Optimization of Supercritical Fluid Extraction of Total Alkaloids, Peimisine, Peimine and Peiminine from the Bulb of Fritillaria thunbergii Miq, and Evaluation of Antioxidant Activities of the Extracts
Abstract
:1. Introduction
2. Results
2.1. UPLC Chromatogram
2.2. Optimization Strategy
2.3. Effect of the Factors
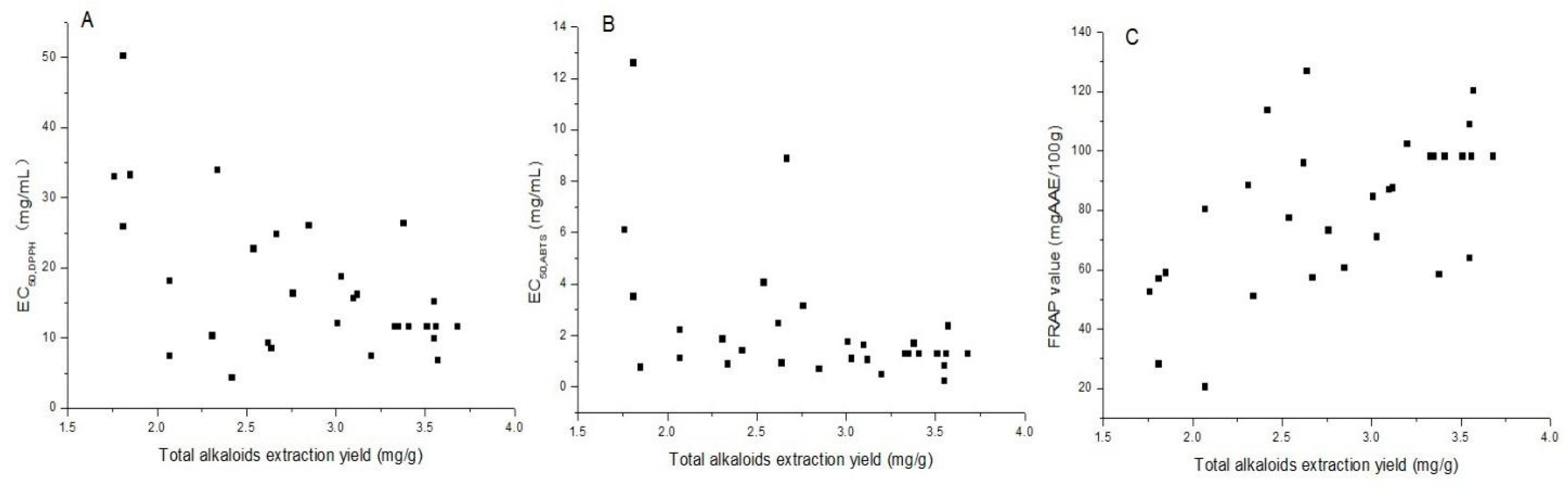
2.4. Antioxidant Activities and Correlation with Total Alkaloids
2.5. Optimization of Extraction Conditions and Verification Tests
3. Materials and Methods
3.1. Materials and Reagents
3.2. Supercritical Fluid Extraction
3.3. Soxhlet Extraction
3.4. UPLC-ELSD Analysis
3.5. Determination of Total Alkaloids Content
3.6. DPPH Radical Scavenging Assay
3.7. ABTS Radical Scavenging Assay
3.8. Ferric Reduction Activity Power (FRAP)
3.9. Experimental Design and Evaluation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SFE | Supercritical fluid extraction |
| SC-CO2 | Supercritical carbon dioxide |
| DPPH-RSA | DPPH radical scavenging activity |
| ABTS-RSA | ABTS radical scavenging activity |
| FRAP | Ferric reducing capacity |
| CCD | Central composite design |
References
- Rix, E.M. Fritillaria: A Revised Classification; The Fritillaria Group of the Alpine Garden Society: Edinburgh, UK, 2001. [Google Scholar]
- Xiao, P.G.; Jiang, Y.; Li, P.; Luo, Y.B.; Liu, Y. The botanical origin and pharmacophylogenetic treatment of Chinese material medica Beimu. Acta Phytotaxon. Sin. 2007, 45, 473–487. [Google Scholar] [CrossRef]
- Hao, D.C.; Gu, X.J.; Xiao, P.G.; Peng, Y. Phytochemical and biological research of Fritillaria medicinal resources. Chin. J. Nat. Med. 2013, 11, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Jiang, Y.; Li, P. Chemistry, bioactivity and geographical diversity of steroidal alkaloids from the Liliaceae family. Nat. Prod. Rep. 2006, 23, 735–752. [Google Scholar] [CrossRef] [PubMed]
- Stermitz, F.R.; Tawara, J.N.; Boeckl, M.; Pomeroy, M.; Foderaro, T.A.; Todd, F.G. Piperidine alkaloid content of Picea (spruce) and Pinus (pine). Phytochemistry 1994, 35, 951–953. [Google Scholar] [CrossRef]
- Gerson, E.A.; Kelsey, R.G. Foliar storage and extraction methods for quantitative analysis of piperidine alkaloids from ponderosa pine (Pinus ponderosa). Phytochem. Anal. 1999, 10, 322–327. [Google Scholar] [CrossRef]
- Pharmacopoeia Commission of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China; Chemistry Industry Publishing House: Beijing, China, 2010. [Google Scholar]
- Mitra, P.; Ramaswamy, H.S.; Chang, K.S. Pumpkin (Cucurbita maxima) seed oil extraction using supercritical carbon and physicochemical properties of the oil. J. Food Eng. 2009, 95, 208–213. [Google Scholar] [CrossRef]
- Zarena, A.S.; Sachindra, N.M.; Sankar, K.U. Optimisation of ethanol modified supercritical carbon dioxide on the extract yield and antioxidant activity from Garcinia mangostana L. Food Chem. 2012, 130, 203–208. [Google Scholar] [CrossRef]
- Liu, B.; Jiang, H.L.; Shen, B.; Chang, Y.L. Supercritical fluid extraction of sinomenine from Sinomenium acutum (Thumb) Rehd et Wils. J. Chromatogr. A 2005, 1075, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.Y.; Zhang, G.Y.; Cui, Z.J.; Zhang, C.K. Supercritical fluid extraction of quinolizidine alkaloids from Sophora flavescens Ait. and purification by high-speed counter-current chromatography. J. Chromatogr. A 2007, 1145, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Guo, F.; Chang, Y.L.; Jiang, H.L.; Wang, Q. Optimization of extraction of evodiamine and rutaecarpine from fruit of Evodia rutaecarpa using modified supercritical CO2. J. Chromatogr. A 2010, 1217, 7833–7839. [Google Scholar] [CrossRef] [PubMed]
- Arshadi, M.; Hunt, A.J.; Clark, J.H. Supercritical fluid extraction (SFE) as an effective tool in reducing auto-oxidation of dried pine sawdust for power generation. RSC Adv. 2012, 2, 1806–1809. [Google Scholar] [CrossRef]
- Herreroa, M.; Mendiolaa, J.A.; Cifuentesa, A.; Ibáneza, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [PubMed]
- Rashid, J.I.A.; Yusof, N.A.; Abdullah, J.; Hashim, U.; Hajian, R. Surface modifications to boost sensitivities of electrochemical biosensors using gold nanoparticles/silicon nanowires and response surface methodology approach. J. Mater. Sci. 2015, 51, 1083–1097. [Google Scholar] [CrossRef]
- Kou, X.H.; Chen, Q.; Li, X.H.; Li, M.F.; Kan, C.; Chen, B.R.; Zhang, Y.; Xue, Z.H. Quantitative assessment of bioactive compounds and the antioxidant activity of 15 jujube cultivars. Food Chem. 2015, 173, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Deng, Z.Y.; Ramdath, D.D.; Tang, Y.; Chen, P.X.; Liu, R.H.; Liu, Q.; Tsao, R. Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on a-glucosidase and pancreatic lipase. Food Chem. 2015, 172, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Smolskait, L.; Venskutonis, P.R.; Talou, T. Comprehensive evaluation of antioxidant and antimicrobial properties of different mushroom species. LWT-Food Sci. Technol. 2015, 60, 462–471. [Google Scholar] [CrossRef]
- Raudonis, R.; Raudone, L.; Jakstas, V.; Janulis, V. Comparative evaluation of post-column free radical scavenging and ferric reducing antioxidant power assays for screening of antioxidants in strawberries. J. Chromatogr. A 2012, 1233, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Lia, D.Q.; Zhao, J.; Lia, S.P. High-performance liquid chromatography coupled with post-columndual-bioactivity assay for simultaneous screening of xanthine oxidaseinhibitors and free radical scavengers from complex mixture. J. Chromatogr. A 2014, 1345, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Helmja, K.; Vahera, M.; Püssa, T.; Kaljurand, M. Analysis of the stable free radical scavenging capability of artificial polyphenol mixtures and plant extracts by capillary electrophoresis and liquid chromatography-diode array detection-tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 2417–2423. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Shen, Y.Y.; Wang, H.F.; Zhang, N.P.; Xu, S.; Zhang, L. Application of response surface methodology to optimise extraction of flavonoids from Fructus sophorae. Food Chem. 2013, 138, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, B.; Chen, M.; Chen, T. Supercritical fluid extraction of ecdysterone from the roots of Achyranthes bidentata BL. J. Sep. Sci. 2008, 31, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wu, Y.; Qian, G.; Chang, Y. Optimum extraction of osthole and imperatorin from the fruits of Cnidium monnieri (L.) cusson by supercritical fluid. Sep. Sci. Technol. 2013, 48, 652–658. [Google Scholar] [CrossRef]
- Xiao, J.; Tian, B.; Xie, B.; Yang, E.; Shi, J.; Sun, Z. Supercritical fluid extraction and identification of isoquinoline alkaloids from leaves of Nelumbo nucifera Gaertn. Eur. Food Res. Technol. 2010, 231, 407–414. [Google Scholar] [CrossRef]
- Choi, Y.H.; Chin, Y.W.; Kim, J.; Jeon, S.H.; Yoo, K.P. Strategies for supercritical fluid extraction of hyoscyamine and scopolamine salts using basified modifiers. J. Chromatogr. A 1999, 863, 47–55. [Google Scholar] [CrossRef]
- Korkmaz, A.; Reiter, R.J.; Tan, D.X.; Manchester, L.C. Melatonin: From pineal gland to healthy foods. Food Chem. 2011, 123, 33–36. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Fuentes-Broto, L. Melatonin: A multitasking molecule. Prog. Brain Res. 2010, 181, 127–151. [Google Scholar] [PubMed]
- Herraiz, T.; Galisteo, J. Hydroxyl radical reactions and the radical scavenging activity of β-carboline alkaloids. Food Chem. 2015, 172, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, B.; Yi, R.N.; Cao, L.F.; Li, S.B.; Tan, R.R.; Chen, M.; Li, X.X.; Wang, C.; Li, Y.F.; Kurihara, H.; et al. Comparing antioxidant capacity of purine alkaloids: A new, efficient trio for screening and discovering potential antioxidants in vitro and in vivo. Food Chem. 2015, 176, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Shirwaikar, A.; Rajendran, K.; Punitha, I.S.R. In vitro antioxidant studies on the benzyl tetra isoquinoline alkaloid berberine. Biol. Pharm. Bull. 2006, 29, 1906–1910. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Temel, E.; Alasalvar, C.; Gökçe, H.; Güder, A.; Albayrak, Ç.; Alpaslan, Y.B.; Alpaslan, G.; Dilek, N. DFT calculations, spectroscopy and antioxidant activity studies on (E)-2-nitro-4-[(phenylimino)methyl]phenol. Spectrochim. Acta A 2015, 136, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Majo, D.D.; Neve, L.L.; Guardia, M.L.; Casuccio, A.; Giammanco, M. The influence of two different pH levels on the antioxidant properties of flavonols, flavan-3-ols, phenolic acids and aldehyde compounds analysed in synthetic wine and in a phosphate buffer. J. Food Compos. Anal. 2011, 24, 265–269. [Google Scholar] [CrossRef]
- Dapkevicius, A.; Vau Beek, T.A.; Niederlander, H.A.G. Evalution and comparison of two improved techniques for the on-line detection of antioxidants in liquid chromatography eluates. J. Chromatogr. A 2001, 912, 73–82. [Google Scholar] [CrossRef]
- Lemanska, K.; Szymusiak, H.; Tyrakowska, B.; Zielinski, R.; Soffers, A.E.M.F.; Rietjens, I.M.C.M. The influence of pH on antioxidant action of hydroxyflavones. Free Radic. Biol. Med. 2001, 31, 869–881. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Li, Y.; Li, Q.; Zheng, Y.F.; Fu, Y.; Li, P. Screening and characterization of natural antioxidants in four Glycyrrhiza species by liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 8181–8191. [Google Scholar] [CrossRef] [PubMed]
- Paz, M.; Gúllon, P.; Barroso, M.F.; Carvalho, A.P.; Domingues, V.F.; Gomes, A.M.; Becker, H.; Longhinotti, E.; Delerue-Matos, C. Brazilian fruit pulps as functional foods and additives: Evaluation of bioactive compounds. Food Chem. 2015, 172, 462–468. [Google Scholar] [CrossRef] [PubMed]
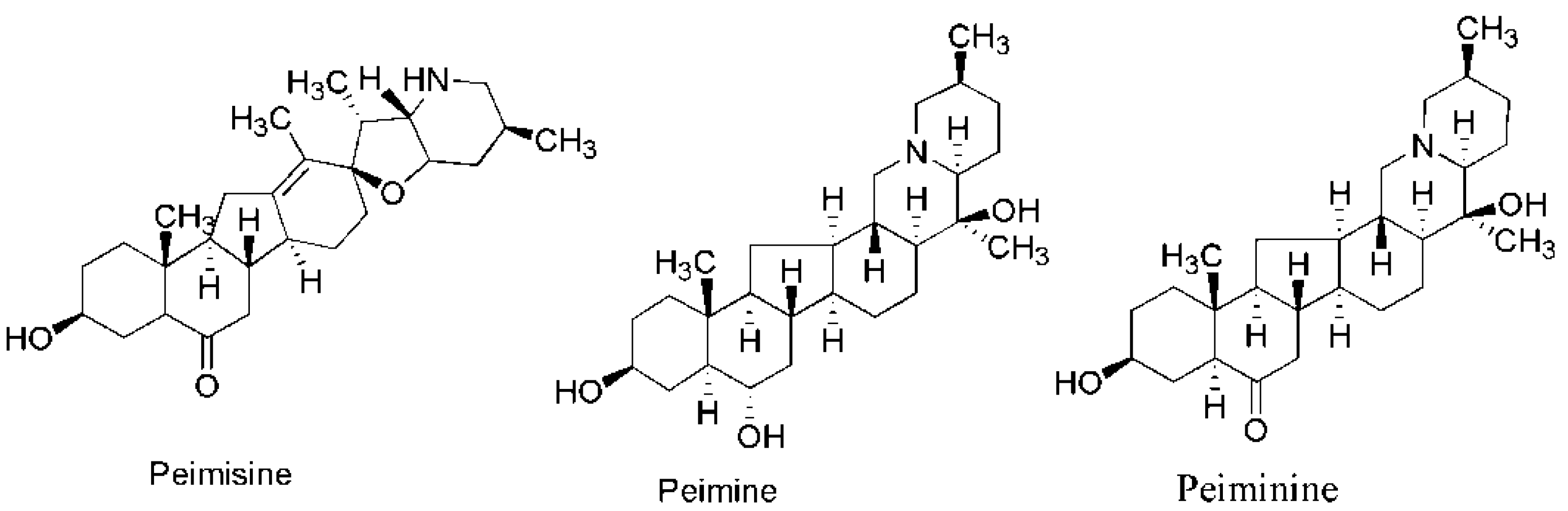

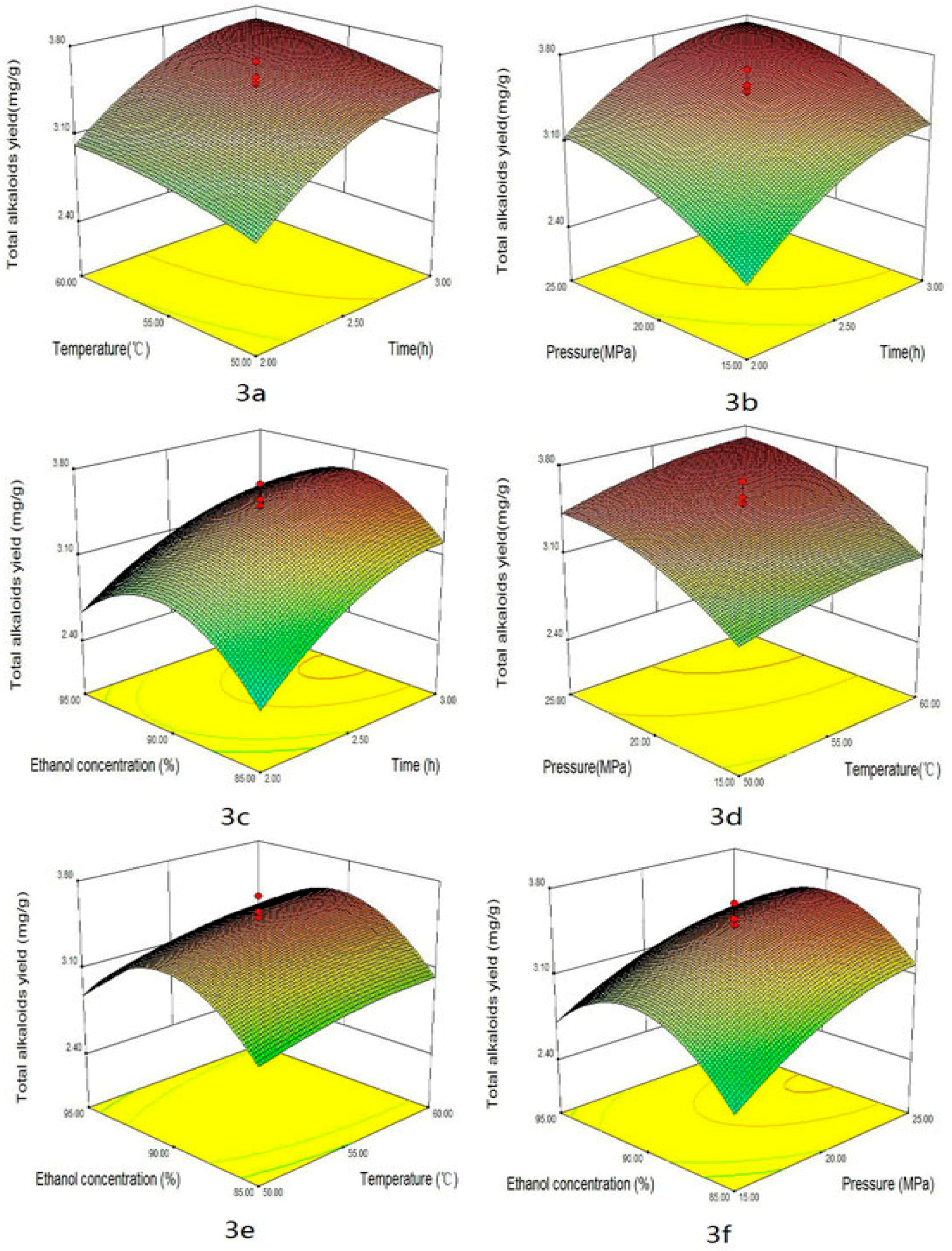
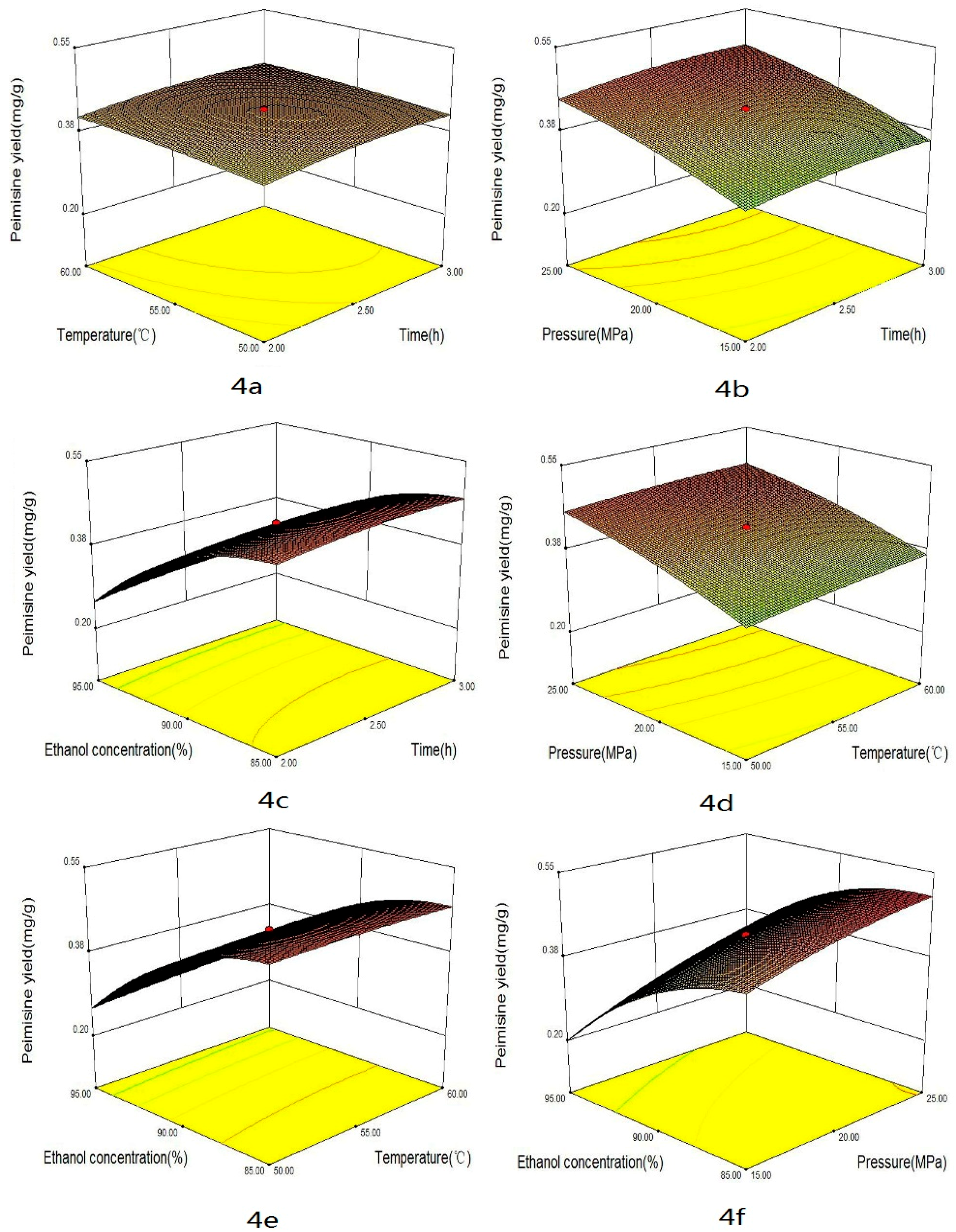
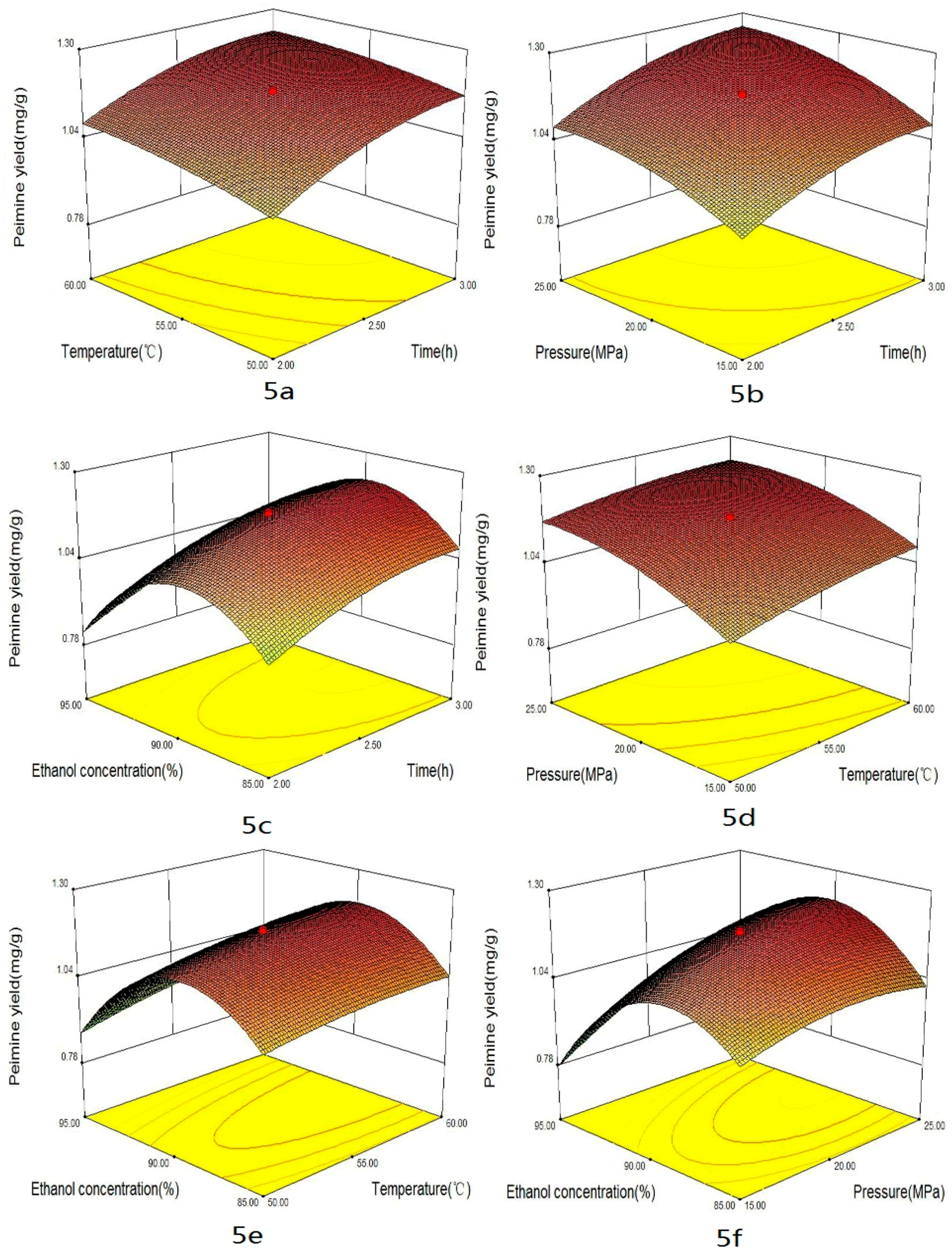

| Trial No. | X1 | X2 | X3 | X4 | Total Alkaloids | Peimisine | Peimine | Peiminine | DPPH | ABTS | FRAP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/g | mg/g | mg/g | mg/g | EC50, mg/mL | EC50, mg/mL | (mg AAE/100 g) | |||||
| 1 | 1 | −1 | 1 | −1 | 3.1 | 0.5 | 1.0 | 0.7 | 16.2 | 1.1 | 87.5 |
| 2 | 0 | 0 | 0 | 0 | 3.4 | 0.4 | 1.2 | 1.2 | 11.6 | 1.3 | 98.2 |
| 3 | 0 | 0 | 0 | 0 | 3.7 | 0.4 | 1.2 | 1.2 | 11.6 | 1.3 | 98.2 |
| 4 | 0 | 0 | 0 | 2 | 1.8 | 0.1 | 0.3 | 0.3 | 33.1 | 6.1 | 52.6 |
| 5 | 0 | 0 | 0 | 0 | 3.3 | 0.4 | 1.2 | 1.1 | 11.6 | 1.3 | 98.2 |
| 6 | −1 | 1 | 1 | −1 | 2.8 | 0.5 | 0.9 | 0.9 | 16.4 | 3.2 | 73.1 |
| 7 | −1 | −1 | −1 | −1 | 1.9 | 0.4 | 0.8 | 0.6 | 33.3 | 0.8 | 58.9 |
| 8 | −1 | −1 | 1 | 1 | 2.6 | 0.3 | 0.8 | 0.7 | 9.3 | 2.5 | 95.9 |
| 9 | −1 | −1 | 1 | −1 | 2.3 | 0.5 | 0.9 | 0.7 | 34.0 | 0.9 | 51.1 |
| 10 | 1 | 1 | 1 | 1 | 3.2 | 0.3 | 1.1 | 1.0 | 7.5 | 0.5 | 102.3 |
| 11 | 1 | 1 | 1 | −1 | 3.4 | 0.5 | 1.1 | 1.1 | 26.4 | 1.7 | 58.4 |
| 12 | 0 | 0 | 0 | 0 | 3.5 | 0.4 | 1.2 | 1.1 | 11.6 | 1.3 | 98.2 |
| 13 | −1 | 1 | 1 | 1 | 2.9 | 0.3 | 0.9 | 0.8 | 26.0 | 0.7 | 60.5 |
| 14 | 1 | 1 | −1 | 1 | 3.0 | 0.2 | 0.9 | 0.9 | 12.1 | 1.7 | 84.6 |
| 15 | 0 | −2 | 0 | 0 | 3.0 | 0.4 | 1.0 | 0.9 | 18.8 | 1.1 | 71.0 |
| 16 | 1 | 1 | −1 | −1 | 2.7 | 0.4 | 1.0 | 0.7 | 24.9 | 8.9 | 57.2 |
| 17 | −1 | 1 | −1 | −1 | 1.8 | 0.4 | 0.9 | 0.6 | 25.9 | 3.5 | 56.8 |
| 18 | 1 | −1 | −1 | 1 | 2.5 | 0.2 | 0.7 | 0.8 | 22.7 | 4.1 | 77.3 |
| 19 | 0 | 0 | −2 | 0 | 2.4 | 0.3 | 0.9 | 0.9 | 4.4 | 1.4 | 113.8 |
| 20 | 0 | 0 | 0 | 0 | 3.4 | 0.4 | 1.2 | 1.2 | 11.6 | 1.3 | 98.2 |
| 21 | −1 | −1 | −1 | 1 | 2.1 | 0.2 | 0.6 | 0.7 | 18.1 | 1.1 | 80.3 |
| 22 | 1 | −1 | −1 | −1 | 2.6 | 0.4 | 1.0 | 0.6 | 8.5 | 0.9 | 126.9 |
| 23 | 2 | 0 | 0 | 0 | 3.6 | 0.4 | 1.2 | 1.2 | 9.9 | 0.2 | 109.0 |
| 24 | −1 | 1 | −1 | 1 | 2.3 | 0.2 | 0.7 | 0.7 | 10.3 | 1.9 | 88.4 |
| 25 | 0 | 0 | 0 | −2 | 1.8 | 0.4 | 0.5 | 0.2 | 50.3 | 12.6 | 28.2 |
| 26 | 0 | 2 | 0 | 0 | 3.6 | 0.4 | 1.2 | 1.1 | 6.9 | 2.4 | 120.4 |
| 27 | 1 | −1 | 1 | 1 | 3.1 | 0.3 | 1.0 | 0.9 | 15.7 | 1.6 | 87.0 |
| 28 | 0 | 0 | 2 | 0 | 3.6 | 0.5 | 1.2 | 1.2 | 15.2 | 0.8 | 63.8 |
| 29 | 0 | 0 | 0 | 0 | 3.6 | 0.4 | 1.2 | 1.2 | 11.6 | 1.3 | 98.2 |
| 30 | −2 | 0 | 0 | 0 | 2.1 | 0.4 | 0.9 | 0.9 | 7.5 | 2.2 | 20.4 |
| Source | Total Alkaloids | Peimisine | Peimine | Peiminine | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sum of Squares | F-Value | p-Value | Sum of Squares | F-Value | p-Value | Sum of Squares | F-Value | p-Value | Sum of Squares | F-Value | p-Value | |
| Model | 10.76 | 41.50 | <0.01 | 0.37 | 2147.68 | <0.01 | 1.37 | 3398.91 | <0.01 | 2.02 | 50.64 | <0.01 |
| X1 | 2.67 | 144.29 | <0.01 | 2.58 × 10−3 | 211.46 | <0.01 | 0.15 | 5113.40 | <0.01 | 0.10 | 36.53 | <0.01 |
| X2 | 0.32 | 17.51 | <0.01 | 1.58 × 10−3 | 129.69 | <0.01 | 0.03 | 1174.37 | <0.01 | 0.08 | 26.28 | <0.01 |
| X3 | 1.89 | 101.86 | <0.01 | 0.07 | 5561.83 | <0.01 | 0.13 | 4687.03 | <0.01 | 0.11 | 40.33 | <0.01 |
| X4 | 0.04 | 2.39 | 0.08 | 0.23 | 18,787.21 | <0.01 | 0.057 | 1998.27 | <0.01 | 0.02 | 5.27 | 0.04 |
| X1X2 | 6.25 × 10−6 | 6.25 × 10−6 | 0.68 | 5.06 × 10−6 | 0.41 | 0.51 | 6.00 × 10−4 | 20.89 | <0.01 | 9.03 × 10−3 | 3.17 | 0.09 |
| X1X3 | 0.02 | 1.17 | 0.26 | 6.81 × 10−5 | 5.57 | 0.32 | 1.02 × 10−3 | 35.63 | <0.01 | 5.63 × 10−3 | 1.98 | 0.18 |
| X1X4 | 0.07 | 3.72 | 0.13 | 3.06 × 10−6 | 0.25 | 0.87 | 3.24 × 10−4 | 11.27 | <0.01 | 0.01 | 3.87 | 0.06 |
| X2X3 | 6.01 × 10−3 | 6.01 × 10−3 | 0.42 | 7.66 × 10−5 | 6.27 | 0.16 | 1.48 × 10−3 | 51.58 | <0.01 | 0.02 | 8.44 | 0.01 |
| X2X4 | 8.56 × 10−3 | 8.56 × 10−3 | 0.52 | 6.63 × 10−4 | 54.28 | 0.10 | 4.56 × 10−3 | 158.54 | <0.01 | 2.03 × 10−3 | 0.71 | 0.41 |
| X3X4 | 0.04 | 2.11 | 0.12 | 3.05 × 10−3 | 249.87 | <0.01 | 0.05 | 1593.52 | <0.01 | 0.04 | 14.76 | <0.01 |
| X12 | 0.88 | 47.36 | <0.01 | 2.05 × 10−3 | 167.42 | <0.01 | 0.04 | 1501.71 | <0.01 | 0.06 | 19.87 | <0.01 |
| X22 | 0.09 | 4.70 | 0.03 | 3.38 × 10−4 | 27.67 | 0.93 | 6.10 × 10−3 | 212.36 | <0.01 | 0.07 | 24.49 | <0.01 |
| X32 | 0.50 | 27.02 | <0.01 | 3.03 × 10−3 | 248.02 | 0.03 | 0.05 | 1578.38 | <0.01 | 0.07 | 24.49 | <0.01 |
| X42 | 5.19 | 280.27 | <0.01 | 0.06 | 4988.22 | <0.01 | 0.93 | 32,195.41 | <0.01 | 1.60 | 562.62 | <0.01 |
| Residual | 0.28 | 1.83 × 10−4 | 4.31 × 10−4 | 0.04 | ||||||||
| Lack of Fit | 0.19 | 1.02 | 0.52 | 1.42 × 10−4 | 1.72 | 0.29 | 1.99 × 10−4 | 0.43 | 0.88 | 0.04 | 4.41 | 0.06 |
| Pure Error | 0.09 | R2 | 0.97 | 4.13 × 10−5 | R2 | 0.99 | 2.31 × 10−4 | R2 | 0.99 | 4.35 × 10−3 | R2 | 0.98 |
| Cor total | 11.04 | 0.37 | 1.37 | 2.06 | ||||||||
| Extraction Condition | Total Alkaloids Yield (mg/g) | Peimisine Yields (mg/g) | Peimine Yields (mg/g) | Peiminine Yields (mg/g) | DPPH EC50(mg/mL) | ABTS EC50 (mg/mL) | FRAP (mg AAE/100 g) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SFE: extraction | 3.8 E | 3.8 P | 0.5 E | 0.5 P | 1.3 E | 1.3 P | 1.3 E | 1.3 P | 5.5 | 0.3 | 118.2 |
| Soxhlet extraction | 3.0 | 0.3 | 1.0 | 1.0 | 8.4 | 1.8 | 58.2 | ||||
| Independent Variables | Model | Symbol Coded Factor Level | ||||
|---|---|---|---|---|---|---|
| −2 | −1 | 0 | +1 | +2 | ||
| Extraction time (h) | X1 | 1.5 | 2 | 2.5 | 3 | 3.5 |
| Extraction temperature (°C) | X2 | 45 | 50 | 55 | 60 | 65 |
| Extraction pressure (MPa) | X3 | 10 | 15 | 20 | 25 | 30 |
| Ethanol concentration (%) | X4 | 80 | 85 | 90 | 95 | 100 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, X.; Yang, L.; Cui, W.-X.; Zhang, M.-X.; Li, Z.-H.; Liu, B.; Wang, Q. Optimization of Supercritical Fluid Extraction of Total Alkaloids, Peimisine, Peimine and Peiminine from the Bulb of Fritillaria thunbergii Miq, and Evaluation of Antioxidant Activities of the Extracts. Materials 2016, 9, 524. https://doi.org/10.3390/ma9070524
Ruan X, Yang L, Cui W-X, Zhang M-X, Li Z-H, Liu B, Wang Q. Optimization of Supercritical Fluid Extraction of Total Alkaloids, Peimisine, Peimine and Peiminine from the Bulb of Fritillaria thunbergii Miq, and Evaluation of Antioxidant Activities of the Extracts. Materials. 2016; 9(7):524. https://doi.org/10.3390/ma9070524
Chicago/Turabian StyleRuan, Xiao, Li Yang, Wen-Xia Cui, Men-Xing Zhang, Zhao-Hui Li, Ben Liu, and Qiang Wang. 2016. "Optimization of Supercritical Fluid Extraction of Total Alkaloids, Peimisine, Peimine and Peiminine from the Bulb of Fritillaria thunbergii Miq, and Evaluation of Antioxidant Activities of the Extracts" Materials 9, no. 7: 524. https://doi.org/10.3390/ma9070524






