Non-Equilibrium Plasma Processing for the Preparation of Antibacterial Surfaces
Abstract
:1. Introduction
1.1. Materials Related Infections and Common Antibacterial Approaches
1.2. Plasma Technology
- Plasma Medicine, i.e., the therapeutic use of cold AP air plasmas on living tissues for non-invasive surgery, wound sterilization and healing, blood clotting, teeth bleaching, cancer treatments, and other applications [35].
- Sterilization and decontamination of materials and devices [36].
- Surface modification of biomaterials, Tissue Engineering scaffolds, biosensors and medical devices [37] aimed to optimize the response of biological entities (proteins, bacteria, cells, fluids, and tissues) in contact with the modified material and drive, and, consequently, their behavior in vitro and in vivo.
2. Non-Fouling Plasma Deposited Coatings
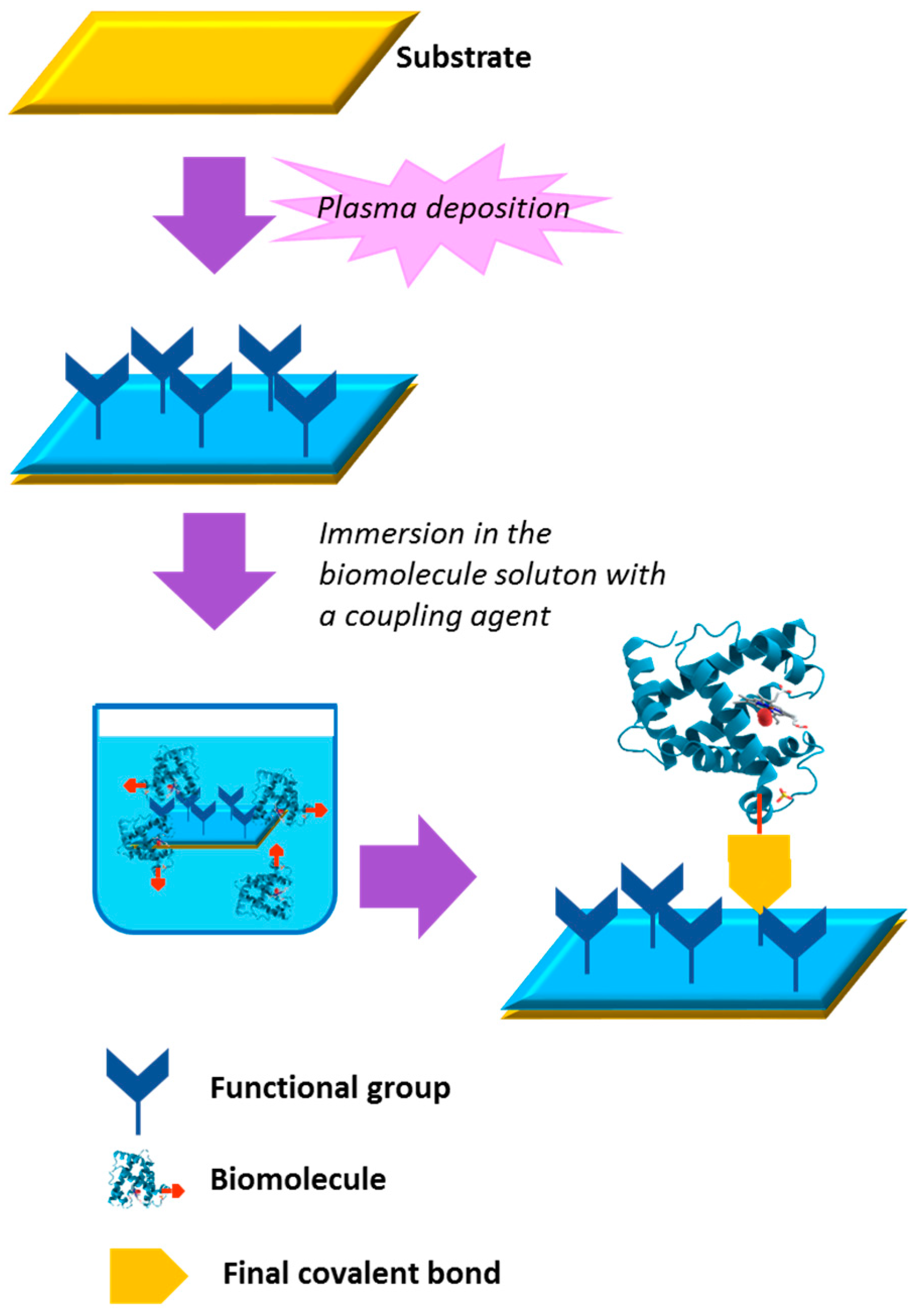
3. Bio-Conjugated Plasma Modified Surfaces
4. Plasma Deposited Composite Coatings Embedding Organic Antibacterial Agents
5. Plasma Deposited Composite Coatings Embedding Inorganic Antibacterial Agents
6. Conclusions and Perspectives
Acknowledgments
Conflicts of Interest
References
- Hetrick, E.M.; Schoenfisch, M.H. Reducing implant-related infections: Active release strategies. Chem. Soc. Rev. 2006, 35, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Speziale, P.; Arciola, C.R. Antibiotic-loaded biomaterials and the risks for the spread of antibiotic resistance following their prophylactic and therapeutic clinical use. Biomaterials 2010, 31, 6363–6377. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Cutter, C.N.; Willett, J.L.; Siragusa, G.R. Improved antimicrobial activity of nisin-incorporated polymer films by formulation change and addition of food grade chelator. Lett. Appl. Microbiol. 2001, 33, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Yan, T.; Du, J.; He, F.; Luo, H.; Wan, Y. Introduction of broad spectrum antibacterial properties to bacterial cellulose nanofibers via immobilising ε-polylysine nanocoatings. Food Hydrocoll. 2014, 36, 204–211. [Google Scholar] [CrossRef]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern approaches to marine antifouling coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef]
- Shi, X.; Zhu, X. Biofilm formation and food safety in food industries. Trends Food Sci. Technol. 2009, 20, 407–413. [Google Scholar] [CrossRef]
- Vasilev, K.; Griesser, S.S.; Griesser, H.J. Antibacterial surfaces and coatings produced by plasma techniques. Plasma Process. Polym. 2011, 8, 1010–1023. [Google Scholar] [CrossRef]
- Thissen, H.; Gengenbach, T.; du Toit, R.; Sweeney, D.F.; Kingshott, P.; Griesser, H.J.; Meagher, L. Clinical observations of biofouling on PEO coated silicone hydrogel contact lenses. Biomaterials 2010, 31, 5510–5519. [Google Scholar] [CrossRef] [PubMed]
- Procaccini, R.A.; Studdert, C.A.; Pellice, S.A. Silver doped silica-methyl hybrid coatings. Structural evolution and antibacterial properties. Surf. Coat. Technol. 2014, 244, 92–97. [Google Scholar] [CrossRef]
- Cometa, S.; Iatta, R.; Ricci, M.A.; Ferretti, C.; De Giglio, E. Analytical characterization and antimicrobial properties of novel copper nanoparticle-loaded electrosynthesized hydrogel coatings. J. Bioact. Compat. Polym. 2013, 28, 508–522. [Google Scholar] [CrossRef]
- De Giglio, E.; Cafagna, D.; Cometa, S.; Allegretta, A.; Pedico, A.; Giannossa, L.C.; Sabbatini, L.; Mattioli-Belmonte, M.; Iatta, R. An innovative, easily fabricated, silver nanoparticle-based titanium implant coating: Development and analytical characterization. Anal. Bioanal. Chem. 2013, 405, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Lucke, M.; Schmidmaier, G.; Sadoni, S.; Wildemann, B.; Schiller, R.; Haas, N.P.; Raschke, M. Gentamicin coating of metallic implants reduces implant-related osteomyelitis in rats. Bone 2003, 32, 521–531. [Google Scholar] [CrossRef]
- Kim, W.H.; Lee, S.B.; Oh, K.T.; Moon, S.K.; Kim, K.M.; Kim, K.N. The release behavior of CHX from polymer-coated titanium surfaces. Surf. Interface Anal. 2008, 40, 202–204. [Google Scholar] [CrossRef]
- Rojas, I.A.; Slunt, J.B.; Grainger, D.W. Polyurethane coatings release bioactive antibodies to reduce bacterial adhesion. J. Control. Release 2000, 63, 175–189. [Google Scholar] [CrossRef]
- Etienne, O.; Picart, C.; Taddei, C.; Haikel, Y.; Dimarcq, J.L.; Schaaf, P.; Voegel, J.C.; Ogier, J.A.; Egles, C. Multilayer polyelectrolyte films functionalized by insertion of defensin: A new approach to protection of implants from bacterial colonization. Antimicrob. Agents Chemother. 2004, 48, 3662–3669. [Google Scholar] [CrossRef] [PubMed]
- Amorosi, C.; Michel, M.; Avérous, L.; Toniazzo, V.; Ruch, D.; Ball, V. Plasma polymer films as an alternative to (PSS-PAH) n or (PSS-PDADMAC) n films to retain active enzymes in exponentially growing polyelectrolyte multilayers. Colloids Surfaces B Biointerfaces 2012, 97, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Boulmedais, F.; Frisch, B.; Etienne, O.; Lavalle, P.; Picart, C.; Ogier, J.; Voegel, J.C.; Schaaf, P.; Egles, C. Polyelectrolyte multilayer films with pegylated polypeptides as a new type of anti-microbial protection for biomaterials. Biomaterials 2004, 25, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Micelles, L.B.C.; Nguyen, P.M.; Zacharia, N.S.; Verploegen, E.; Hammond, P.T. Extended Release Antibacterial Layer-by-Layer Films Incorporating. Mater. Sci. 2007, 02139, 8932–8936. [Google Scholar]
- Simchi, A.; Tamjid, E.; Pishbin, F.; Boccaccini, A.R. Recent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applications. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Barbé, C.J.; Kong, L.; Finnie, K.S.; Calleja, S.; Hanna, J.V.; Drabarek, E.; Cassidy, D.T.; Blackford, M.G. Sol-gel matrices for controlled release: From macro to nano using emulsion polymerisation. J. Sol-Gel Sci. Technol. 2008, 46, 393–409. [Google Scholar] [CrossRef]
- Fan, Y.; Duan, K.; Wang, R. A composite coating by electrolysis-induced collagen self-assembly and calcium phosphate mineralization. Biomaterials 2005, 26, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lin, C.J.; Hu, R.; Zhang, F.; Lin, L.W. A novel nano-micro structured octacalcium phosphate/protein composite coating on titanium by using an electrochemically induced deposition. J. Biomed. Mater. Res. A 2008, 87, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Layrolle, P.; Stigter, M.; De Groot, K. Biomimetic and electrolytic calcium phosphate coatings on titanium alloy: Physicochemical characteristics and cell attachment. Biomaterials 2004, 25, 583–592. [Google Scholar] [CrossRef]
- Chen, R.; Cole, N.; Willcox, M.D.P.; Park, J.; Rasul, R.; Carter, E.; Kumar, N. Synthesis, characterization and in vitro activity of a surface-attached antimicrobial cationic peptide. Biofouling 2009, 25, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Tsuru, K.; Hayakawa, S.; Osaka, A. Preparation of alginic acid layers on stainless-steel substrates for biomedical applications. Biomaterials 2003, 24, 2889–2894. [Google Scholar] [CrossRef]
- Dettin, M.; Bagno, A.; Gambaretto, R.; Iucci, G.; Conconi, M.T.; Tuccitto, N.; Menti, A.M.; Grandi, C.; Di Bello, C.; Licciardello, A.; et al. Covalent surface modification of titanium oxide with different adhesive peptides: Surface characterization and osteoblast-like cell adhesion. J. Biomed. Mater. Res. A 2009, 90, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.J.; Hsieh, M.F.; Huang, C.W.; Perng, L.H.; Wen, H.W.; Chin, T.S. Antimicrobial effects and human gingival biocompatibility of hydroxyapatite sol-gel coatings. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 76, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; He, J.; Wang, C.; Yao, K.; Gou, Z. Copper-containing mesoporous bioactive glass coatings on orbital implants for improving drug delivery capacity and antibacterial activity. Biotechnol. Lett. 2014, 36, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Kurczewska, J.; Sawicka, P.; Ratajczak, M.; Gajęcka, M.; Schroeder, G. Vancomycin-modified silica: Synthesis, controlled release and biological activity of the drug. Int. J. Pharm. 2015, 486, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. Oscillations inionied gases. Phys. Rev. 1928, 14, 627–637. [Google Scholar]
- D’Agostino, R.; Favia, P.; Oehr, C.; Wertheimer, M.R. Low-temperature plasma processing of materials: Past, present, and future. Plasma Process. Polym. 2005, 2, 7–15. [Google Scholar] [CrossRef]
- Plasma 2010 Committee; Plasma Science Committee; National Research Council. Plasma Science: Advancing Knowledge in the National Interest; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Donnelly, V.M.; Kornblit, A. Plasma etching: Yesterday, today, and tomorrow. J. Vac. Sci. Technol. A 2013, 31. [Google Scholar] [CrossRef]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied plasma medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Laroussi, M. Low temperature plasma-based sterilization: Overview and state-of-the-art. Plasma Process. Polym. 2005, 2, 391–400. [Google Scholar] [CrossRef]
- Sardella, E.; Favia, P.; Gristina, R.; Nardulli, M.; d’Agostino, R. Plasma-aided micro- and nanopatterning processes for biomedical applications. Plasma Process. Polym. 2006, 3, 456–469. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef] [PubMed]
- Milella, A.; Di Mundo, R.; Palumbo, F.; Favia, P.; Fracassi, F.; d’Agostino, R. Plasma nanostructuring of polymers: Different routes to superhydrophobicity. Plasma Process. Polym. 2009, 6, 460–466. [Google Scholar] [CrossRef]
- Di Mundo, R.; Gristina, R.; Sardella, E.; Intranuovo, F.; Nardulli, M.; Milella, A.; Palumbo, F.; D’Agostino, R.; Favia, P. Micro-/nanoscale structuring of cell-culture substrates with fluorocarbon plasmas. Plasma Process. Polym. 2010, 7, 212–223. [Google Scholar] [CrossRef]
- Harris, L.G.; Tosatti, S.; Wieland, M.; Textor, M.; Richards, R.G. Staphylococcus aureus adhesion to titanium oxide surfaces coated with non-functionalized and peptide-functionalized poly(l-lysine)-grafted- poly(ethylene glycol) copolymers. Biomaterials 2004, 25, 4135–4148. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Zhu, S.; Ishihara, K.; Brash, J.L. Protein resistant surfaces: Comparison of acrylate graft polymers bearing oligo-ethylene oxide and phosphorylcholine side chains. Biointerphases 2006, 1, 50. [Google Scholar] [CrossRef] [PubMed]
- Choukourov, A.; Gordeev, I.; Polonskyi, O.; Artemenko, A.; Hanyková, L.; Krakovský, I.; Kylián, O.; Slavínská, D.; Biederman, H. Polyethylene (ethylene oxide)-like plasma polymers produced by plasma-assisted vacuum evaporation. Plasma Process. Polym. 2010, 7, 445–458. [Google Scholar] [CrossRef]
- Brétagnol, F.; Lejeune, M.; Papadopoulou-Bouraoui, A.; Hasiwa, M.; Rauscher, H.; Ceccone, G.; Colpo, P.; Rossi, F. Fouling and non-fouling surfaces produced by plasma polymerization of ethylene oxide monomer. Acta Biomater. 2006, 2, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Morra, M. On the molecular basis of fouling resistance. J. Biomater. Sci. Polym. Ed. 2000, 11, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer (Guildf) 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- Griesser, H.J.; Hall, H.; Jenkins, T.A.; Griesser, S.S.; Vasilev, K. Intelligent Surfaces in Biotechnology; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2012; pp. 183–241. [Google Scholar]
- Favia, P.; d’Agostino, R. Plasma treatments and plasma deposition of polymers for biomedical applications. Surf. Coat. Technol. 1998, 98, 1102–1106. [Google Scholar] [CrossRef]
- Sardella, E.; Detomaso, L.; Gristina, R.; Senesi, G.S.; Agheli, H.; Sutherland, D.S.; d’Agostino, R.; Favia, P. Nano-structured cell-adhesive and cell-repulsive plasma-deposited coatings: Chemical and topographical effects on keratinocyte adhesion. Plasma Process. Polym. 2008, 5, 540–551. [Google Scholar] [CrossRef]
- Sardella, E.; Gristina, R.; Ceccone, G.; Gilliland, D.; Papadopoulou-Bouraoui, A.; Rossi, F.; Senesi, G.S.; Detomaso, L.; Favia, P.; d’Agostino, R. Control of cell adhesion and spreading by spatial microarranged PEO-like and pdAA domains. Surf. Coat. Technol. 2005, 200, 51–57. [Google Scholar] [CrossRef]
- Palumbo, F.; Favia, P.; Vulpio, M.; D’Agostino, R. RF plasma deposition of PEO-like films: Diagnostics and process control. Plasmas Polym. 2001, 6, 163–174. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.B.; Andrade, J.D. Blood compatibility of polyethylene oxide surfaces. Prog. Polym. Sci. 1995, 20, 1043–1079. [Google Scholar] [CrossRef]
- Sardella, E.; Gristina, R.; Senesi, G.S.; D’Agostino, R.; Favia, P. Homogeneous and micro-patterned plasma-deposited PEO-like coatings for biomedical surfaces. Plasma Process. Polym. 2004, 1, 63–72. [Google Scholar] [CrossRef]
- Ilhm, L.; Jacobsen, O.; Miiller, R.H.; Mak, E.; Davis, S.S. Surface characteristics and and the interaction of colloidal particles with mouse peritoneal macrophages. Biomaterials 1986, 8, 113–117. [Google Scholar]
- Beyer, D.; Knoll, W.; Ringsdorf, H.; Wang, J.; Timmons, R.B.; Sluka, P. Reduced protein adsorption on plastics via direct plasma deposition of triethylene glycol monoallyl ether. J. Biomed. Mater. Res. 1997, 36, 181–189. [Google Scholar] [CrossRef]
- Favia, P.; Sardella, E.; Gristina, R.; d’Agostino, R. Novel plasma processes for biomaterials: Micro-scale patterning of biomedical polymers. Surf. Coat. Technol. 2003, 169–170, 707–711. [Google Scholar] [CrossRef]
- Krsko, P.; Kaplan, J.B.; Libera, M. Spatially controlled bacterial adhesion using surface-patterned poly(ethylene glycol) hydrogels. Acta Biomater. 2009, 5, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Johnston, E.E.; Bryers, J.D.; Ratner, B.D. Plasma deposition and surface characterization of oligoglyme, dioxane, and crown ether nonfouling films. Langmuir 2005, 21, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Kingshott, P.; Wei, J.; Bagge-Ravn, D.; Gadegaard, N.; Gram, L. Covalent attachment of poly(ethylene glycol) to surfaces, critical for reducing bacterial adhesion. Langmuir 2003, 19, 6912–6921. [Google Scholar] [CrossRef]
- Rodriguez-Emmenegger, C.; Kylián, O.; Houska, M.; Brynda, E.; Artemenko, A.; Kousal, J.; Alles, A.B.; Biederman, H. Substrate-independent approach for the generation of functional protein resistant surfaces. Biomacromolecules 2011, 12, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Şen, Y.; Baǧci, U.; Güleç, H.A.; Mutlu, M. Modification of Food-Contacting Surfaces by Plasma Polymerization Technique: Reducing the Biofouling of Microorganisms on Stainless Steel Surface. Food Bioprocess Technol. 2012, 5, 166–175. [Google Scholar] [CrossRef]
- Li, M.S.; Zhao, Z.P.; Wang, M.X.; Zhang, Y. Controllable modification of polymer membranes by LDDLT plasma flow: Antibacterial layer onto PE hollow fiber membrane module. Chem. Eng. J. 2015, 265, 16–26. [Google Scholar] [CrossRef]
- Johnston, E.; Bryers, J.E.; Ratner, B.D. Interaction between Pseudomonas aeuginosa and plasma-deposited PEO-like thin films during initial attachment and growth. Am. Chem. Soc. Polym. Prepr. Division Polym. Chem. 1997, 38, 1016–1017. [Google Scholar]
- Stoffels, E. “Tissue processing” with atmospheric plasmas. Contrib. Plasma Phys. 2007, 47, 40–48. [Google Scholar] [CrossRef]
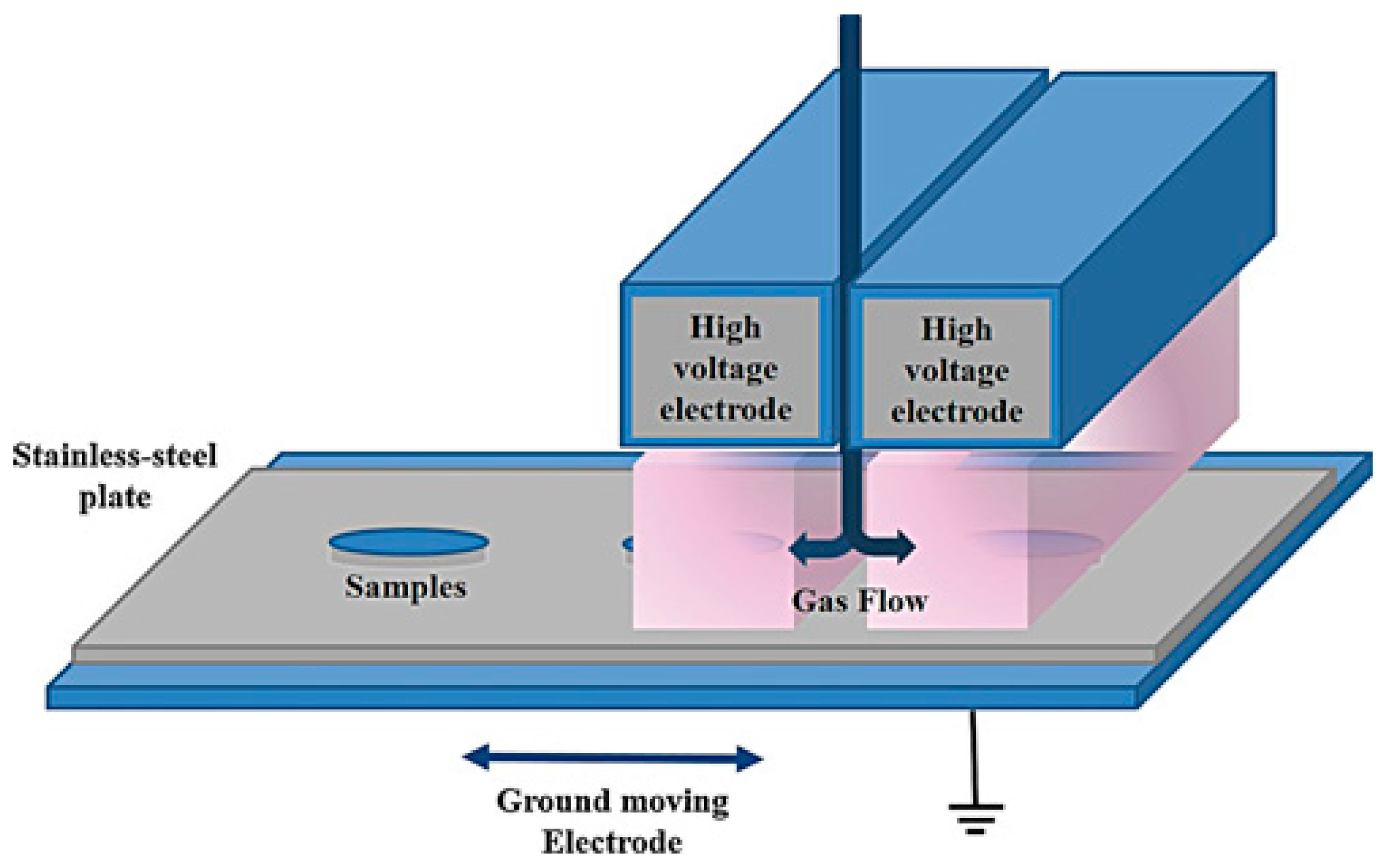
- Da Ponte, G.; Sardella, E.; Fanelli, F.; D’Agostino, R.; Gristina, R.; Favia, P. Plasma deposition of PEO-like coatings with aerosol-assisted dielectric barrier discharges. Plasma Process. Polym. 2012, 9, 1176–1183. [Google Scholar] [CrossRef]
- Da Ponte, G.; Sardella, E.; Fanelli, F.; Van Hoeck, A.; d’Agostino, R.; Paulussen, S.; Favia, P. Atmospheric pressure plasma deposition of organic films of biomedical interest. Surf. Coat. Technol. 2011, 205, S525–S528. [Google Scholar] [CrossRef]
- Mauchauffé, R.; Moreno-Couranjou, M.; Boscher, N.D.; Van De Weerdt, C.; Duwez, A.S.; Choquet, P. Robust bio-inspired antibacterial surfaces based on the covalent binding of peptides on functional atmospheric plasma this films. J. Mater. Chem. B. 2014, 2, 5168–5177. [Google Scholar] [CrossRef]
- Quintieri, L.; Pistillo, B.R.; Caputo, L.; Favia, P.; Baruzzi, F. Bovine lactoferrin and lactoferricin on plasma-deposited coating against spoilage Pseudomonas spp. Innov. Food Sci. Emerg. Technol. 2013, 20, 215–222. [Google Scholar] [CrossRef]
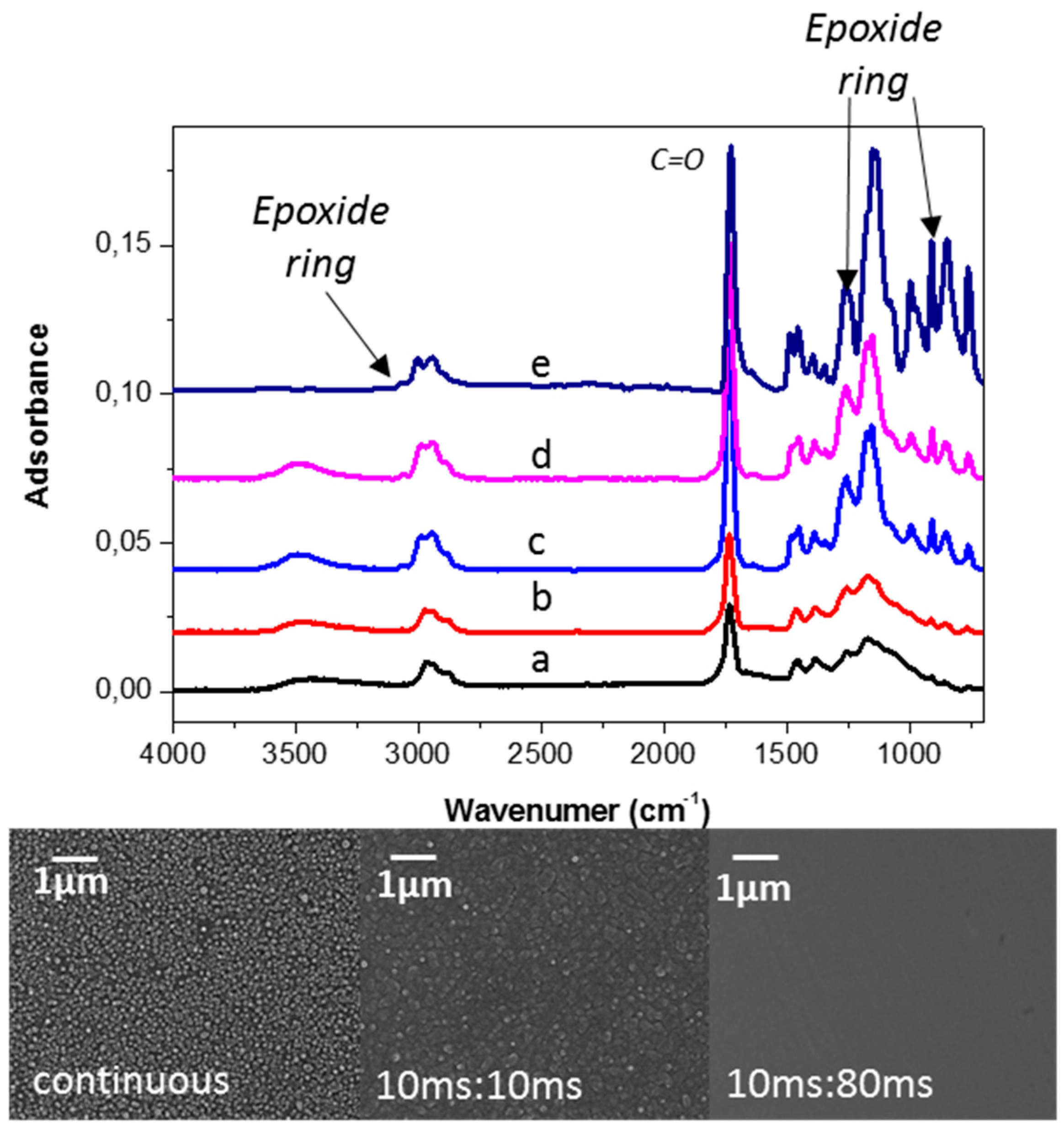
- Camporeale, G.; Moreno-Couranjou, M.; Bonot, S.; Mauchauffé, R.; Boscher, N.D.; Bebrone, C.; Van de Weerdt, C.; Cauchie, H.M.; Favia, P.; Choquet, P. Atmospheric-Pressure Plasma Deposited Epoxy-Rich Thin Films as Platforms for Biomolecule Immobilization-Application for Anti-Biofouling and Xenobiotic-Degrading Surfaces. Plasma Process. Polym. 2015, 1208–1219. [Google Scholar] [CrossRef]
- Jampala, S.N.; Sarmadi, M.; Somers, E.B.; Wong, A.C.L.; Denes, F.S. Plasma-enhanced synthesis of bactericidal quaternary ammonium thin layers on stainless steel and cellulose surfaces. Langmuir 2008, 24, 8583–8591. [Google Scholar] [CrossRef] [PubMed]
- Schofield, W.C.E.; Badyal, J.P.S. A substrate-independent approach for bactericidal surfaces. ACS Appl. Mater. Interfaces 2009, 1, 2763–2767. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E. Cationic peptides: Effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 2001, 1, 156–164. [Google Scholar] [CrossRef]
- Magliulo, M.; Mallardi, A.; Mulla, M.Y.; Cotrone, S.; Pistillo, B.R.; Favia, P.; Vikholm-Lundin, I.; Palazzo, G.; Torsi, L. Electrolyte-gated organic field-effect transistor sensors based on supported biotinylated phospholipid bilayer. Adv. Mater. 2013, 25, 2090–2094. [Google Scholar] [CrossRef] [PubMed]
- De Bartolo, L.; Morelli, S.; Piscioneri, A.; Lopez, L.C.; Favia, P.; d’Agostino, R.; Drioli, E. Novel membranes and surface modification able to activate specific cellular responses. Biomol. Eng. 2007, 24, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Favia, P.; Palumbo, F.; d’Agostino, R.; Lamponi, S.; Magnani, A.; Barbucci, R. Immobilization of Heparin and Highly-Sulphated Hyaluronic Acid onto Plasma-Treated Polyethylene. Plasmas Polym. 1998, 3, 77–96. [Google Scholar] [CrossRef]
- Kastellorizios, M.; Michanetzis, G.P.A.K.; Pistillo, B.R.; Mourtas, S.; Klepetsanis, P.; Favia, P.; Sardella, E.; D’Agostino, R.; Missirlis, Y.F.; Antimisiaris, S.G. Haemocompatibility improvement of metallic surfaces by covalent immobilization of heparin-liposomes. Int. J. Pharm. 2012, 432, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.C.; Gristina, R.; Ceccone, G.; Rossi, F.; Favia, P.; d’Agostino, R. Immobilization of RGD peptides on stable plasma-deposited acrylic acid coatings for biomedical devices. Surf. Coat. Technol. 2005, 200, 1000–1004. [Google Scholar] [CrossRef]
- Bazaka, K.; Jacob, M.V.; Chrzanowski, W.; Ostrikov, K. Anti-bacterial surfaces: Natural agents, mechanisms of action, and plasma surface modification. RSC Adv. 2015, 5, 48739–48759. [Google Scholar] [CrossRef]
- Heyse, P.; Roeffaers, M.B.J.; Paulussen, S.; Hofkens, J.; Jacobs, P.A.; Sels, B.F. Protein immobilization using atmospheric-pressure dielectric-barrier discharges: A route to a straightforward manufacture of bioactive films. Plasma Process. Polym. 2008, 5, 186–191. [Google Scholar] [CrossRef]
- Heyse, P.; Van Hoeck, A.; Roeffaers, M.B.J.; Raffin, J.P.; Steinbüchel, A.; Stöveken, T.; Lammertyn, J.; Verboven, P.; Jacobs, P.A.; Hofkens, J.; et al. Exploration of atmospheric pressure plasma nanofilm technology for straightforward bio-active coating deposition: Enzymes, plasmas and polymers, an elegant synergy. Plasma Process. Polym. 2011, 8, 965–974. [Google Scholar] [CrossRef]
- Da Ponte, G.; Sardella, E.; Fanelli, F.; Paulussen, S.; Favia, P. Atmospheric pressure plasma deposition of poly lactic acid-like coatings with embedded elastin. Plasma Process. Polym. 2014, 11, 345–352. [Google Scholar] [CrossRef]
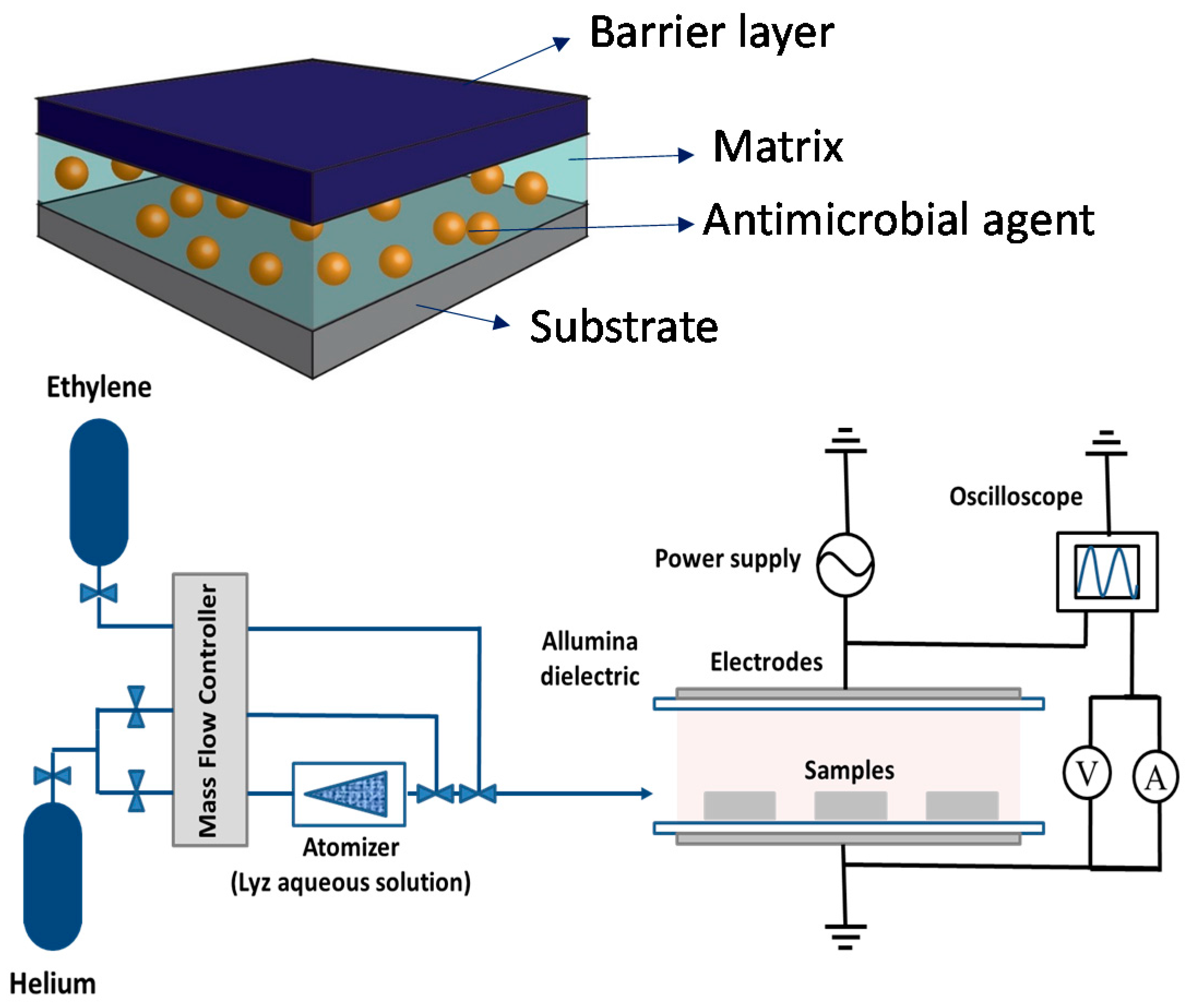
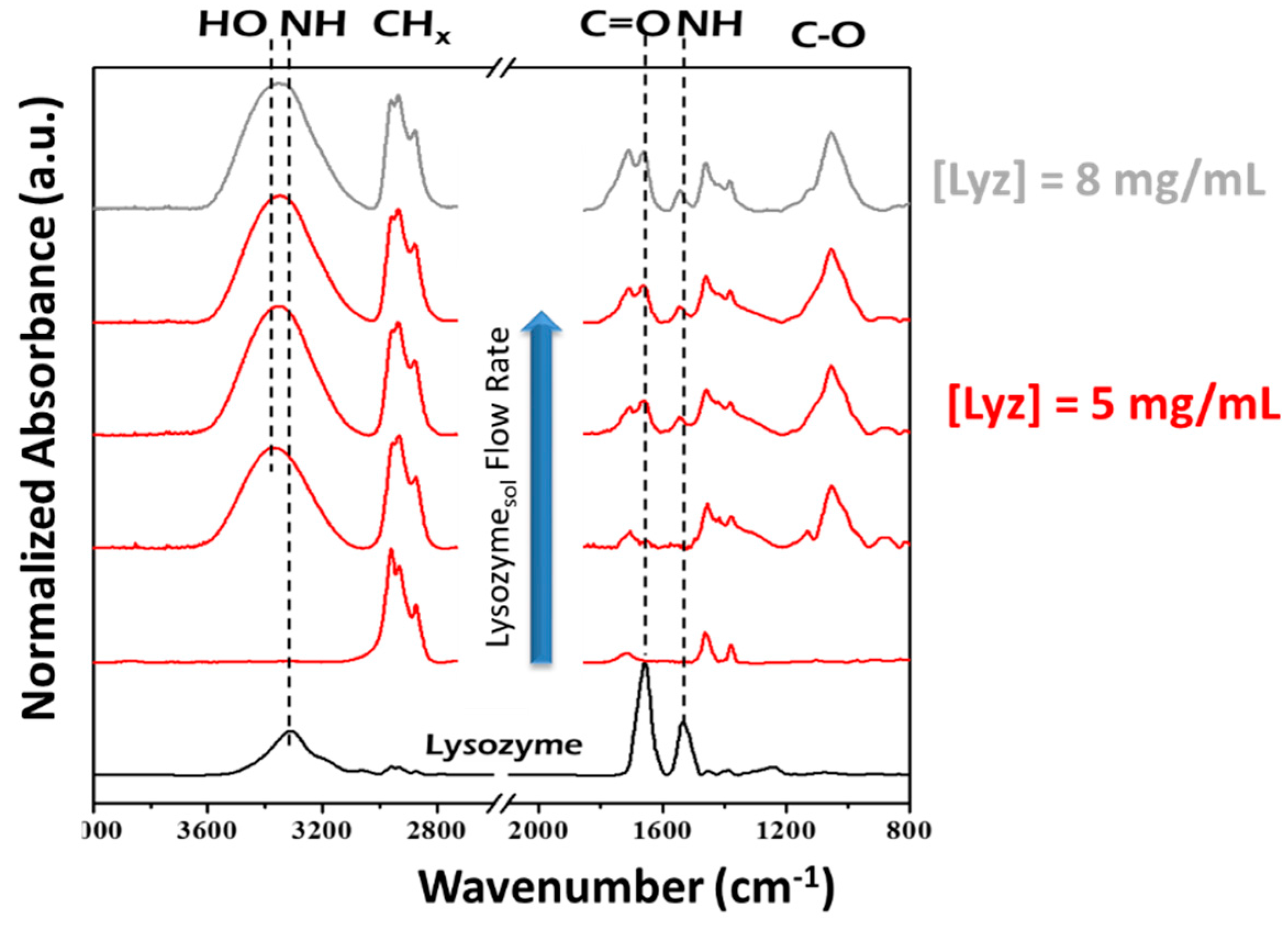
- Palumbo, F.; Camporeale, G.; Yang, Y.W.; Wu, J.S.; Sardella, E.; Dilecce, G.; Calvano, C.D.; Quintieri, L.; Caputo, L.; Baruzzi, F.; et al. Direct Plasma Deposition of Lysozyme-Embedded Bio-Composite Thin Films. Plasma Process. Polym. 2015, 12, 1302–1310. [Google Scholar] [CrossRef]
- Yang, Y.W.; Camporeale, G.; Sardella, E.; Dilecce, G.; Wu, J.S.; Palumbo, F.; Favia, P. Deposition of hydroxyl functionalized films by means of ethylene aerosol-assisted atmospheric pressure plasma. Plasma Process. Polym. 2014, 11, 1102–1111. [Google Scholar] [CrossRef]
- Palza, H. Antimicrobial polymers with metal nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [PubMed]
- Sintubin, L.; De Windt, W.; Dick, J.; Mast, J.; Van Der Ha, D.; Verstraete, W.; Boon, N. Lactic acid bacteria as reducing and capping agent for the fast and efficient production of silver nanoparticles. Appl. Microbiol. Biotechnol. 2009, 84, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Rock, C.O. Membrane lipid homeostasis in bacteria. Nat. Rev. Microbiol. 2008, 6, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S.; Hans, M.; Mücklich, F.; Solioz, M. Contact killing of bacteria on copper is suppressed if bacterial-metal contact is prevented and is induced on iron by copper ions. Appl. Environ. Microbiol. 2013, 79, 2605–2611. [Google Scholar] [CrossRef] [PubMed]
- Mulley, G.; Jenkins, A.T.A.; Waterfield, N.R. Inactivation of the antibacterial and cytotoxic properties of silver ions by biologically relevant compounds. PLoS ONE 2014, 9, 2–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.A.; Lynch, I. Mechanisms of silver nanoparticle release, transformation and toxicity: A critical review of current knowledge and recommendations for future studies and applications. Materials 2013, 6, 2295–2350. [Google Scholar] [CrossRef]
- Bayston, R.; Vera, L.; Mills, A.; Ashraf, W.; Stevenson, O.; Howdle, S.M. In vitro antimicrobial activity of silver-processed catheters for neurosurgery. J. Antimicrob. Chemother. 2009, 65, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Kvitek, L.; Panacek, A.; Prucek, R.; Soukupova, J.; Vanickova, M.; Kolar, M.; Zboril, R. Antibacterial activity and toxicity of silver—Nanosilver versus ionic silver. J. Phys. Conf. Ser. 2011, 304, 012029. [Google Scholar] [CrossRef]
- Bruellhoff, K.; Fiedler, J.; Möller, M.; Groll, J.; Brenner, R.E. Surface coating strategies to prevent biofilm formation on implant surfaces. Int. J. Artif. Organs 2010, 33, 646–653. [Google Scholar] [PubMed]
- Deng, X.; Nikiforov, A.Y.; Coenye, T.; Cools, P.; Aziz, G.; Morent, R.; De Geyter, N.; Leys, C. Antimicrobial nano-silver non-woven polyethylene terephthalate fabric via an atmospheric pressure plasma deposition process. Sci. Rep. 2015, 5, 10138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Nikiforov, A.; Vujosevic, D.; Vuksanovic, V.; Mugoša, B.; Cvelbar, U.; De Geyter, N.; Morent, R.; Leys, C. Antibacterial activity of nano-silver non-woven fabric prepared by atmospheric pressure plasma deposition. Mater. Lett. 2015, 149, 95–99. [Google Scholar] [CrossRef]
- Zille, A.; Fernandes, M.M.; Francesko, A.; Tzanov, T.; Fernandes, M.; Oliveira, F.R.; Almeida, L.; Amorim, T.; Carneiro, N.; Esteves, M.F.; et al. Size and Aging Effects on Antimicrobial Efficiency of Silver Nanoparticles Coated on Polyamide Fabrics Activated by Atmospheric DBD Plasma. ACS Appl. Mater. Interfaces 2015, 7, 13731–13744. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, K.; Sah, V.; Anselme, K.; Ndi, C.; Mateescu, M.; Dollmann, B.; Martinek, P.; Ys, H.; Ploux, L.; Griesser, H.J. Tunable antibacterial coatings that support mammalian cell growth. Nano Lett. 2010, 10, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Ploux, L.; Mateescu, M.; Anselme, K.; Vasilev, K. Antibacterial properties of silver-loaded plasma polymer coatings. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef]
- Kumar, V.; Jolivalt, C.; Pulpytel, J.; Jafari, R.; Arefi-Khonsari, F. Development of silver nanoparticle loaded antibacterial polymer mesh using plasma polymerization process. J. Biomed. Mater. Res. A 2013, 101A, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.C.; Hess, D.W. Dissolution and swelling behaviour of plasma-polymerized polyethylene glycol-like hydrogel films for use as drug-delivery reservoir. ECS Trans. 2008, 6, 1–12. [Google Scholar]
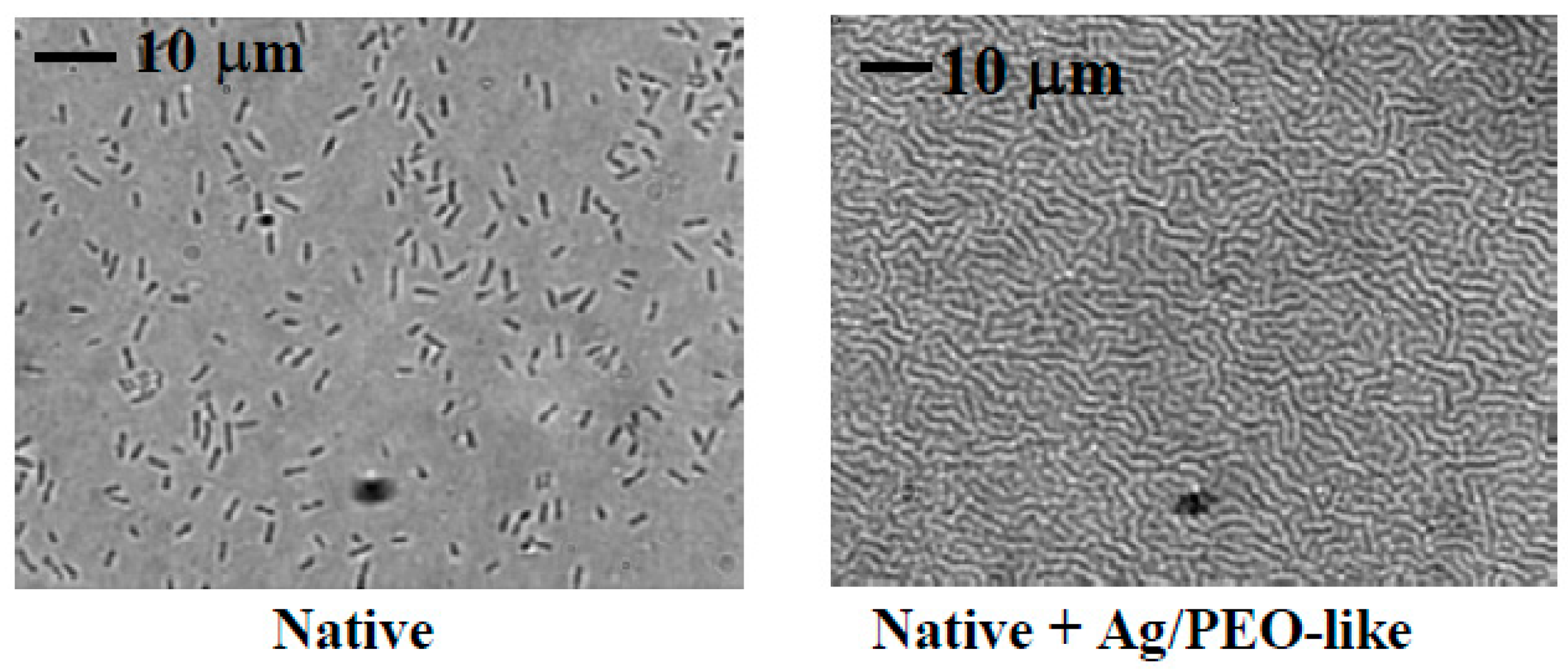
- Balazs, D.J.; Triandafillu, K.; Sardella, E.; Iacoviello, G.; Favia, P.; d’Agostino, R.; Harms, H.; Mathieu, H.J. PECVD modification of PVC endotracheal tubes to inhibit bacterial adhesion: PEO-like and nano-composite Ag/PEO-like coatings. In Plasma Processes and Polymers; d’Agostino, R., Favia, P., Oher, C., Wertheimer, M.R., Eds.; Wiley VCH: Weinheim, Germany, 2005; pp. 351–372. [Google Scholar]
- Liu, X.; Zhang, C.; Yang, J.; Lin, D.; Zhang, L.; Chen, X.; Zha, L. Silver nanoparticles loading pH responsive hybrid microgels: pH tunable plasmonic coupling demonstrated by surface enhanced Raman scattering. RSC Adv. 2013, 3, 3384. [Google Scholar] [CrossRef]
- Song, W.-H.; Ryu, H.S.; Hong, S.-H. Antibacterial properties of Ag (or Pt)-containing calcium phosphate coatings formed by micro-arc oxidation. J. Biomed. Mater. Res. A 2009, 88, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, W.; Qiao, Y.; Jiang, X.; Liu, X.; Ding, C. Antibacterial activity and increased bone marrow stem cell functions of Zn-incorporated TiO2 coatings on titanium. Acta Biomater. 2012, 8, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Duque, L.; Förch, R. Plasma polymerization of zinc acetyl acetonate for the development of a polymer-based zinc release system. Plasma Process. Polym. 2011, 8, 444–451. [Google Scholar] [CrossRef]
- Poulter, N.; Munoz-Berbel, X.; Johnson, A.L.; Dowling, A.J.; Waterfield, N.; Jenkins, A.T.A. An organo-silver compound that shows antimicrobial activity against Pseudomonas aeruginosa as a monomer and plasma deposited film. Chem. Commun. (Camb.) 2009, 2009, 7312–7314. [Google Scholar] [CrossRef] [PubMed]
- Liguori, A.; Traldi, E.; Toccaceli, E.; Laurita, R.; Pollicino, A.; Focarete, M.L.; Colombo, V.; Gherardi, M. Co-Deposition of Plasma-Polymerized Polyacrylic Acid and Silver Nanoparticles for the Production of Nanocomposite Coatings Using a Non-Equilibrium Atmospheric Pressure Plasma Jet. Plasma Process. Polym. 2015, 13, 623–632. [Google Scholar] [CrossRef]
- Beier, O.; Pfuch, A.; Horn, K.; Weisser, J.; Schnabelrauch, M.; Schimanski, A. Low temperature deposition of antibacterially active silicon oxide layers containing silver nanoparticles, prepared by atmospheric pressure plasma chemical vapor deposition. Plasma Process. Polym. 2013, 10, 77–87. [Google Scholar] [CrossRef]
- Deng, X.; Leys, C.; Vujosevic, D.; Vuksanovic, V.; Cvelbar, U.; De Geyter, N.; Morent, R.; Nikiforov, A. Engineering of Composite Organosilicon Thin Films with Embedded Silver Nanoparticles via Atmospheric Pressure Plasma Process for Antibacterial Activity. Plasma Process. Polym. 2014, 11, 921–930. [Google Scholar] [CrossRef]
- Choi, H.W.; Dauskardt, R.H.; Lee, S.-C.; Lee, K.-R.; Oh, K.H. Characteristic of silver doped DLC films on surface properties and protein adsorption. Diam. Relat. Mater. 2008, 17, 252–257. [Google Scholar] [CrossRef]
- Baba, K.; Hatada, R.; Flege, S.; Ensinger, W.; Shibata, Y.; Nakashima, J.; Sawase, T.; Morimura, T. Preparation and antibacterial properties of Ag-containing diamond-like carbon films prepared by a combination of magnetron sputtering and plasma source ion implantation. Vacuum 2013, 89, 179–184. [Google Scholar] [CrossRef]
- Saulou, C.; Despax, B.; Raynaud, P.; Zanna, S.; Seyeux, A.; Marcus, P.; Audinot, J.N.; Mercier-Bonin, M. Plasma-mediated nanosilver-organosilicon composite films deposited on stainless steel: Synthesis, surface characterization, and evaluation of anti-adhesive and anti-microbial properties on the model yeast saccharomyces cerevisiae. Plasma Process. Polym. 2012, 9, 324–338. [Google Scholar] [CrossRef]
- Körner, E.; Aguirre, M.H.; Fortunato, G.; Ritter, A.; Rühe, J.; Hegemann, D. Formation and distribution of silver nanoparticles in a functional plasma polymer matrix and related Ag+ release properties. Plasma Process. Polym. 2010, 7, 619–625. [Google Scholar] [CrossRef]
- Chakravadhanula, V.S.K.; Kübel, C.; Hrkac, T.; Zaporojtchenko, V.; Strunskus, T.; Faupel, F.; Kienle, L. Surface segregation in TiO2-based nanocomposite thin films. Nanotechnology 2012, 23, 495701. [Google Scholar] [CrossRef] [PubMed]
- Drábik, M.; Pešička, J.; Biederman, H.; Hegemann, D. Long-term aging of Ag/a-C:H:O nanocomposite coatings in air and in aqueous environment. Sci. Technol. Adv. Mater. 2015, 16, 025005. [Google Scholar] [CrossRef]
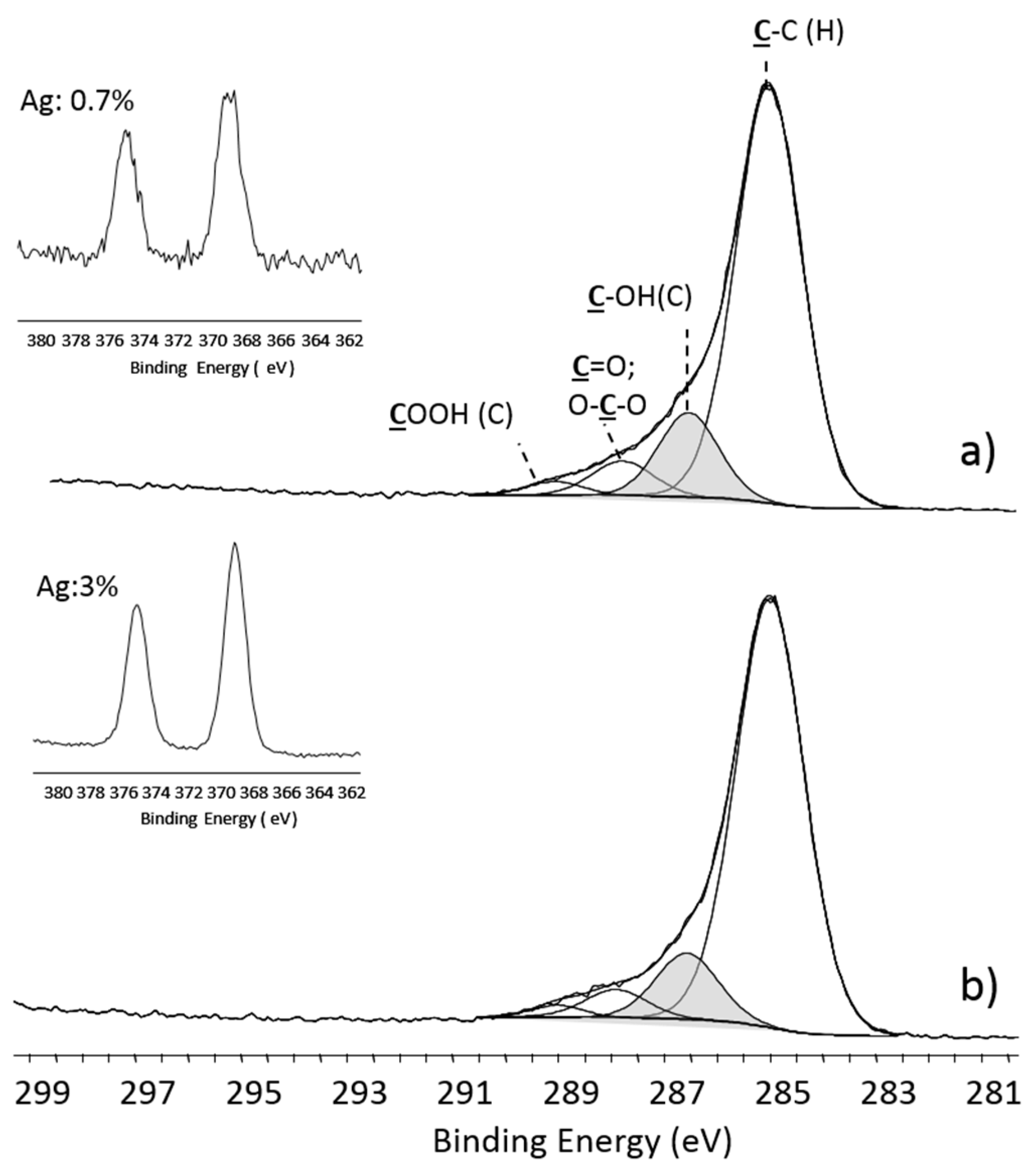
- Escobar Galindo, R.; Manninen, N.K.; Palacio, C.; Carvalho, S. Advanced surface characterization of silver nanocluster segregation in Ag-TiCN bioactive coatings by RBS, GDOES, and ARXPS. Anal. Bioanal. Chem. 2013, 405, 6259–6269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
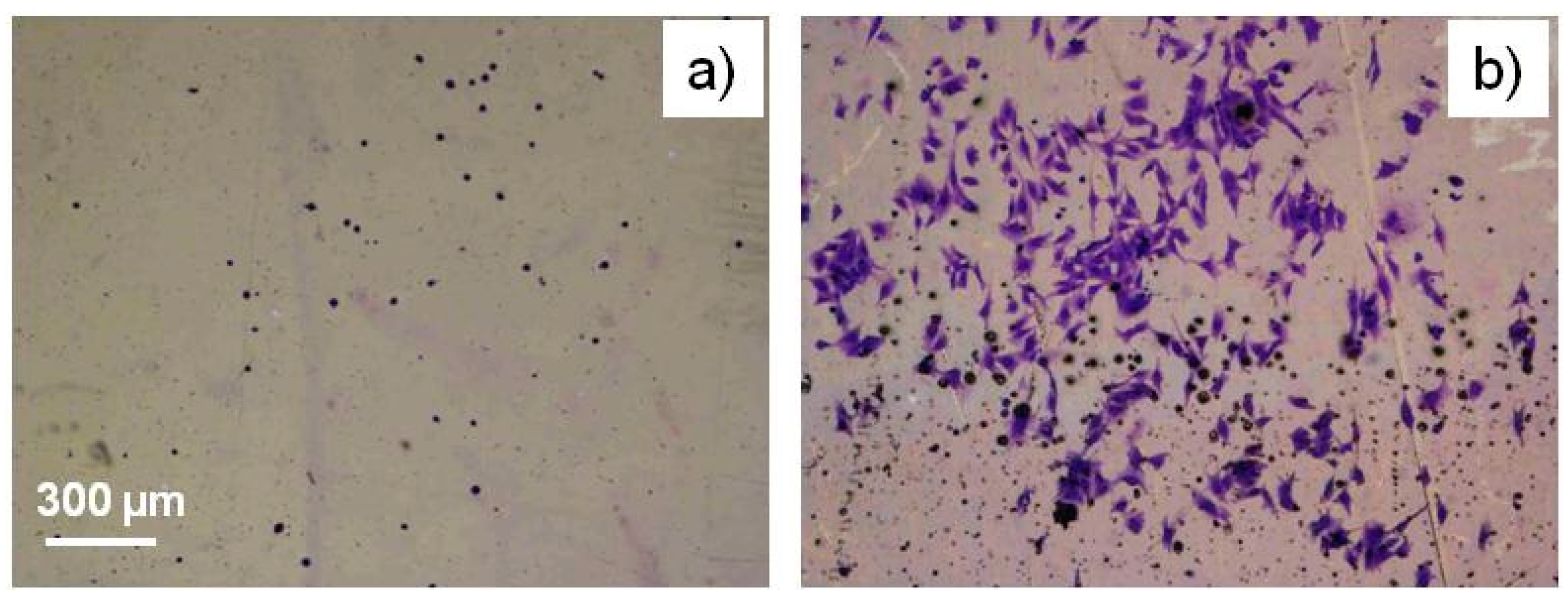
- Katsikogianni, M.G.; Foka, A.; Sardella, E.; Ingrosso, C.; Favia, P.; Mangone, A.; Spiliopoulou, I.; Missirlis, Y.F. Fluid Flow and Sub-Bactericidal Release of Silver from Organic Nanocomposite Coatings Enhance ica Operon Expression in Staphylococcus epidermidis. Sci. Res. 2013, 4, 30–40. [Google Scholar]
- Kajiyama, S.; Tsurumoto, T.; Osaki, M.; Yanagihara, K.; Shindo, H. Quantitative analysis of Staphylococcus epidermidis biofilm on the surface of biomaterial. J. Orthop. Sci. 2009, 14, 769–775. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, R.; Favia, P.; Fracassi, F.; Sardella, E.; Costigliola, A.; Mangone, A. Processes for the Production by Plasma of Nanometric Thickness Coatings Allowing Controlled Release of Silver Ions of Other Elements, or of Molecules of Biomedical Interest, from Solid Products, and Products thus Coated. Patent WO 2013021409 A8, 4 April 2013. [Google Scholar]
- Kvítek, L.; Panácek, A.; Soukupova, J.; Kolar, M.; Vecerova, R.; Prucek, R.; Holecova, M.; Zboril, R.; Kvı, L.; Vec, R.; et al. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles. J. Phys. Chem. 2008, 112, 5825–5834. [Google Scholar] [CrossRef]
- Laroussi, M. From killing bacteria to destroying cancer cells: 20 years of plasma medicine. Plasma Process. Polym. 2014, 11, 1138–1141. [Google Scholar] [CrossRef]
- Randeniya, L.K.; De Groot, G.J.J.B. Non-Thermal Plasma Treatment of Agricultural Seeds for Stimulation of Germination, Removal of Surface Contamination and Other Benefits: A Review. Plasma Process. Polym. 2015, 12, 608–623. [Google Scholar] [CrossRef]


| Drug Delivery Systems | |||
| Technology | Antibacterial Agent | Bacterial Target | Ref. |
| Drop Casting/Dip Coating | Chlorexidin | - | [14] |
| Immunoglobulin G | Escherichia coli | [15] | |
| Layer-by-Layer | Defensin | Micrococcus luteus E. coli | [16] |
| PEGylated polylysine | E. coli | [18] | |
| Triclosan | Staphylococcus aureus | [19] | |
| Sol-gel | Ag+/Zn2+ | Staphylococcus mutans | [28] |
| CuO | S. aureus E. coli | [29] | |
| Electrochemical Deposition | Cu2+ | S. aureus E. coli | [11] |
| Ag+ | S. aureus Pseudomonas. Aeruginosa E. coli | [12] | |
| Collagen | - | [22] | |
| Penicillin/Streptomicin | - | [23] | |
| Covalent Immobilization | |||
| Surface Reactive groups | Antibacterial Ingredient | Bacterial target | Ref. |
| -COOH/-F | Melimine | P. aeruginosa S. aureus | [25] |
| -NH2 | Algnic acid | - | [26] |
| -COCl | Vancomycin | S. aureus | [30] |
| Samples | Reduction of Adherent Population [%] |
|---|---|
| Stainless steel | 0 |
| ppGMA layers | 0 |
| Dsp B immobilized at pH 8.5 | 84 ± 11 |
| Released Lysozyme [µg/mL] | 15 Min | 1 Day | 7 Days | |
| Without barrier coating | 20 | 28 | - | |
| With barrier coating | 2 | 15 | 18 |
| Sample | Inhibition Halo Diameter [mm] |
|---|---|
| C2H4/Lyzsol coating | 8 ± 1 |
| C2H4/H2O plasma deposited coating (control) | 0 |
| Lyz std solution (10 µg/mL) | 0 |
| Lyz std solution (30 µg/mL) | 6 ± 1 |
| Lyz std solution (300 µg/mL) | 12 ± 1 |
| Blank (negative control) | 0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sardella, E.; Palumbo, F.; Camporeale, G.; Favia, P. Non-Equilibrium Plasma Processing for the Preparation of Antibacterial Surfaces. Materials 2016, 9, 515. https://doi.org/10.3390/ma9070515
Sardella E, Palumbo F, Camporeale G, Favia P. Non-Equilibrium Plasma Processing for the Preparation of Antibacterial Surfaces. Materials. 2016; 9(7):515. https://doi.org/10.3390/ma9070515
Chicago/Turabian StyleSardella, Eloisa, Fabio Palumbo, Giuseppe Camporeale, and Pietro Favia. 2016. "Non-Equilibrium Plasma Processing for the Preparation of Antibacterial Surfaces" Materials 9, no. 7: 515. https://doi.org/10.3390/ma9070515







