1. Introduction
Heavy metals are one of the most important classes of pollutants due to their wide sources, non-degradability and high toxicity [
1,
2]. With the development of industrialization and urbanization, the needs for metals have increased drastically, and therefore have created a widespread contamination of heavy metals to water bodies and soils, especially in developing countries [
3]. Lead is one of the most dangerous heavy metals, and is used as an industrial raw material for storage battery manufacturing, printing, pigments, fuels, photography materials and explosives manufacturing [
4]. The use of lead compounds also causes large amounts of industrial wastewater containing the high quantity of Pb(II) ions. Lead is a potent neurotoxin, therefore childhood lead poisoning has an impact on many developmental and biological processes, most notably intelligence, behavior, and overall life achievement [
5]. The reason for this is that children can absorb 40% to 50% of an oral dose of water-soluble lead compared with 3% to 10% for adults. From 2009 to 2011, 10 major lead poisoning episodes caused by heavily polluted enterprises occurred in China, affecting more than 4000 children [
6]. From 2015 to 2016, a public health crisis has resulted from the switching of the water supply from Lake Huron to a more corrosive water source from the Flint River, which has become an important environmental issue because of the Pb(II) pollution [
7]. It was reported that the elevated blood lead levels (≥5 µg/dL) increase from 2.4% to 4.9% after the water source change, and neighborhoods with the highest water lead levels experienced a 6.6% increase in this region [
5].
Because Pb(II) ions are non-biodegradable and tend to bioaccumulate in cells of living organisms, stricter environmental requirements and urgent treatment methods are needed for their removal from water and wastewater [
8]. Since primary treatment (grid, grit chamber and primary settling tank) and secondary treatment (biological treatment) cannot completely treat wastewater, tertiary treatment (advanced treatment) of Pb(II) ions in wastewater was carried out by several methods, such as nanofiltration (NF), reverse osmosis (RO), solvent extraction, reduction, ion-exchange, electro-chemical reduction, evaporation, precipitation and adsorption. Among these existing methods, adsorption has become a popular and effective technique due to its better performance, availability of various adsorbents, ease of handling and no production of toxic secondary compounds [
9]. One of major industrial-scale techniques for the removal of Pb(II) ions is the chelating resin as a traditional adsorption tool, which has been used for the recovery of Pb(II) ions in wastewater for many years. Its economic cost is still relatively high when the metals are dissolved in the large volume of solution with a relatively low concentration [
10]. Furthermore, traditional separation methods, including centrifugation and filtration, are time consuming and tedious. By addressing these problems, using high removal capacity and easy separation materials is an ideal approach to remove Pb(II) ions [
11,
12,
13,
14]. Nanomaterials with high efficiencies in the advanced treatment of wastewater can overcome the shortcomings of the traditional methods, which can replace or operate together with the traditional methods for advanced treatment of wastewater.
Nanoscale zero-valent iron (nZVI) is known to be a promising, effective reductant because of its large specific surface area, small particle size, excellent reactivity and high injectability into an aqueous solution. Despite being highly effective for heavy metals, bare iron nanoparticles tend to rapidly agglomerate and are highly susceptible to oxidation [
15]. Bare iron nanoparticles lack durability and their mechanical strength is also weak. To overcome these shortcomings, technologies have been developed using some materials as mechanical supports to enhance the dispersion of nZVI particles [
16]. Many studies have been carried out on the use of porous materials to support nZVI, for example, nZVI particles supported on resin, were used to remove Pb(II) ions in aqueous solution, and the results showed that the reaction rates for Pb(II) ions were up to 30 times higher than that using iron fillings or powder [
17]. Kaolinite was selected as the supporting material loaded with 20 wt % nZVI (K-nZVI), and the removal efficiency of Pb(II) ions was obviously higher than using bare nZVI [
18]. The reduced graphene oxide-supported nanoscale zero-valent iron (nZVI/rGO) composites have been considered simple and effective for the treatment of contaminants, such as cadmium, uranium, arsenic and trichloroethylene [
19,
20,
21,
22,
23,
24]. The nZVI/rGO composites were also used to remove Pb(II) ions in aqueous solution, and the operating parameters, such as contact time, solution pH, initial Pb(II) concentration and temperature, have been investigated [
25,
26]. However, there is no information available in the literature regarding the optimization of Pb(II) ions removal by nZVI/rGO composites.
To achieve an optimal control and management of Pb pollution, new concepts involving efficient operation and design should be developed and understood [
27]. Hence, a number of tools, such as artificial neural network (ANN), response surface methodology (RSM), partial least square (PLS), modified simplex method (MSM) and uniform experimental design (UED), can be selected to optimize the process for the removal of Pb ions. The past decade has especially seen a host of data analysis tools based on biological phenomena developed into well-established modeling techniques, such as artificial intelligence (AI) and evolutionary computing [
28]. ANN is one of the major tools for AI that has been employed to predict the solid–liquid interface adsorption capacity of activated carbon for the removal of phenol from aqueous solution [
29]. However, this study required a large number of batch experiments to be performed. The experimental design technique is a powerful tool in the field of engineering, which helps in better understanding and improving efficiency of the process [
9]. RSM is a set of mathematical and statistical techniques that has been found to be a useful method for studying the effect of several factors influencing the response as well as optimizing the parameters in the removal process. RSM also identifies the relationship between the controllable input parameters and the response variable. Compared with the orthogonal experiment, the advantage of the RSM is that it can continuously analyze the various levels of the experiment, while the orthogonal experiment can only analyze the experiment point. Another important advantage for the use of statistical models in process optimization is that it requires very less number of experiments to be performed and hence, minimizes time, as well as cost [
30]. Box–Behnken design is a spherical, revolving RSM design that consists of a central point and the middle points of the edges of the cube circumscribed on the sphere [
31]. It consists of three interlocking 2
2 factorial designs having points, all lying on the surface of a sphere surrounding the center of the design [
32]. RSM has also been applied for the optimization of several chemical and physical processes [
33,
34], and the number of experiments is decided accordingly.
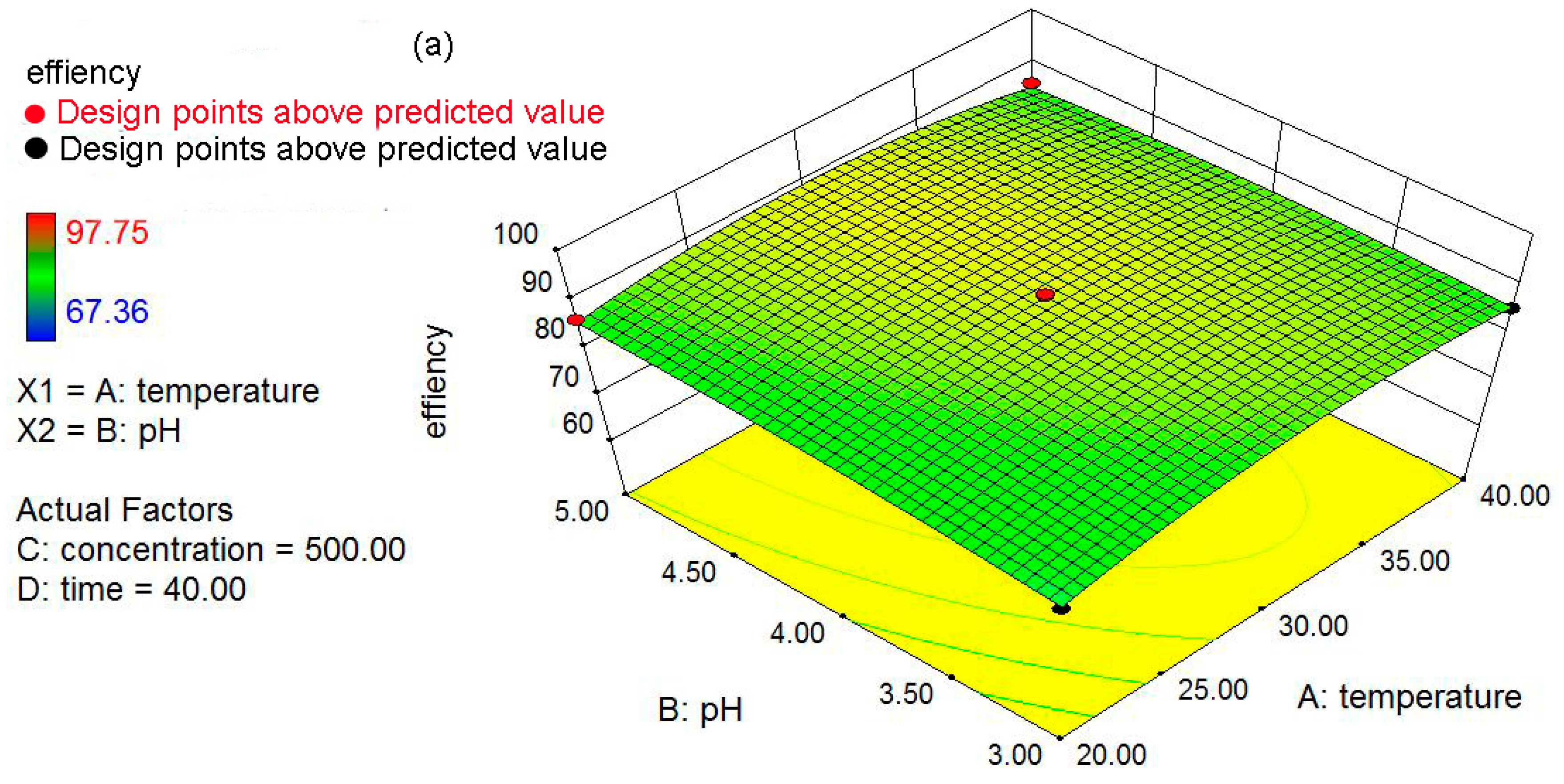
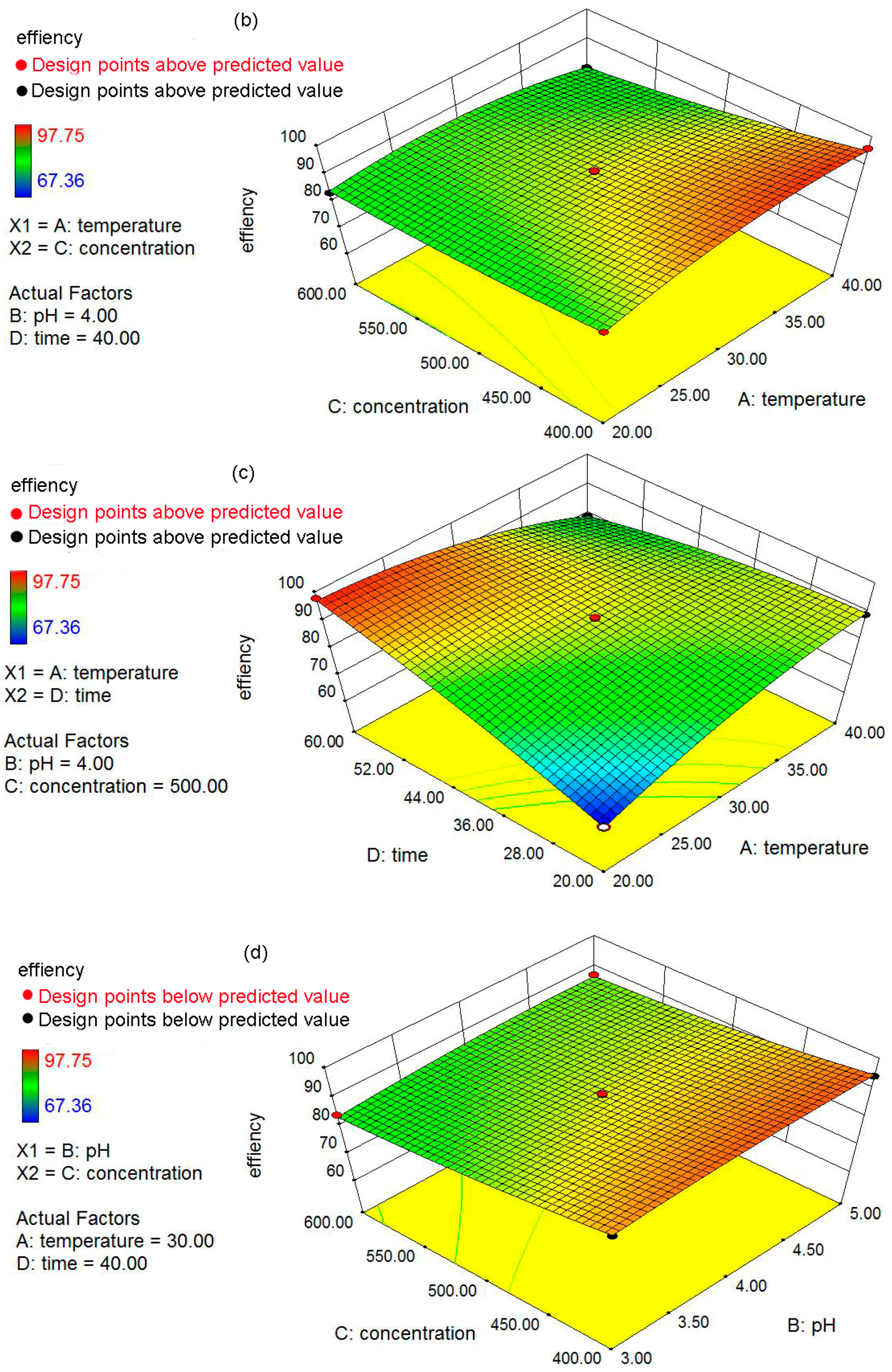
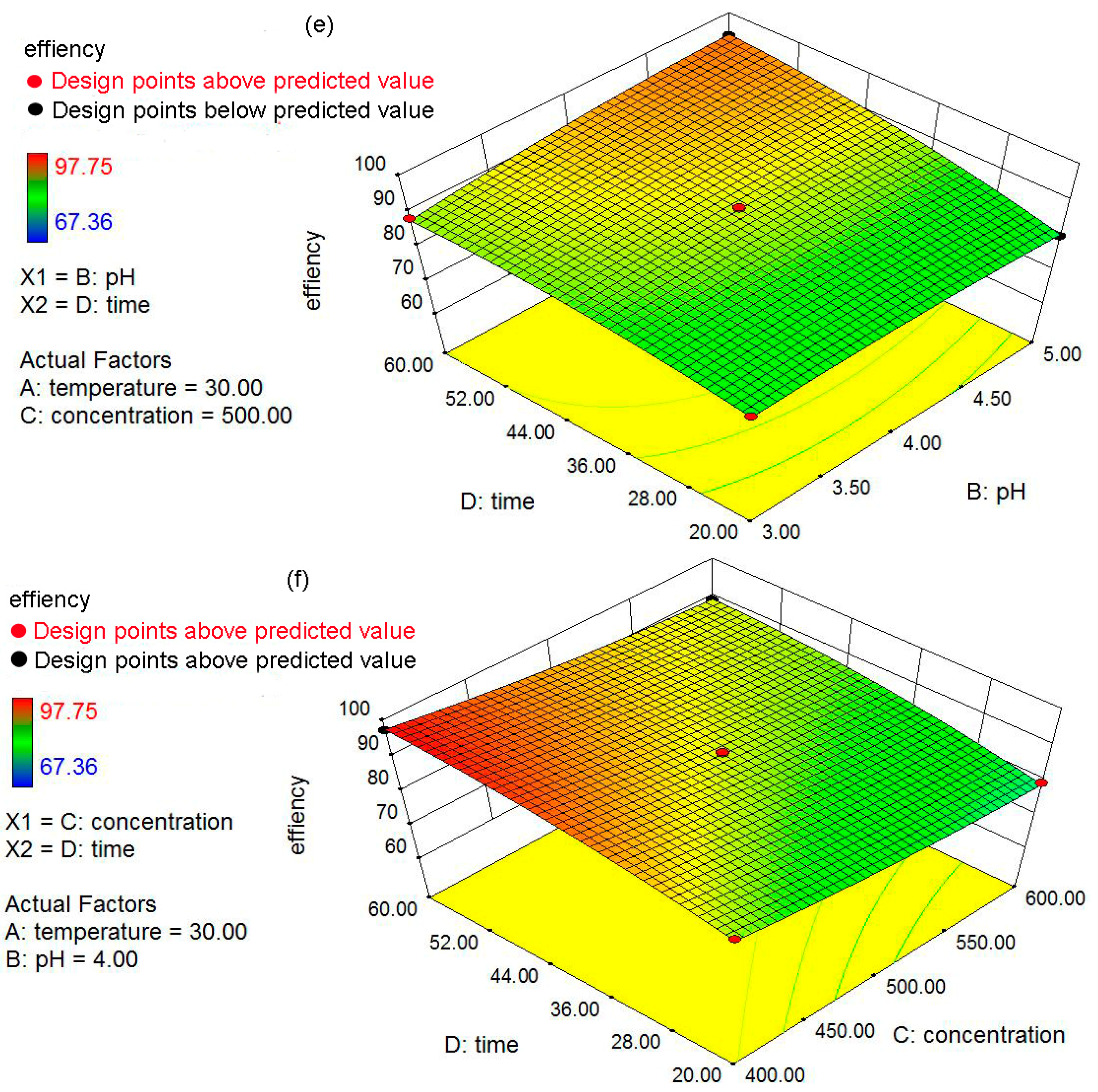
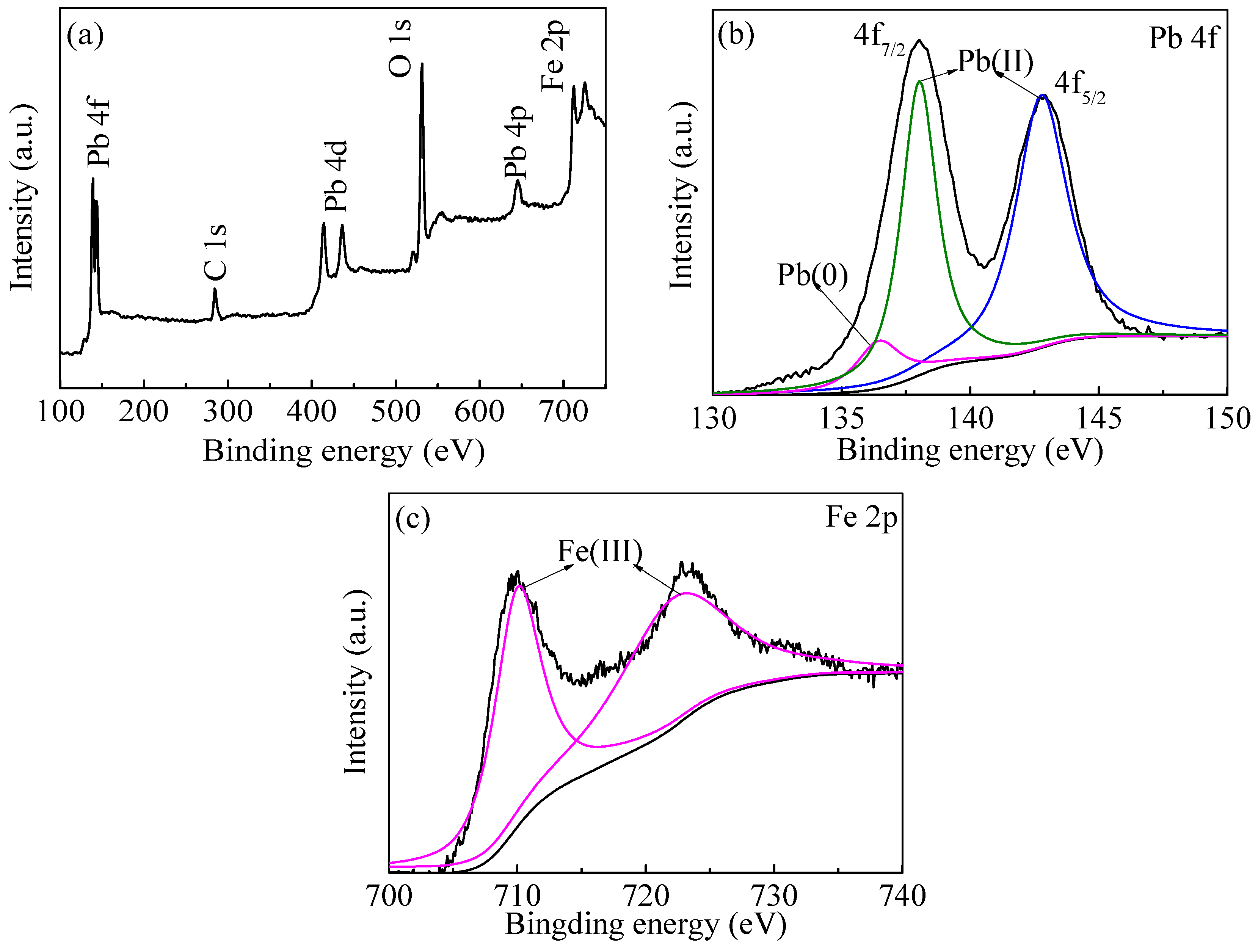
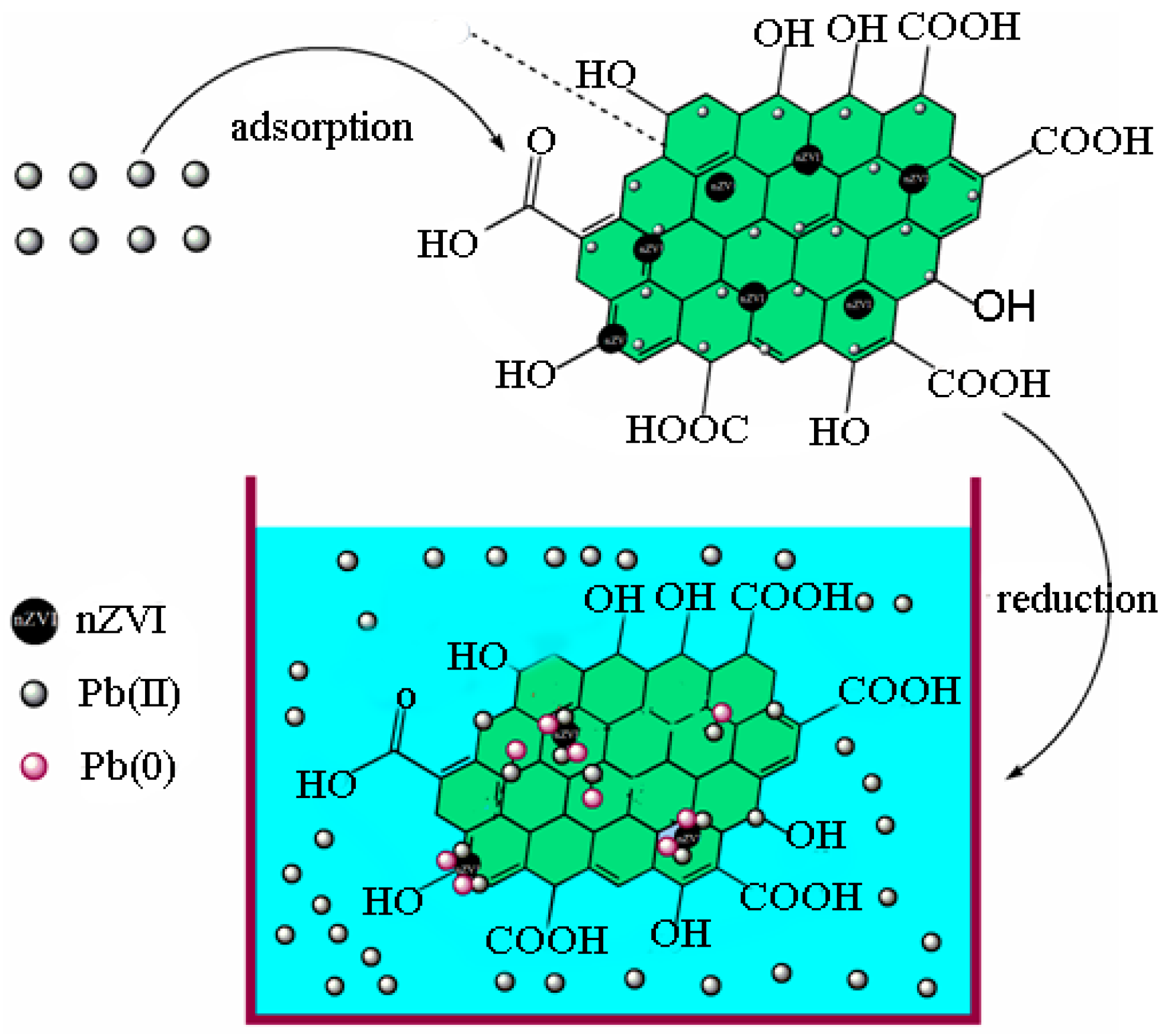
The objectives of the present study were: (a) to prepare and characterize the nZVI and nZVI/rGO; (b) to optimize the process parameters (temperature, pH, initial concentration and contact time) for maximum removal efficiency using the Box–Behnken design; and (c) to fit the obtained removal data to the adsorption isotherms and kinetic models. In addition, the mechanisms for the removal of Pb(II) ions by the nZVI/rGO composites were investigated based on the X-ray photoelectron spectroscopy (XPS) analysis.
4. Conclusions
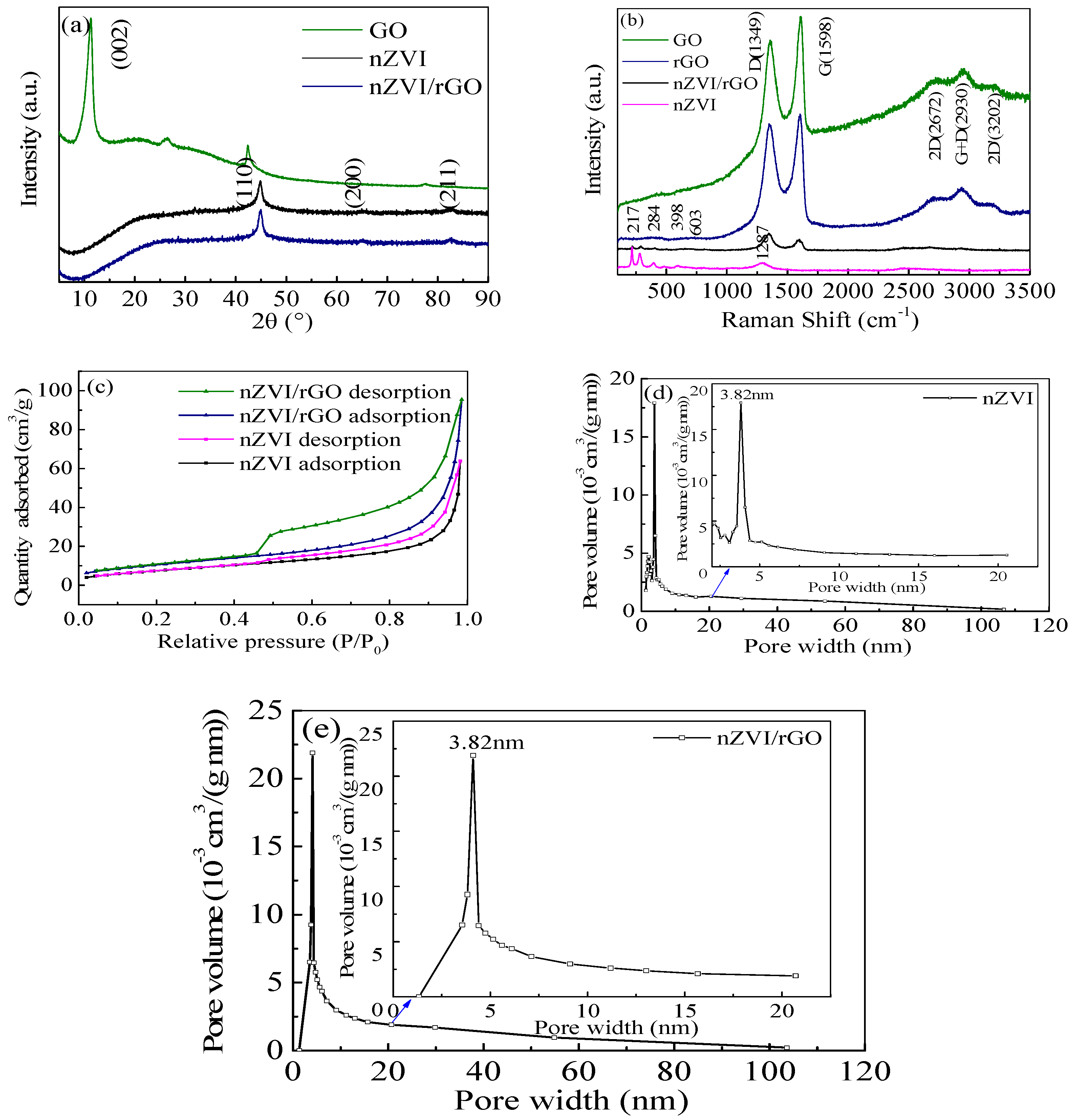
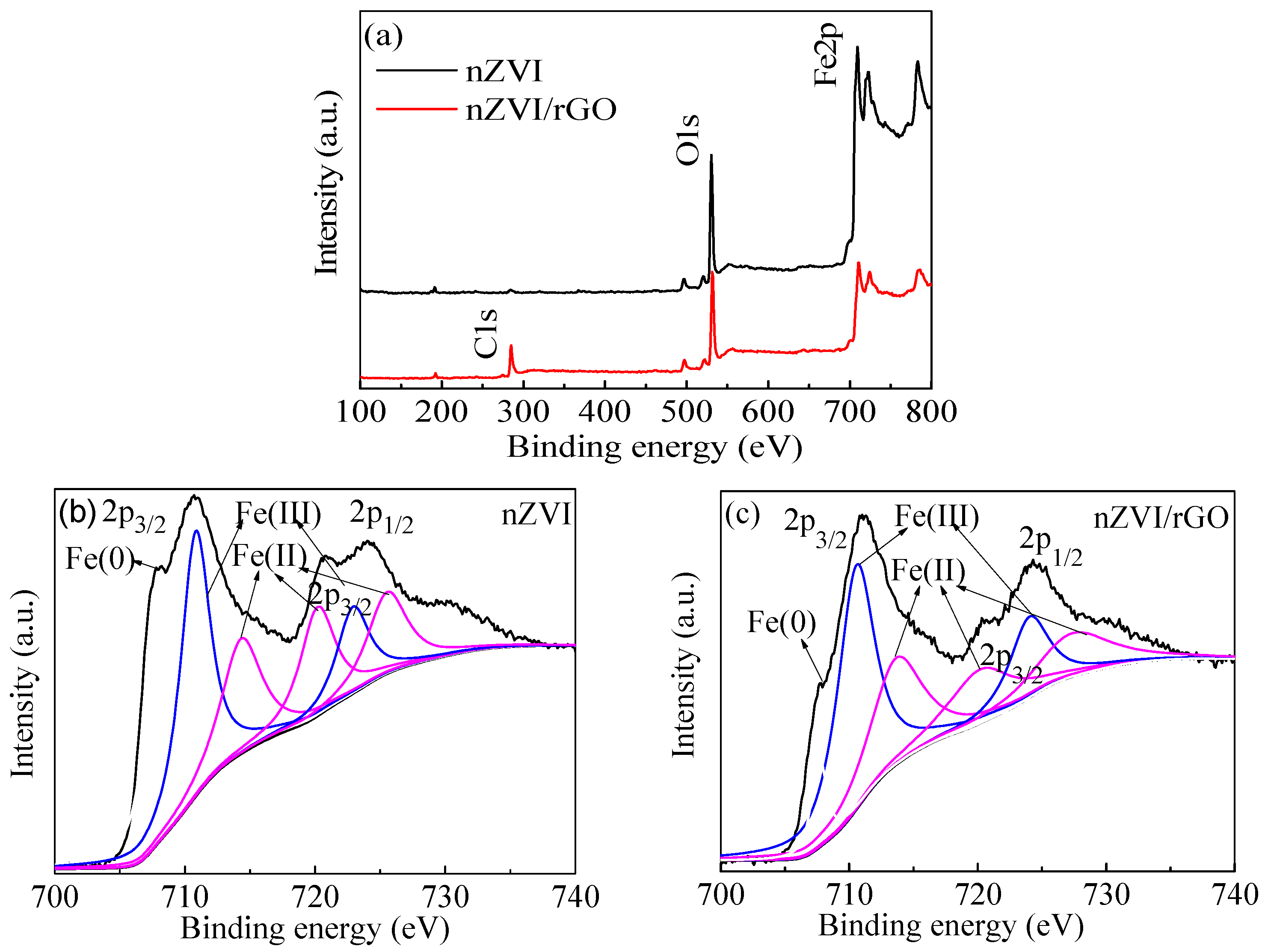
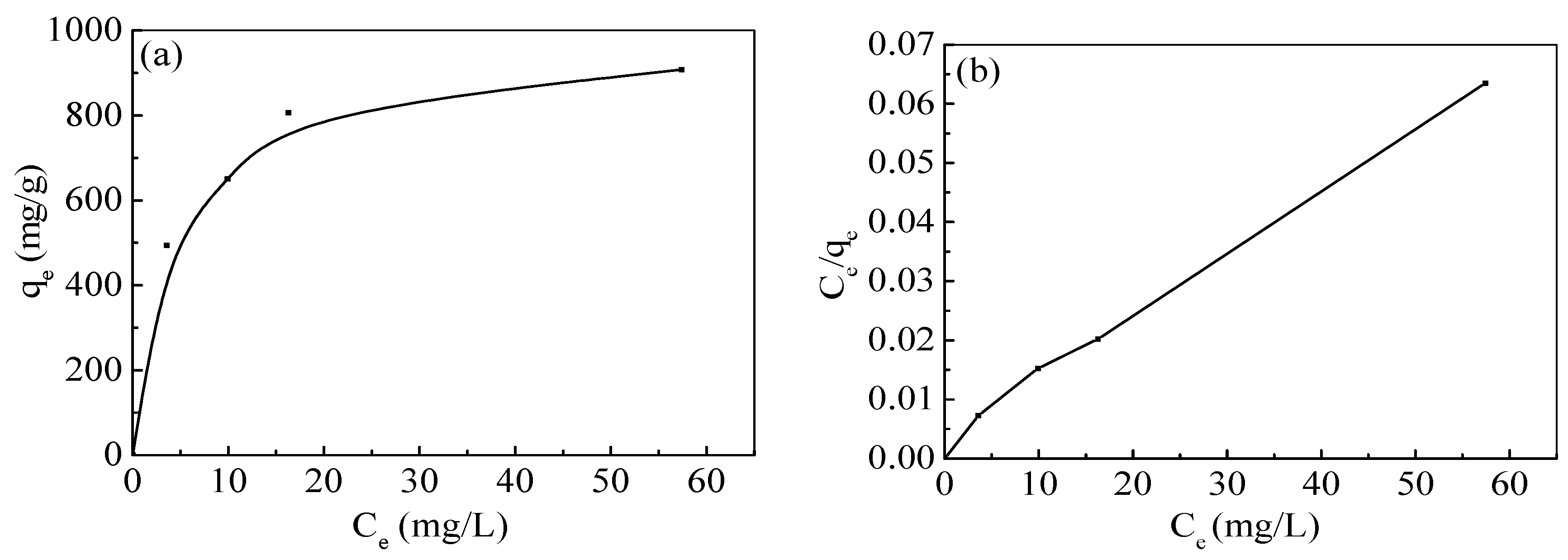
In the present study, the nZVI/rGO composites were prepared by chemical deposition method, which were characterized by SEM, XRD, Raman spectroscopy, N2-sorption and XPS. Using RSM with the quadratic model, the optimal temperature, pH, initial concentration and contact time for Pb(II) ions removal by the nZVI/rGO composites in the present experiment were 21.30 °C, 5.00, 400.00 mg/L and 60.00 min, respectively. The adsorption results could be fitted to the Langmuir isotherm, and the removal kinetics of Pb(II) ions was well described by the pseudo-second order kinetic model. This study suggests that the removal of Pb(II) ions by the nZVI/rGO composites was a physical adsorption. The maximum removal capacity of Pb(II) ions by the nZVI/rGO composites was 904 mg/g, which was higher than that by other materials. In addition, the dominant mechanism for the removal of Pb(II) ions is based on the adsorption by the nZVI/rGO composites. Although the rGO is less cost-effective than other traditional materials, rGO has been synthesized in large scale and at low price in recent years. Therefore, the nZVI/rGO composites have a great potential to be used as suitable material for the immobilization and remediation of heavy metal ions from large volumes of aqueous solutions in environmental cleanup. As prospective material for permeable reactive barriers, the nZVI/rGO composites present a great potential for the removal of Pb(II) ions. Another potential use of these composites is for high-end applications, e.g., advanced treatment of drinking water in some special cases.