Electrochemical Study and Characterization of an Amperometric Biosensor Based on the Immobilization of Laccase in a Nanostructure of TiO2 Synthesized by the Sol-Gel Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Apparatus
2.3. Preparation of Titania Sol
2.4. Preparation of Biosensor
3. Results and Discussion
3.1. Particle Size
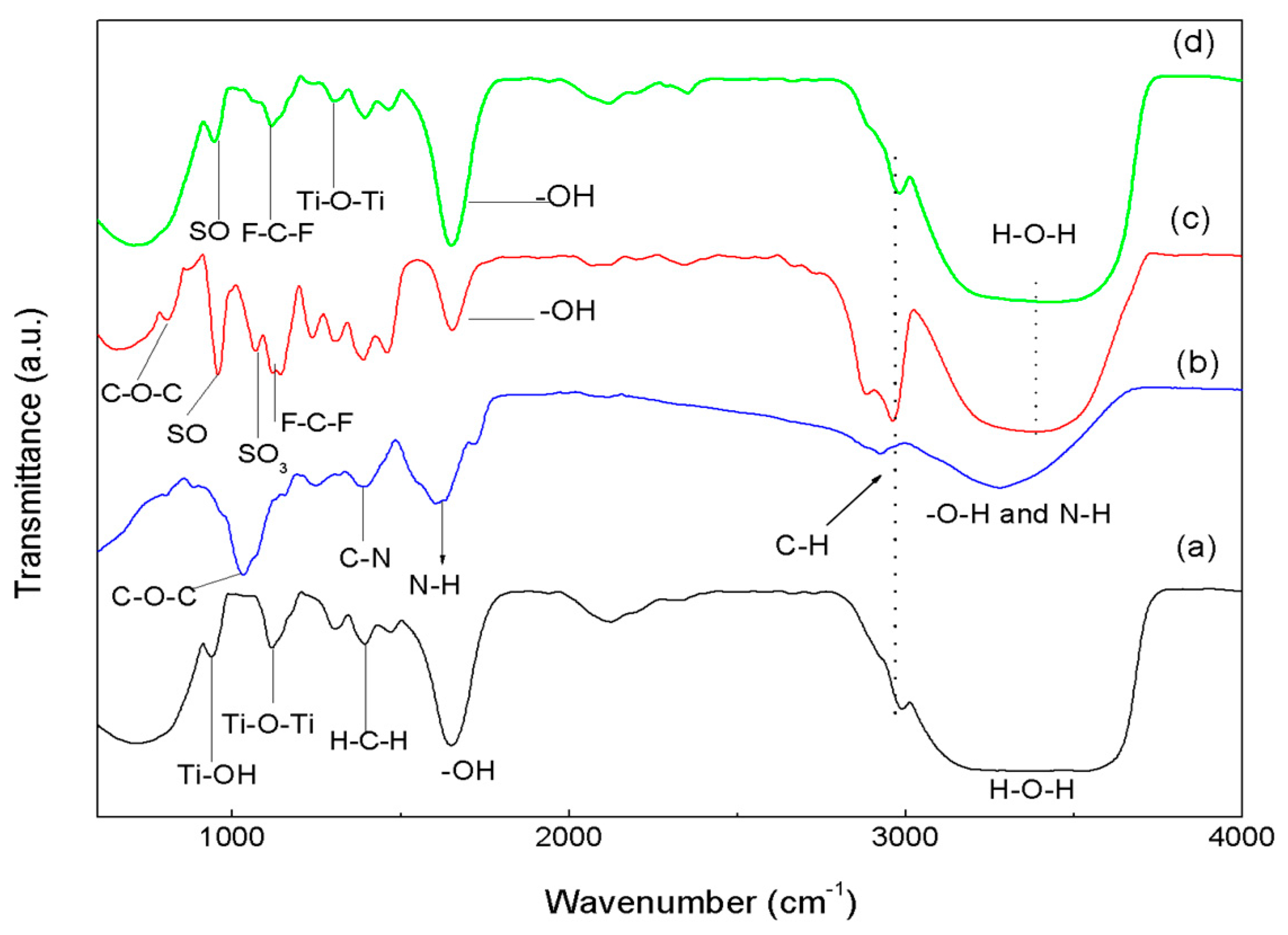
3.2. FTIR Spectroscopy
3.3. Electrochemical Impedance Spectra (EIS) Characterization of the Biosensor
3.4. Cyclic Voltammetry (CV)
3.5. Cyclic Voltammetry Behavior of Catechol
3.6. Amperometric Response of Catechol
3.7. Biosensor Stability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| FTIR | Fourier Transform Infrared |
| CV | Cyclic Voltammetry |
| EIS | Electrochemical Impedance Spectroscopy |
| SCE | Satured Calomel Electrode |
| GE | Graphite Electrode |
| NAF | Nafion |
| LAC | Laccase |
| TiO2 | Titania sol |
References
- Wang, L.; Ran, O.; Tian, Y.; Ye, S.; Xu, J.; Xian, Y.; Peng, R.; Jin, L. Covalent grafting tyrosinase and its application in phenolic compounds detection. Microchim. Acta 2010, 171, 217–223. [Google Scholar] [CrossRef]
- Vianello, F.; Ragusa, S.; Cambria, M.T.; Rigo, A. A high sensitivity amperometric biosensor using laccase as biorecognition element. Biosens. Bioelectron. 2006, 2, 2155–2160. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xiong, H.; Zhang, X.; Wang, S. A novel tyrosinase biosensor based on chitosan-carbon-coated nickel nanocomposite film. Bioelectrochemistry 2012, 84, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Kafi, A.K.M.; Chen, A. A novel amperometric biosensor for the detection of nitrophenol. Talanta 2009, 79, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Rawal, R.; Shabnam; Kuhad, R.C.; Pundir, C.S. An amperometric polyphenol biosensor based on laccase immobilized on epoxy resin membrane. Anal. Methods 2011, 3, 709–714. [Google Scholar] [CrossRef]
- Han, R.; Cui, L.; Ai, S.; Yin, H.; Liu, X.; Qiu, Y. Amperometric biosensor based on tyrosinase immobilized in hydrotalcite-like compounds film for the determination of polyphenols. J. Solid State Electrochem. 2012, 16, 449–456. [Google Scholar] [CrossRef]
- Portaccio, M.; Di Martino, S.; Maiuri, P.; Durante, D.; De Luca, P.; Lepore, M.; Bencivenga, U.; Rossi, S.; De Maio, A.; Mita, D.G. Biosensors for phenolic compounds: The catechol as a substrate model. J. Mol. Catal. B Enzym. 2006, 4, 97–102. [Google Scholar] [CrossRef]
- Yu, J.; Liu, S.; Ju, H. Mediator-free phenol sensor based on titania sol-gel encapsulation matrix for immobilization of tyrosinase by a vapor deposition method. Biosens. Bioelectron. 2003, 19, 509–514. [Google Scholar] [CrossRef]
- Das, P.; Barbora, L.; Das, M.; Goswami, P. Highly sensitive and stable laccase based amperometric biosensor developed on nano-composite matrix for detecting pyrocatechol in environmental samples. Sens. Actuat. B Chem. 2014, 192, 737–744. [Google Scholar] [CrossRef]
- Kochana, J.; Nowak, P.; Jarosz-Wilkołazka, A.; Bieroń, M. Tyrosinase/laccase bienzyme biosensor for amperometric determination of phenolic compounds. Microchem. J. 2008, 89, 171–174. [Google Scholar] [CrossRef]
- Romero-Arcos, M.; Garnica-Romo, M.G.; Martínez-Flores, H.E.; Vázquez-Marrufo, G.; Ramírez-Bon, R.; González-Hernández, J.; Barbosa-Cánovas, G.V. Enzyme immobilization by amperometric biosensors with tio2 nanoparticles used to detect phenol compounds. Food Eng. Rev. 2016, 8, 235–250. [Google Scholar] [CrossRef]
- Rajesh, W.T.; Kaneto, K. Amperometric phenol biosensor based on covalent immobilization of tyrosinase onto an electrochemically prepared novel copolymer poly (N-3-aminopropyl pyrrole-CO-pyrrole) film. Sens. Actuat. B Chem. 2004, 102, 271–277. [Google Scholar] [CrossRef]
- Liu, Y.; Qu, X.; Guo, H.; Chen, H.; Liu, B.; Dong, S. Facile preparation of amperometric laccase biosensor with multifunction based on the matrix of carbon nanotubes–chitosan composite. Biosens. Bioelectron. 2006, 21, 2195–2201. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, B.; Gorton, L.; Ruzgas, T.; Jönsson, L.J. Characterization of graphite electrodes modified with laccase from trametes versicolor and their use for bioelectrochemical monitoring of phenolic compounds in flow injection analysis. Anal. Chim. Acta 2003, 487, 3–14. [Google Scholar] [CrossRef]
- Jarosz-Wilkołazk, A.; Ruzgas, T.; Gorton, L. Amperometric detection of mono- and diphenols at Cerrena unicolor laccase-modified graphite electrode: Correlation between sensitivity and substrate structure. Talanta 2005, 66, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Sánchez, C.; Tzanov, T.; Gübitz, G.M.; Cavaco-Paulo, A. Voltammetric monitoring of laccase-catalysed mediated reactions. Bioelectrochemistry 2002, 58, 149–156. [Google Scholar] [CrossRef]
- Quan, D.; Kim, Y.; Shin, W. Characterization of an amperometric laccase electrode covalently immobilized on platinum surface. J. Electroanal. Chem. 2004, 561, 181–189. [Google Scholar] [CrossRef]
- Tortolini, C.; Rea, S.; Carota, L.; Cannistraro, S.; Mazzei, F. Influence of the immobilization procedures on the electroanalytical performances of trametes versicolor laccase based bioelectrode. Microchem. J. 2012, 100, 8–13. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Chiu, C.-C. Amperometric biosensors based on multiwalled carbon nanotube-Nafion-tyrosinase nanobiocomposites for the determination of phenolic compounds. Sens. Actuat. B Chem. 2007, 125, 10–16. [Google Scholar] [CrossRef]
- Wang, J.J. Sol-gel materials for electrochemical biosensors. Anal. Chim. Acta 1999, 399, 21–27. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ahammad, A.J.; Jin, J.-H.; Ahn, S.J.; Lee, J.J. A comprehensive review of glucose biosensors based on nanostructured metal-oxides. Sensors 2010, 10, 4855–4886. [Google Scholar] [CrossRef] [PubMed]
- Etienne, M.; Zhang, L.; Vilà, N.; Walcarius, A. Mesoporous materials-based electrochemical enzymatic biosensors. Electroanalysis 2015, 27, 2028–2054. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Lyu, Y.-K.; Choi, H.N.; Lee, W.-Y. Amperometric Tyrosinase Biosensor Based on Carbon Nanotube–Titania–Nafion Composite Film. Electroanalysis 2007, 19, 1048–1054. [Google Scholar] [CrossRef]
- Venckatesh, R.; Balachandaran, K.; Sivaraj, R. Synthesis and characterization of nano TiO2-SiO2: PVA composite—A novel route. Int. Nano Lett. 2012, 2, 1–5. [Google Scholar] [CrossRef]
- Porkodi, K.; Arokiamary, S.D. Synthesis and spectroscopic characterization of nanostructured anatase titania: A photocatalyst. Mater. Charact. 2007, 58, 495–503. [Google Scholar] [CrossRef]
- Watson, S.; Beydoun, D.; Scott, J.; Amal, R. Preparation of nanosized crystalline TiO2 particles at low temperature for photocatalysis. J. Nanopart. Res. 2004, 6, 193–207. [Google Scholar] [CrossRef]
- Yuan, S.; Hu, S. Characterization and electrochemical studies of Nafion/nano-TiO2 film modified electrodes. Electrochim. Acta 2004, 49, 4287–4293. [Google Scholar] [CrossRef]
- Walcarius, A.; Minteer, S.D.; Wang, J.; Lin, Y.; Merkoçi, A. Nanomaterials for bio-functionalized electrodes: Recent trends. J. Mater. Chem. B 2013, 1, 4878–4908. [Google Scholar] [CrossRef]
- Zhang, T.; Tian, B.; Kong, J.; Yang, P.; Liu, B. A sensitive mediator-free tyrosinase biosensor based on an inorganic–organic hybrid titania sol-gel matrix. Anal. Chim. Acta 2003, 489, 199–206. [Google Scholar] [CrossRef]
- Sousa, P.C.; Polo, A.; Torres, R.; de Torres, C.I.; Alves, W. Chemical modification of a nanocrystalline TiO2 film for efficient electric connection of glucose oxidase. J. Colloid Interface Sci. 2010, 346, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Yuan, R.; Chai, Y.; Li, W.; Zhang, Y.; Wang, C. Amperometric biosensor for hydrogen peroxide based on horseradish peroxidase onto gold nanowires and TiO2 nanoparticles. Bioprocess Biosyst. Eng. 2011, 34, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Kochana, J.; Gala, A.; Parczewski, A.; Adamski, J. Titania sol-gel-derived tyrosinase-based amperometric biosensor for determination of phenolic compounds in water samples. Examination of interference effects. Anal. Bioanal. Chem. 2008, 391, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kuang, D.; Feng, Y.; Zhang, F.; Liu, M. Glucose biosensor based on glucose oxidase immobilized on a nanofilm composed of mesoporous hydroxyapatite, titanium dioxide, and modified with multi-walled carbon nanotubes. Microchim. Acta 2012, 176, 73–80. [Google Scholar] [CrossRef]
- Zheng, M.; Gu, M.; Jin, Y.; Jin, G. Preparation, structure and properties of TiO2–PVP hybrid films. Mater. Sci. Eng. B 2000, 77, 55–59. [Google Scholar] [CrossRef]
- Tavares, A.P.M.; Silva, C.G.; Dražić, G.; Silva, A.M.T.; Loureiro, J.M.; Faria, J.L. Laccase immobilization over multi-walled carbon nanotubes: Kinetic, thermodynamic and stability studies. J. Colloid Interface Sci. 2015, 454, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M.; Krywko-Cendrowska, A.; Krysiński, P.; Rogalski, J. Encapsulation of laccase in a conducting polymer matrix: A simple route towards polypyrrole microcontainers. Synth. Metals 2009, 159, 1731–1738. [Google Scholar] [CrossRef]
- Wang, K.; Tang, J.; Zhang, Z.; Gao, Y.; Chen, G. Laccase on Black Pearl 2000 modified glassy carbon electrode: Characterization of direct electron transfer and biological sensing properties for pyrocatechol. Electrochim. Acta 2012, 70, 112–117. [Google Scholar] [CrossRef]
- Kato, K.; Inukai, K.; Fujikurab, K.; Kasuga, T. Effective encapsulation of laccase in an aluminium silicate nanotube hydrogel. New J. Chem. 2014, 38, 3591–3599. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, H.; Chen, J.; Qian, P.; Zhai, Y. Modification of nafion membrane using interfacial polymerization for vanadium redox flow battery applications. J. Membr. Sci. 2008, 311, 98–103. [Google Scholar] [CrossRef]
- Kumar, A.S.; Po-Hsun, L.; Chen, S.M. Electrochemical synthesis and characterization of TiO2 nanoparticles and their use as a platform for flavin adenine dinucleotide immobilization and efficient electrocatalysis. Nanotechnology 2008, 19, 255501. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Rawal, R.; Kumar, D.; Pundir, C.S. Amperometric determination of total phenolic content in wine by laccase immobilized onto silver nanoparticles/zinc oxide nanoparticles modified gold electrode. Anal. Biochem. 2012, 430, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Mayorga-Martínez, C.C.; Cadevall, M.; Guix, M.; Ros, J.; Merkoci, A. Bismuth nanoparticles for phenolic compounds biosensing application. Biosens. Bioelectron. 2013, 40, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, G.; Guix, M.; Ambrosi, A.; Ramírez Silva, M.T.; Palomar Pardave, M.E.; Merkoçi, A. Stable and sensitive flow-through monitoring of phenol using a carbon nanotube based screen printed biosensor. Nanotechnology 2010, 21, 245502. [Google Scholar] [CrossRef] [PubMed]
- Kochana, J. Influence of Nafion in Titania Sol-gel Matrix on Analytical Characteristic of Amperometric Phenol Biosensor Based on Tyrosinase. Acta Chim. Slov. 2012, 59, 760–765. [Google Scholar] [PubMed]
- Yang, C.-C.; Chiu, S.-J.; Lee, K.-T.; Chien, W.-C.; Lin, C.-T.; Huang, C.-A. Study of poly(vinyl alcohol)/titanium oxide composite polymer membranes and their application on alkaline direct alcohol fuel cell. J. Power Sources 2008, 184, 44–51. [Google Scholar] [CrossRef]
- Wang, S.; Tan, Y.; Zhao, D.; Liu, G. Amperometric tyrosinase biosensor based on Fe3O4 nanoparticles–chitosan nanocomposite. Biosens. Bioelectron. 2008, 23, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Di Fusco, M.; Tortolini, C.; Deriu, D.; Mazzei, F. Laccase-based biosensor for the determination of polyphenol index in wine. Talanta 2010, 81, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, T.; Itoh, T.; Sumiya, T.; Hanaoka, T.; Mizukami, F.; Ono, M. Amperometric detection of phenolic compounds with enzyme immobilized in mesoporous silica prepared by electrophoretic deposition. Sens. Actuators B 2011, 153, 361–368. [Google Scholar] [CrossRef]
- Li, D.; Luo, L.; Pang, Z.; Ding, L.; Wang, Q.; Ke, H.; Huang, F.; Wei, Q. Novel phenolic biosensor based on a magnetic polydopamine-laccase-nickel nanoparticle loaded carbon nanofiber composite. ACS Appl. Mater. Interfaces 2014, 6, 5144–5151. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]





| Days | Analytical Characteristics | NAF/LAC | TiO2/LAC | TiO2/NAF/LAC |
|---|---|---|---|---|
| 1 | Sensitivity (µA·L/µmol) | 2.6 | 2.71 | 2.94 |
| Linear Range/µM | 1.25–150 | 1.25–150 | 0.75–150 | |
| Detection limit/µM | 1.25 | 1.25 | 0.75 | |
| R2 | 0.9944 | 0.9986 | 0.9966 | |
| 7 | Sensitivity (µA·L/µmol) | 2.78 | 2.62 | 2.85 |
| Linear Range/µM | 2.5–125 | 2.5–150 | 1.25–150 | |
| Detection limit/µM | 2.5 | 2.5 | 1.25 | |
| R2 | 0.951 | 0.9962 | 0.9988 | |
| 15 | Sensitivity (µA·L/µmol) | 2.6 | 2.4 | 2.8 |
| Linear Range/µM | 5–150 | 15–150 | 1.25–100 | |
| Detection limit/µM | 5 | 3.75 | 1.25 | |
| R2 | 0.9993 | 0.9958 | 0.9966 | |
| 22 | Sensitivity (µA·L/µmol) | 2.12 | 1.92 | 2.5 |
| Linear Range/µM | 5–150 | 3.75–150 | 3.75–150 | |
| Detection limit/µM | 5 | 3.75 | 3.75 | |
| R2 | 0.9973 | 0.9988 | 0.9980 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Arcos, M.; Garnica-Romo, M.G.; Martínez-Flores, H.E. Electrochemical Study and Characterization of an Amperometric Biosensor Based on the Immobilization of Laccase in a Nanostructure of TiO2 Synthesized by the Sol-Gel Method. Materials 2016, 9, 543. https://doi.org/10.3390/ma9070543
Romero-Arcos M, Garnica-Romo MG, Martínez-Flores HE. Electrochemical Study and Characterization of an Amperometric Biosensor Based on the Immobilization of Laccase in a Nanostructure of TiO2 Synthesized by the Sol-Gel Method. Materials. 2016; 9(7):543. https://doi.org/10.3390/ma9070543
Chicago/Turabian StyleRomero-Arcos, Mariana, Ma. Guadalupe Garnica-Romo, and Héctor Eduardo Martínez-Flores. 2016. "Electrochemical Study and Characterization of an Amperometric Biosensor Based on the Immobilization of Laccase in a Nanostructure of TiO2 Synthesized by the Sol-Gel Method" Materials 9, no. 7: 543. https://doi.org/10.3390/ma9070543
APA StyleRomero-Arcos, M., Garnica-Romo, M. G., & Martínez-Flores, H. E. (2016). Electrochemical Study and Characterization of an Amperometric Biosensor Based on the Immobilization of Laccase in a Nanostructure of TiO2 Synthesized by the Sol-Gel Method. Materials, 9(7), 543. https://doi.org/10.3390/ma9070543





