibiting High Power and Stable Cycling. Chem. Mater. 2008, 20, 5557–5562. [Google Scholar] [CrossRef]
- Molenda, M.; Bakierska, M.; Majda, D.; Świętosławski, M.; Dziembaj, R. Structural and electrochemical characterization of sulphur-doped lithium manganese spinel cathode materials for lithium ion batteries. Solid State Ion. 2015, 272, 127–132. [Google Scholar] [CrossRef]
- Molenda, M.; Dziembaj, R.; Majda, D.; Dudek, M. Synthesis and characterisation of sulphided lithium manganese spinels LiMn2O4−ySy prepared by sol–gel method. Solid State Ion. 2005, 176, 1705–1709. [Google Scholar] [CrossRef]
- Dziembaj, R.; Molenda, M.; Majda, D.; Walas, S. Synthesis, thermal and electrical properties of Li1+δMn2−δO4 prepared by a sol–gel method. Solid State Ion. 2003, 157, 81–87. [Google Scholar] [CrossRef]
- NOVA Impedance Spectroscopy Tutorial. Available online: http://www.ecochemie.nl/download/NovaTutorials/Impedance_measurements_tutorial.pdf (accessed on 26 July 2016).
- Thackeray, M.M. Manganese oxides for lithium batteries. Prog. Solids. Chem. 1997, 25, 1–71. [Google Scholar] [CrossRef]
- Lin, H.B.; Hu, J.N.; Rong, H.B.; Zhang, Y.M.; Mai, S.W.; Xing, L.D.; Xu, M.Q.; Li, X.P.; Li, W.S. Porous LiMn2O4 cubes architectured with single-crystalline nanoparticles and exhibiting excellent cyclic stability and rate capability as the cathode of a lithium ion battery. J. Mater. Chem. A 2014, 2, 9272–9279. [Google Scholar] [CrossRef]
- Zhu, P.; Chu, X.; Zhou, F.; Sun, R.; Wong, C. Synergistic effect for the preparation of LiMn2O4 microspheres with high electrochemical performance. RSC Adv. 2014, 4, 3293–3298. [Google Scholar] [CrossRef]
- Lvovich, V.F. Impedance Spectroscopy: Applications to Electrochemical and Dielectric Phenomena, 1st ed.; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications, 2nd ed.; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Tang, S.B.; Lai, M.O.; Lu, L. Study on Li+-ion diffusion in nano-crystalline LiMn2O4 thin film cathode grown by pulsed laser deposition using CV, EIS and PITT techniques. Mater. Chem. Phys. 2008, 111, 149–153. [Google Scholar] [CrossRef]
- Ouyang, C.; Shi, S.; Wang, Z.; Huang, X.; Chen, L. Experimental and theoretical studies on dynamic properties of Li ions in LixMn2O4. Solid State Commun. 2004, 130, 501–506. [Google Scholar] [CrossRef]
- Saidi, M.Y.; Barker, J.; Koksbang, R. Thermodynamic and Kinetic Investigation of Lithium Insertion in the Li1−xMn2O4 Spinel Phase. J. Solid State Chem. 1996, 122, 195–199. [Google Scholar] [CrossRef]
- Zhuang, Q.C.; Wei, T.; Du, L.L.; Cui, Y.L.; Fang, L.; Sun, S.G. An Electrochemical Impedance Spectroscopic Study of the Electronic and Ionic Transport Properties of Spinel LiMn2O4. J. Phys. Chem. C 2010, 114, 8614–8621. [Google Scholar] [CrossRef]
- Zhuang, Q.C.; Qiu, X.Y.; Xu, S.D.; Qiang, Y.H.; Sun, S.G. Diagnosis of Electrochemical Impedance Spectroscopy in Lithium-Ion Batteries. In Lithium Ion Batteries—New Developments; Belharouak, I., Ed.; InTech: Rijeka, Croatia, 2012; Volume 8, pp. 189–227. [Google Scholar]
- Molenda, M.; Dziembaj, R.; Kotwica, A.; Łasocha, W. Structural, thermal and electrical properties with a sulphur-substituted oxygen sublattice of lithium-manganese spinel. Mater. Sci. Pol. 2006, 24, 85–93. [Google Scholar]
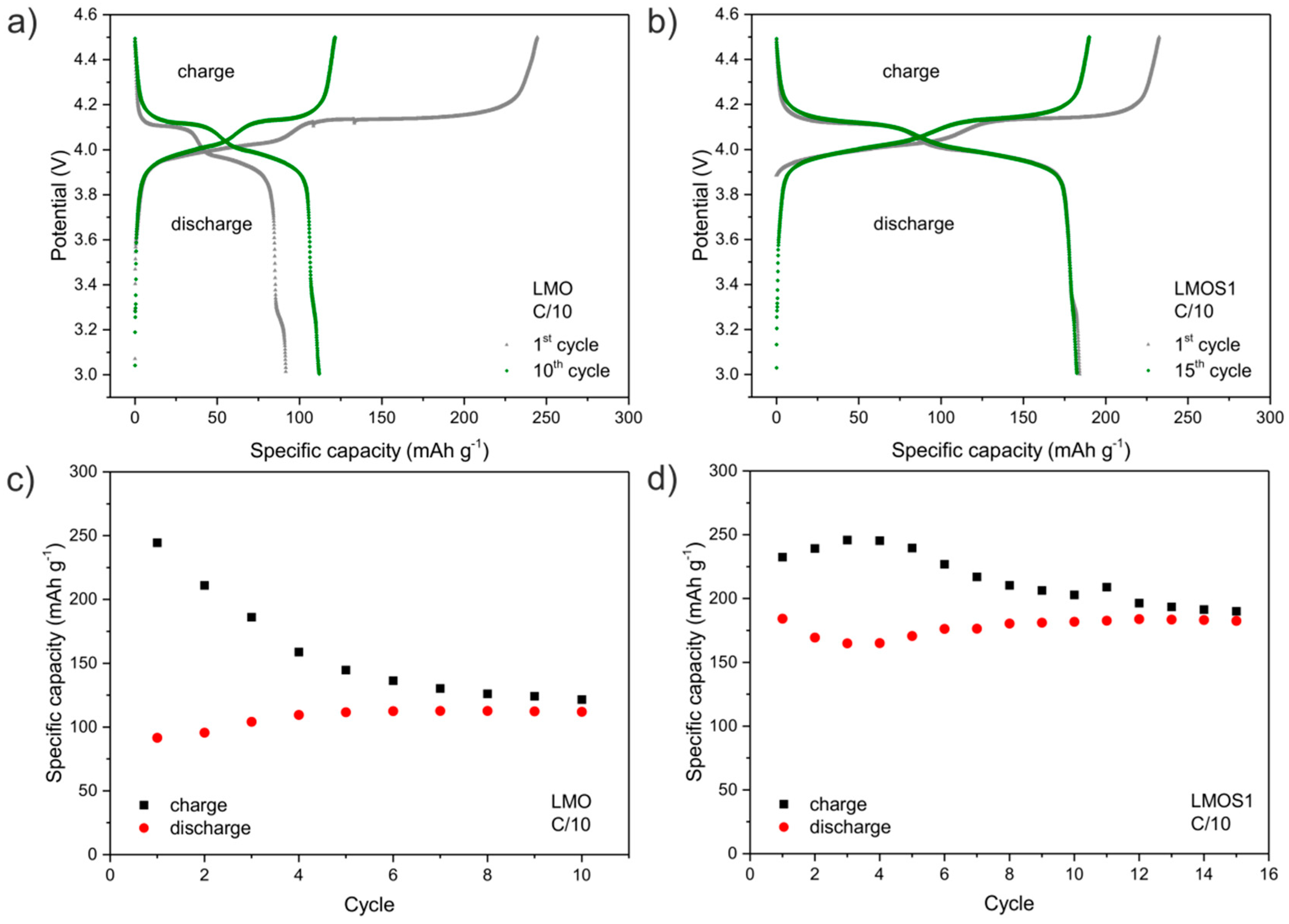
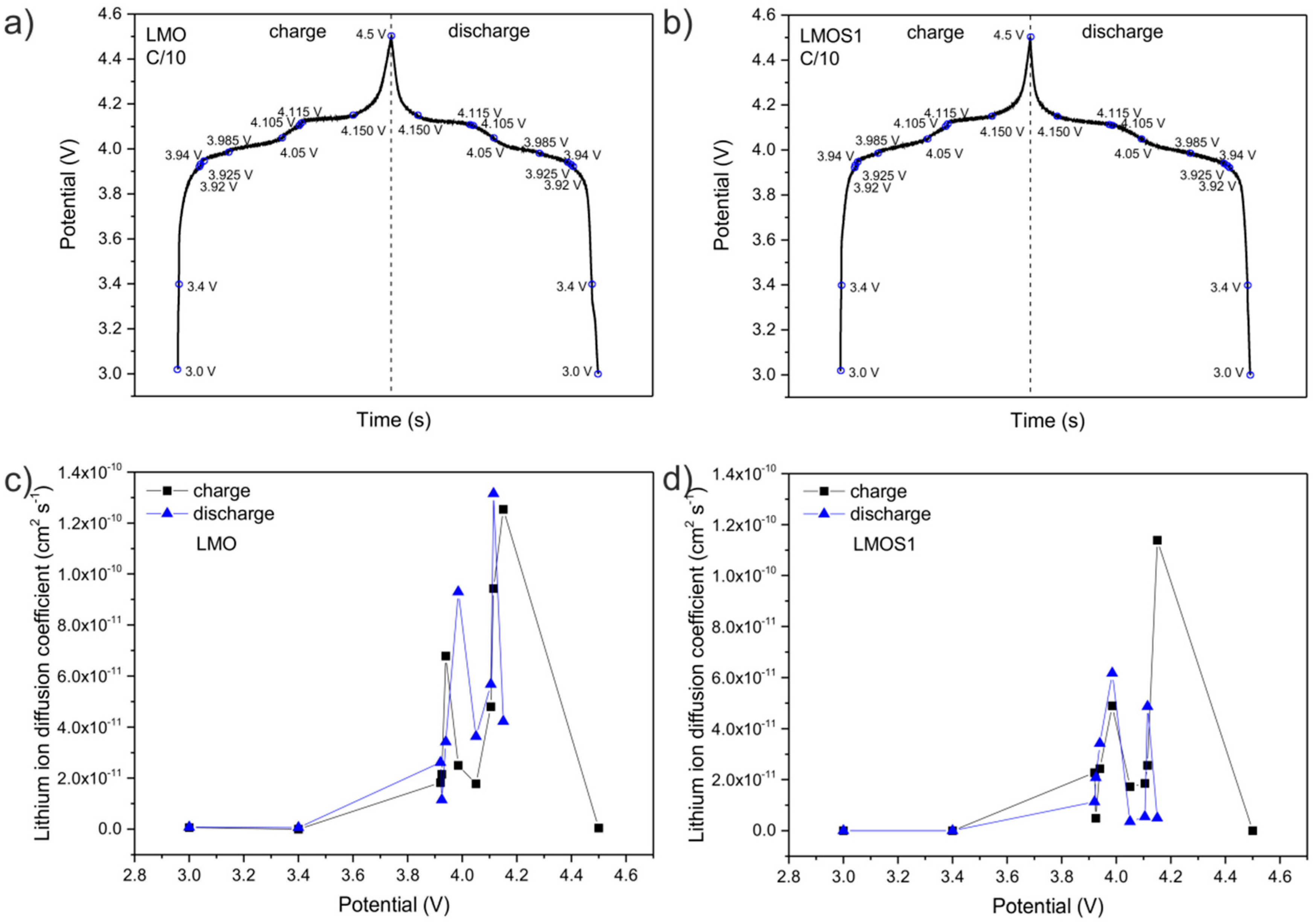
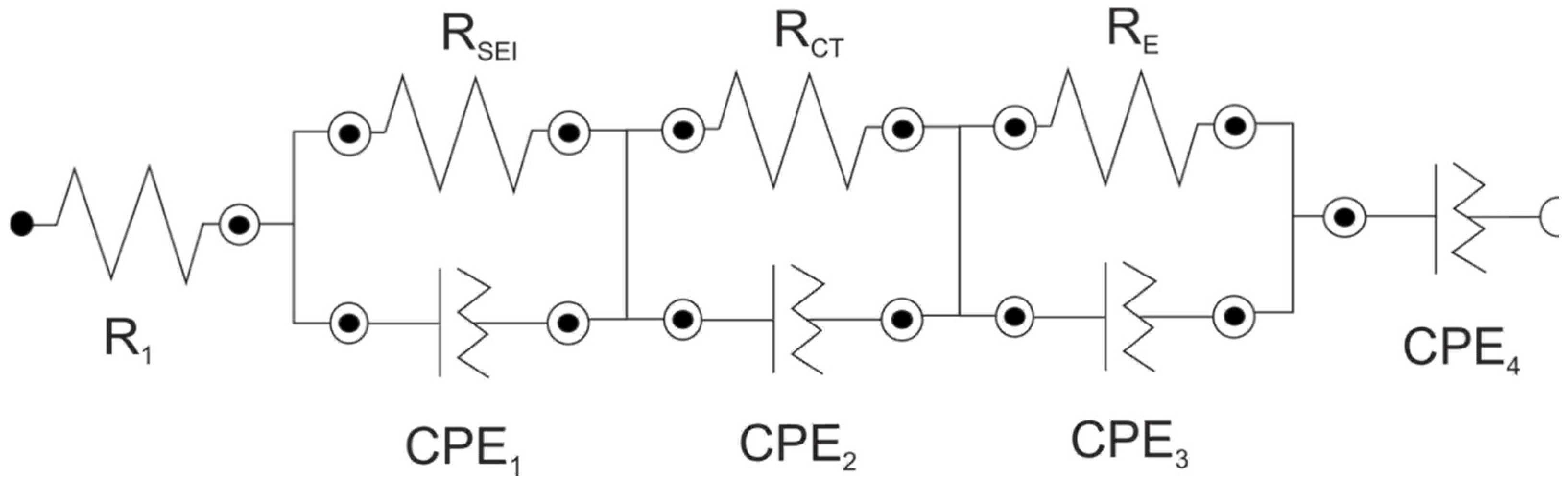
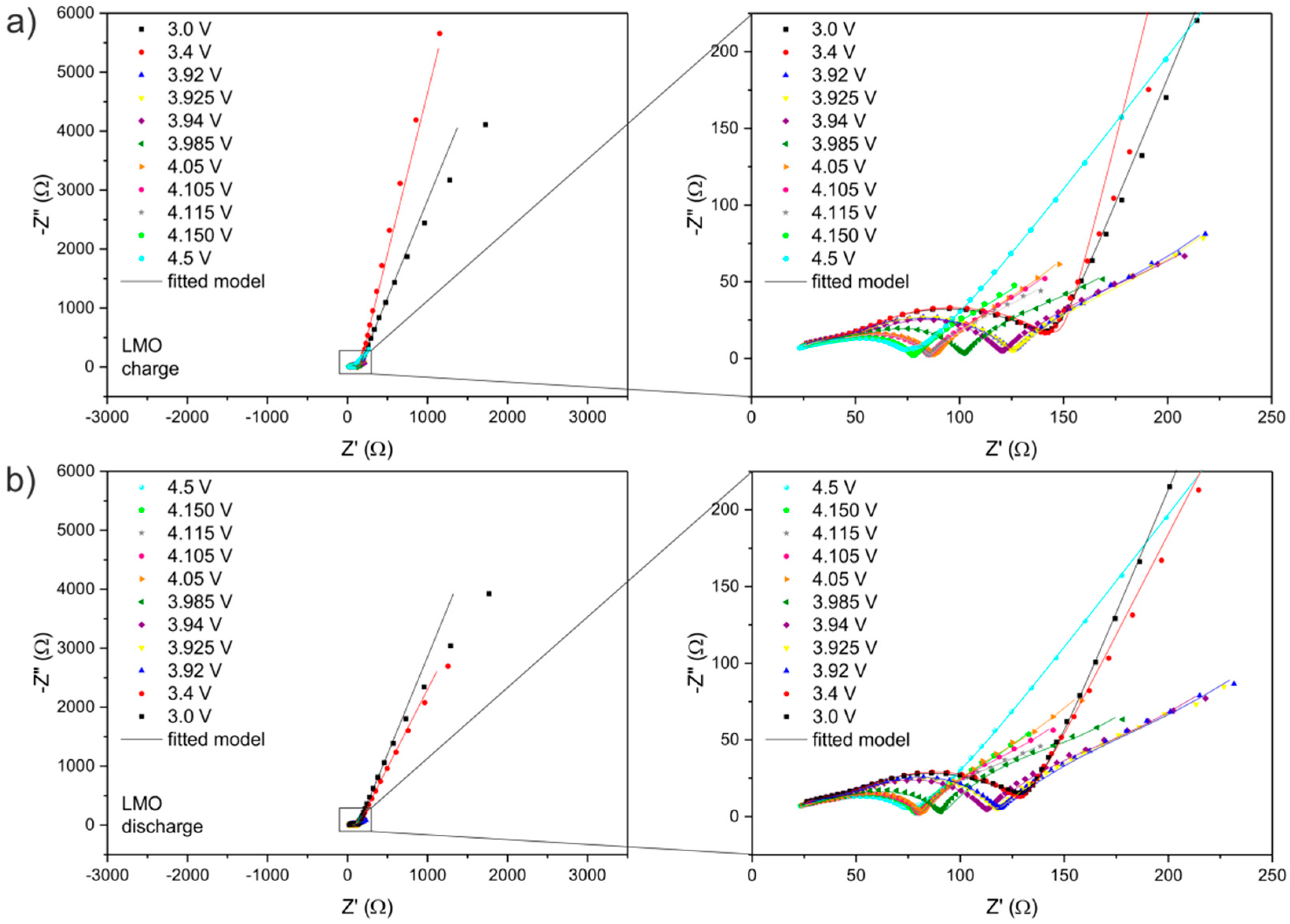
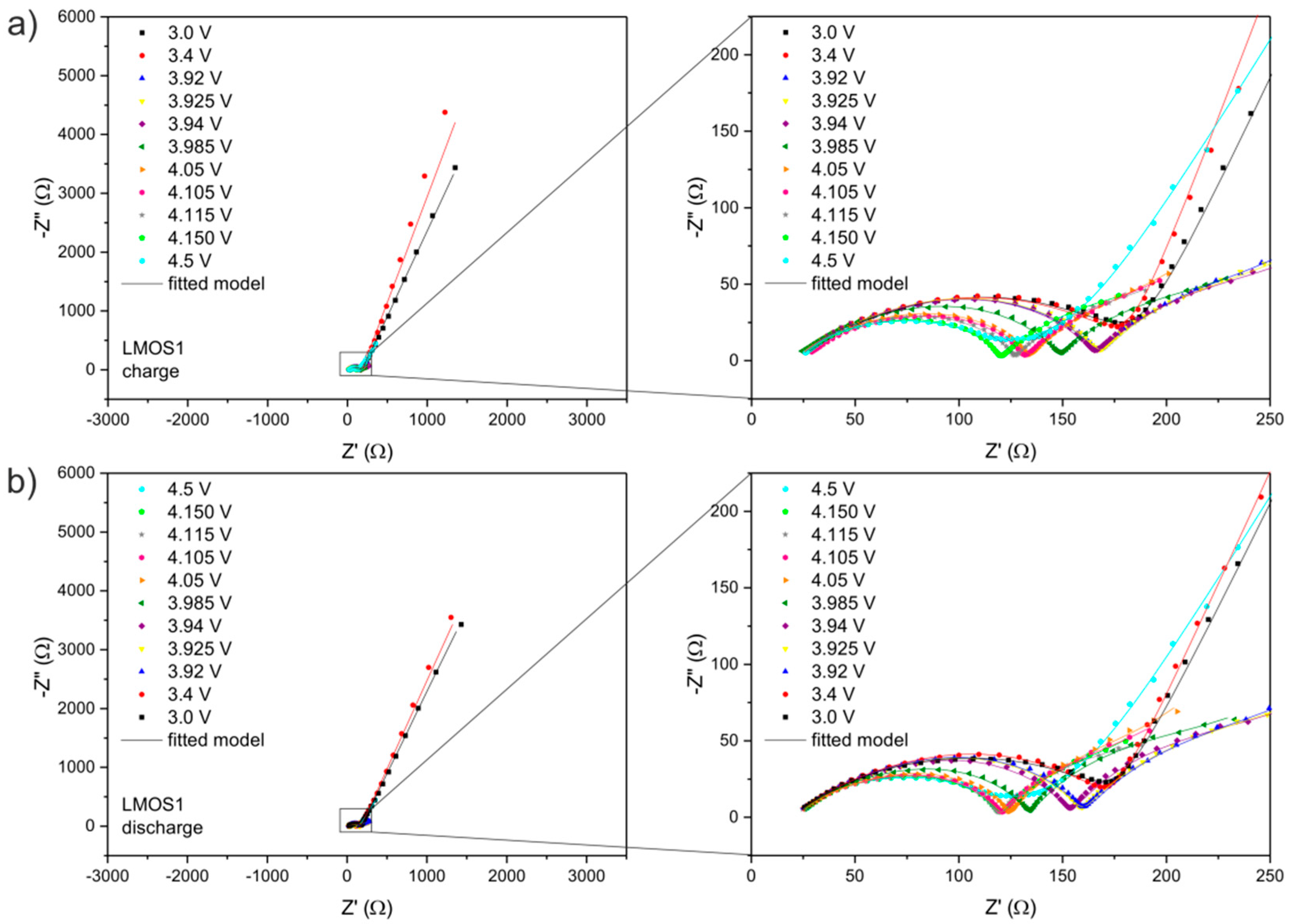
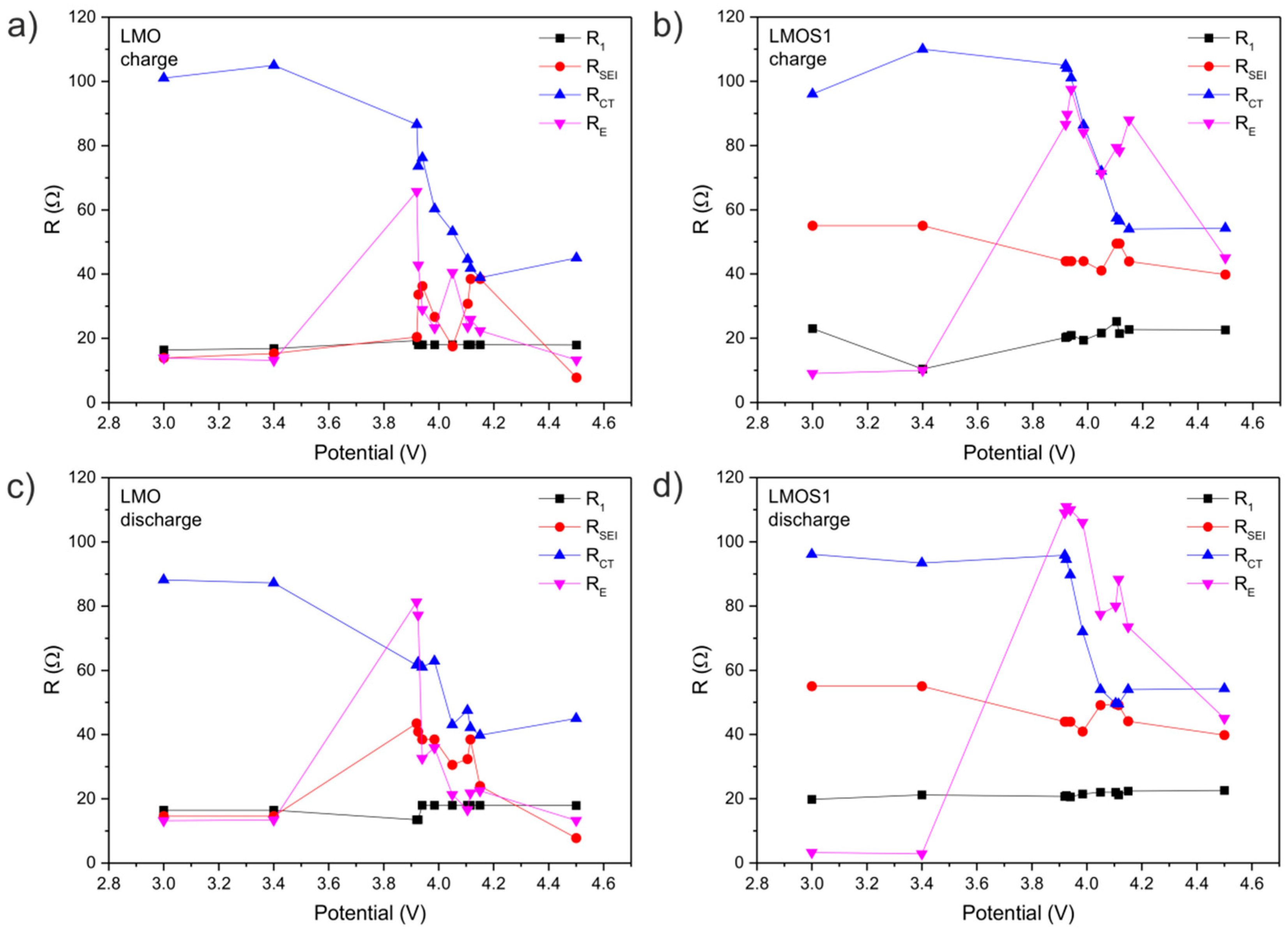
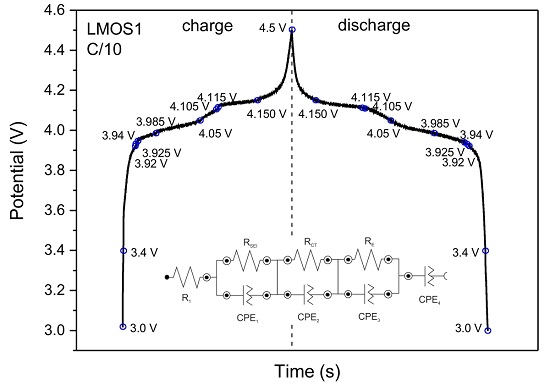
| LixMn2O4 | R1/Ω | RSEI/Ω | RCT/Ω | RE/Ω | CPE1 | CPE2 | CPE3 | CPE4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potential/V | Y0/Ω−1·sN | N | Y0/Ω−1·sN | N | Y0/Ω−1·sN | N | Y0/Ω−1·sN | N | |||||
| charging | 3 | 16 | 14 | 101 | 14 | 4.44E-06 | 0.840 | 4.00E-05 | 0.699 | 7.70E-07 | 0.846 | 2.24E-03 | 0.813 |
| 3.4 | 17 | 15 | 105 | 13 | 8.60E-07 | 0.840 | 4.14E-05 | 0.699 | 5.21E-06 | 0.846 | 2.11E-03 | 0.885 | |
| 3.92 | 19 | 20 | 87 | 66 | 1.22E-06 | 0.841 | 4.22E-05 | 0.684 | 4.57E-02 | 0.696 | 7.99E-02 | 0.649 | |
| 3.925 | 18 | 34 | 74 | 43 | 1.30E-05 | 0.641 | 3.20E-05 | 0.756 | 5.24E-02 | 0.716 | 5.47E-02 | 0.576 | |
| 3.94 | 18 | 36 | 76 | 29 | 6.94E-02 | 0.834 | 6.00E-05 | 0.663 | 2.56E-05 | 0.591 | 6.15E-02 | 0.573 | |
| 3.985 | 18 | 27 | 60 | 23 | 1.19E-01 | 0.826 | 7.84E-05 | 0.515 | 2.50E-05 | 0.939 | 6.73E-02 | 0.522 | |
| 4.05 | 18 | 18 | 53 | 41 | 2.41E-06 | 0.796 | 5.36E-05 | 0.664 | 9.00E-02 | 0.726 | 1.13E-01 | 0.677 | |
| 4.105 | 18 | 31 | 45 | 24 | 1.15E-01 | 0.755 | 5.94E-05 | 0.685 | 1.23E-05 | 0.668 | 1.04E-01 | 0.619 | |
| 4.115 | 18 | 39 | 42 | 26 | 9.10E-02 | 0.708 | 4.65E-05 | 0.728 | 1.20E-05 | 0.668 | 1.33E-01 | 0.619 | |
| 4.15 | 18 | 39 | 39 | 22 | 1.21E-01 | 0.675 | 5.14E-05 | 0.707 | 1.21E-05 | 0.668 | 1.27E-01 | 0.619 | |
| 4.5 | 18 | 8 | 45 | 13 | 6.85E-03 | 0.840 | 3.95E-05 | 0.699 | 1.60E-06 | 0.846 | 1.75E-02 | 0.653 | |
| discharging | |||||||||||||
| 4.15 | 18 | 24 | 40 | 23 | 1.44E-01 | 0.792 | 7.50E-05 | 0.668 | 2.42E-05 | 0.631 | 9.58E-02 | 0.619 | |
| 4.115 | 18 | 39 | 42 | 22 | 9.57E-02 | 0.797 | 1.04E-04 | 0.515 | 1.66E-04 | 0.668 | 1.32E-01 | 0.619 | |
| 4.105 | 18 | 32 | 48 | 17 | 1.01E-01 | 0.817 | 7.83E-05 | 0.632 | 1.47E-05 | 0.668 | 9.34E-02 | 0.619 | |
| 4.05 | 18 | 31 | 43 | 21 | 9.80E-02 | 0.809 | 5.64E-05 | 0.693 | 1.56E-05 | 0.653 | 6.72E-02 | 0.622 | |
| 3.985 | 18 | 39 | 63 | 36 | 8.36E-05 | 0.675 | 4.55E-02 | 0.751 | 1.10E-04 | 0.654 | 1.01E-01 | 0.644 | |
| 3.94 | 18 | 39 | 61 | 33 | 5.35E-02 | 0.760 | 2.62E-05 | 0.780 | 1.09E-05 | 0.659 | 4.21E-02 | 0.515 | |
| 3.925 | 14 | 41 | 63 | 77 | 2.94E-05 | 0.557 | 2.74E-05 | 0.780 | 3.35E-02 | 0.652 | 6.25E-02 | 0.598 | |
| 3.92 | 14 | 44 | 62 | 81 | 3.70E-05 | 0.536 | 2.66E-05 | 0.788 | 3.23E-02 | 0.635 | 6.02E-02 | 0.600 | |
| 3.4 | 17 | 15 | 87 | 13 | 8.49E-07 | 0.840 | 3.96E-05 | 0.699 | 4.83E-06 | 0.846 | 3.03E-03 | 0.772 | |
| 3 | 17 | 15 | 88 | 13 | 8.49E-07 | 0.840 | 3.96E-05 | 0.699 | 4.83E-06 | 0.846 | 2.29E-03 | 0.808 | |
| LixMn2O3.99S0.01 | R1/Ω | RSEI/Ω | RCT/Ω | RE/Ω | CPE1 | CPE2 | CPE3 | CPE4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potential/V | Y0/Ω−1·sN | N | Y0/Ω−1·sN | N | Y0/Ω−1·sN | N | Y0/Ω−1·sN | N | |||||
| charging | 3 | 23 | 55 | 96 | 9 | 9.03E-05 | 0.805 | 2.40E-05 | 0.691 | 7.57E-06 | 0.763 | 2.50E-03 | 0.784 |
| 3.4 | 10 | 55 | 110 | 10 | 8.40E-05 | 0.568 | 5.98E-05 | 0.664 | 6.74E-09 | 0.959 | 2.26E-03 | 0.827 | |
| 3.92 | 20 | 44 | 105 | 87 | 6.83E-05 | 0.575 | 3.30E-05 | 0.722 | 3.30E-02 | 0.728 | 9.77E-02 | 0.681 | |
| 3.925 | 21 | 44 | 104 | 90 | 4.87E-05 | 0.603 | 3.04E-05 | 0.737 | 3.02E-02 | 0.721 | 1.05E-01 | 0.691 | |
| 3.94 | 21 | 44 | 101 | 98 | 4.73E-05 | 0.605 | 2.92E-05 | 0.742 | 3.17E-02 | 0.701 | 1.23E-01 | 0.664 | |
| 3.985 | 19 | 44 | 86 | 84 | 5.77E-05 | 0.590 | 3.18E-05 | 0.734 | 4.15E-02 | 0.705 | 1.88E-01 | 0.686 | |
| 4.05 | 22 | 41 | 72 | 71 | 4.27E-05 | 0.621 | 3.36E-05 | 0.737 | 5.59E-02 | 0.707 | 1.81E-01 | 0.729 | |
| 4.105 | 25 | 50 | 57 | 79 | 36.2E-06 | 0.631 | 3.47E-05 | 0.767 | 5.85E-02 | 0.675 | 2.00E-01 | 0.689 | |
| 4.115 | 22 | 50 | 57 | 78 | 3.96E-05 | 0.624 | 3.55E-05 | 0.763 | 5.81E-02 | 0.685 | 2.56E-01 | 0.698 | |
| 4.15 | 23 | 44 | 54 | 88 | 3.42E-05 | 0.643 | 3.84E-05 | 0.751 | 6.75E-02 | 0.662 | 3.13E-01 | 0.649 | |
| 4.5 | 23 | 40 | 54 | 45 | 9.71E-05 | 0.694 | 3.37E-05 | 0.650 | 6.51E-03 | 0.501 | 1.57E-02 | 0.733 | |
| discharging | |||||||||||||
| 4.15 | 22 | 44 | 54 | 74 | 3.81E-05 | 0.635 | 3.81E-05 | 0.751 | 6.21E-02 | 0.690 | 2.40E-01 | 0.735 | |
| 4.115 | 21 | 49 | 50 | 88 | 3.74E-05 | 0.631 | 3.30E-05 | 0.786 | 5.28E-02 | 0.698 | 2.91E-01 | 0.715 | |
| 4.105 | 22 | 50 | 50 | 80 | 3.70E-05 | 0.632 | 3.22E-05 | 0.790 | 5.08E-02 | 0.703 | 1.95E-01 | 0.733 | |
| 4.05 | 22 | 49 | 54 | 77 | 4.27E-05 | 0.619 | 3.19E-05 | 0.780 | 4.73E-02 | 0.744 | 1.47E-01 | 0.752 | |
| 3.985 | 22 | 41 | 72 | 106 | 4.23E-05 | 0.623 | 2.85E-05 | 0.758 | 3.44E-02 | 0.725 | 1.76E-01 | 0.710 | |
| 3.94 | 21 | 44 | 90 | 110 | 5.54E-05 | 0.594 | 2.77E-05 | 0.747 | 2.80E-02 | 0.723 | 1.24E-01 | 0.687 | |
| 3.925 | 21 | 44 | 95 | 111 | 4.65E-05 | 0.608 | 2.66E-05 | 0.751 | 2.75E-02 | 0.706 | 1.02E-01 | 0.671 | |
| 3.92 | 21 | 44 | 96 | 109 | 4.71E-05 | 0.607 | 2.67E-05 | 0.750 | 2.71E-02 | 0.703 | 9.42E-02 | 0.677 | |
| 3.4 | 21 | 55 | 93 | 3 | 2.40E-05 | 0.678 | 3.81E-05 | 0.747 | 8.03E-07 | 0.983 | 2.48E-03 | 0.792 | |
| 3 | 20 | 55 | 96 | 3 | 2.10E-05 | 0.678 | 4.45E-05 | 0.747 | 2.71E-07 | 0.983 | 2.48E-03 | 0.792 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakierska, M.; Świętosławski, M.; Dziembaj, R.; Molenda, M. Nature of the Electrochemical Properties of Sulphur Substituted LiMn2O4 Spinel Cathode Material Studied by Electrochemical Impedance Spectroscopy. Materials 2016, 9, 696. https://doi.org/10.3390/ma9080696
Bakierska M, Świętosławski M, Dziembaj R, Molenda M. Nature of the Electrochemical Properties of Sulphur Substituted LiMn2O4 Spinel Cathode Material Studied by Electrochemical Impedance Spectroscopy. Materials. 2016; 9(8):696. https://doi.org/10.3390/ma9080696
Chicago/Turabian StyleBakierska, Monika, Michał Świętosławski, Roman Dziembaj, and Marcin Molenda. 2016. "Nature of the Electrochemical Properties of Sulphur Substituted LiMn2O4 Spinel Cathode Material Studied by Electrochemical Impedance Spectroscopy" Materials 9, no. 8: 696. https://doi.org/10.3390/ma9080696