Silk Fiber as the Support and Reductant for the Facile Synthesis of Ag–Fe3O4 Nanocomposites and Its Antibacterial Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Fe3O4–Silk Fiber
2.3. Preparation of the Ag–Fe3O4–Silk Fiber and Ag–Silk Fiber
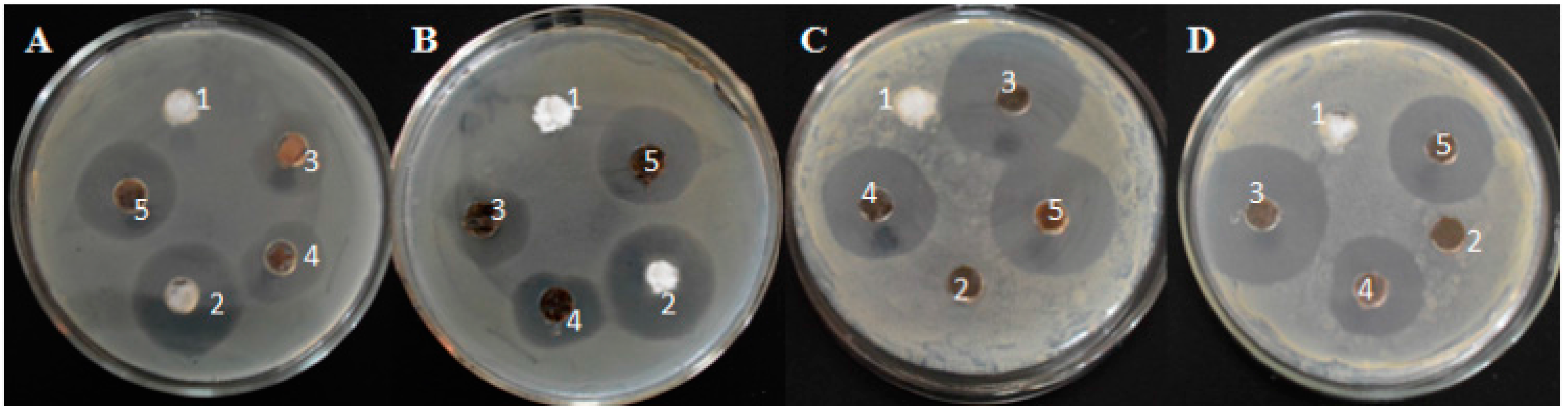
2.4. Antimicrobial Activity of the Ag–Fe3O4–Silk Fiber and Ag–Silk Fiber
2.5. Characterization Methods
2.6. Quantification of Relesased Ag+ by ICP-MS
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carlsson, D.C.; Nystrom, G.; Zhou, Q.; Berglund, L.A.; Nyholm, L.; Strømme, M.M. Electroactive nanofibrillated cellulose aerogel composites with tunable structural and electrochemical properties. J. Mater. Chem. 2012, 22, 19014–19024. [Google Scholar] [CrossRef]
- Dallas, P.; Tucek, J.; Jancik, D.; Kolar, M.; Panacek, A.; Zboril, R. Magnetically controllable silver nanocomposite with multifunctional phosphotriazine matrix and high antimicrobial activity. Adv. Funct. Mater. 2010, 20, 2347–2354. [Google Scholar] [CrossRef]
- Kucheryavy, P.; He, J.; John, V.T.; Maharjan, P.; Spinu, L.; Goloverda, G.Z.; Kolesnichenko, V.L. Superparamagnetic iron oxide nanoparticles with variable size and an iron oxidation state as prospective imaging agents. Langmuir 2013, 29, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Hyeon, T. Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem. Soc. Rev. 2012, 41, 2575–2589. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J. Magnetic nanoparticles for drug delivery. Drug Dev. Res. 2006, 67, 55–60. [Google Scholar] [CrossRef]
- Sunderland, C.J.; Steiert, M.; Talmadge, J.E.; Derfus, A.M.; Barry, S.E. Targeted nanoparticles for detecting and treating cancer. Drug Dev. Res. 2006, 67, 70–97. [Google Scholar] [CrossRef]
- Arruebo, M.; Fernandez-Pacheco, R.; Ibarra, M.R.; Santamaria, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Qiao, R.R.; Yang, C.H.; Gao, M.Y. Superparamagnetic iron oxide nanoparticles: From preparations to in vivo MRI applications. J. Mater. Chem. 2009, 19, 6274–6293. [Google Scholar] [CrossRef]
- Shi, Q.; Vitchuli, N.; Nowak, J.; Noar, J.; Caldwell, J.M.; Breidt, F.; Bourham, M.; McCord, M.; Zhang, X. One-step synthesis of silver nanoparticle-filled nylon 6 nanofibers and their antibacterial properties. J. Mater. Chem. 2011, 21, 10330–10335. [Google Scholar] [CrossRef]
- Koga, H.; Kitaoka, T.; Wariishi, H. In situ synthesis of silver nanoparticles on zinc oxide whiskers incorporated in a paper matrix for antibacterial applications. J. Mater. Chem. 2009, 19, 2135–2140. [Google Scholar] [CrossRef]
- Cheng, F.; Betts, J.W.; Kelly, S.M.; Schaller, J.; Heinze, T. Synthesis and antibacterial effects of aqueous colloidal solutions of silver nanoparticles using aminocellulose as a combined reducing and capping reagent. Green Chem. 2013, 15, 989–998. [Google Scholar] [CrossRef]
- Markova, Z.; Siskova, K.; Filip, J.; Safarova, K.; Prucek, R.; Panacek, A.; Kolar, M.; Zboril, R. Chitosan-based synthesis of magnetically-driven nanocomposites with biogenic magnetite core, controlled silver size, and high antimicrobial activity. Green Chem. 2012, 14, 2550–2558. [Google Scholar] [CrossRef]
- Park, H.H.; Park, S.; Ko, G.; Woo, K. Magnetic hybrid colloids decorated with Ag nanoparticles bite away bacteria and chemisorb viruses. J. Mater. Chem. B 2013, 1, 2701–2709. [Google Scholar] [CrossRef]
- Wu, Z.X.; Li, W.; Webley, P.A.; Zhao, D.Y. General and controllable synthesis of novel mesoporous magnetic iron oxide@ carbon encapsulates for efficient arsenic removal. Adv. Mater. 2012, 24, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Q.H.; Wang, T.M. Facile large-scale synthesis of brain-like mesoporous silica nanocomposites via a selective etching process. Nanoscale 2015, 7, 16442–16450. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, H.N.; Khan, M.S.; Wu, H.F. Graphene oxide as a nanocarrier for gramicidin (GOGD) for high antibacterial performance. RSC Adv. 2014, 4, 50035–50046. [Google Scholar] [CrossRef]
- Fei, X.; Jia, M.H.; Du, X.; Yang, Y.H.; Zhang, R.; Shao, Z.Z.; Zhao, X.; Chen, X. Green synthesis of silk fibroin-silver nanoparticle composites with effective antibacterial and biofilm-disrupting properties. Biomacromolecules 2013, 14, 4483–4488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Ma, X.L.; Cao, C.B.; Wang, M.N.; Zhu, Y.Q. Multifunctional iron oxide/silk-fibroin (Fe3O4–SF) composite microspheres for the delivery of cancer therapeutics. RSC Adv. 2014, 4, 41572–41577. [Google Scholar] [CrossRef]
- Li, G.; Liu, H.; Zhao, H.; Gao, Y.; Wang, J.; Jiang, H.; Boughton, R. Chemical assembly of TiO2 and TiO2@ Ag nanoparticles on silk fiber to produce multifunctional fabrics. J. Colloid Interface Sci. 2011, 358, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Li, J.L.; Hou, X.L.; Sun, T.; Afrin, L.; Wang, X.G. Colorful and antibacterial silk fiber from anisotropic silver nanoparticles. Ind. Eng. Chem. Res. 2013, 52, 4556–4563. [Google Scholar] [CrossRef]
- Huang, X.; Zhuang, J.; Chen, D.; Liu, H.; Tang, F.; Yan, X.; Meng, X.; Zhang, L.; Ren, J. General strategy for designing functionalized magnetic microspheres for different bioapplications. Langmuir 2009, 25, 11657–11663. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.C.; Wang, Q.; Wang, G.; Yang, M.; Dong, W.J.; Yu, J. Facile Hydrogen-bond-assisted polymerization and immobilization method to synthesize hierarchical Fe3O4@poly (4-vinylpyridine-co-divinylbenzene)@ Au nanostructures and their catalytic applications. Chem. Asian J. 2013, 8, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Xu, X.B.; Fang, Q.; Wang, Y.Y.; Li, X.B.; Tan, X.L.; Luo, J.S.; Jiang, X.D.; Wu, W.D.; Yi, Y.G.; et al. Fabrication of silver nanosheets on quartz glass substrates through electroless plating approach. Appl. Phys. A 2014, 114, 485–493. [Google Scholar] [CrossRef]
- Xiong, R.; Lu, C.H.; Wang, Y.R.; Zhou, Z.H.; Zhang, X.X. Nanofibrillated cellulose as the support and reductant for the facile synthesis of Fe3O4/Ag nanocomposites with catalytic and antibacterial activity. J. Mater. Chem. A 2013, 1, 14910–14918. [Google Scholar] [CrossRef]
- Yi, Z.; Luo, J.S.; Tan, X.L.; Yi, Y.; Yao, W.T.; Kang, X.L.; Ye, X.; Zhu, W.K.; Duan, T.; Yi, Y.G.; et al. Mesoporous gold sponges: Electric charge-assisted seed mediated synthesis and application as surface-enhanced Raman scattering substrates. Sci. Report 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, M.; Rice, P.M.; Wang, S.X.; White, R.L.; Sun, S.H. Dumbbell-like bifunctional Au–Fe3O4 nanoparticles. Nano Lett. 2005, 5, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Sun, Y.; Huang, S.; Yu, K.; Zhao, Q.; Peng, F.; Yu, H.; Wang, H.; Yang, J. Crystal engineering and SERS properties of Ag–Fe3O4 nanohybrids: From heterodimer to core–shell nanostructures. J. Mater. Chem. 2011, 21, 17930–17937. [Google Scholar] [CrossRef]
- Sureshkumar, M.; Siswanto, D.Y.; Lee, C.K. Magnetic antimicrobial nanocomposite based on bacterial cellulose and silver nanoparticles. J. Mater. Chem. 2010, 20, 6948–6955. [Google Scholar] [CrossRef]
- Andrade, F.A.C.; Vercik, L.C.D.; Monteiro, F.J.; Rigo, E.C.D. Preparation, characterization and antibacterial properties of silver nanoparticles–hydroxyapatite composites by a simple and eco-friendly method. Ceram. Int. 2016, 42, 2271–2280. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
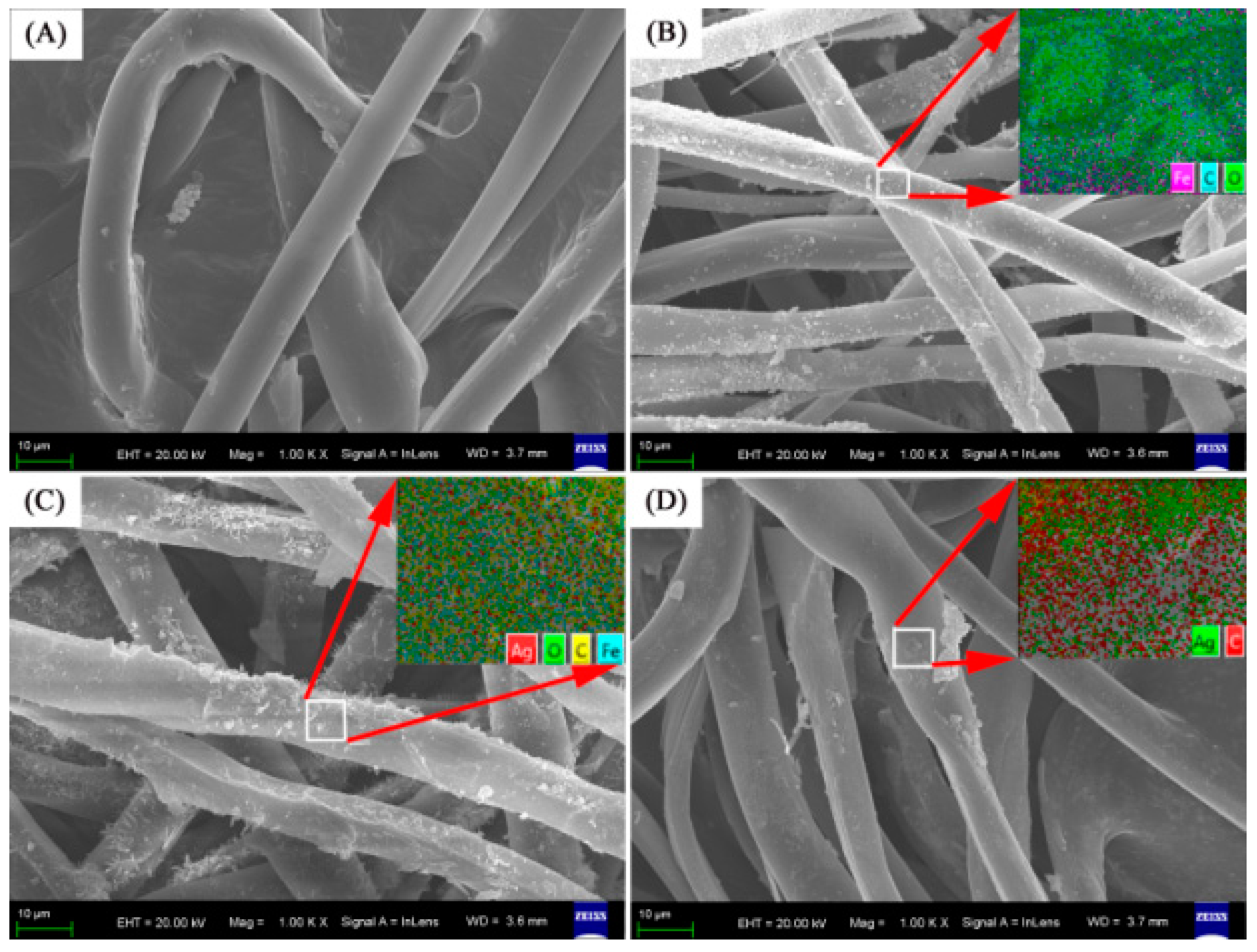
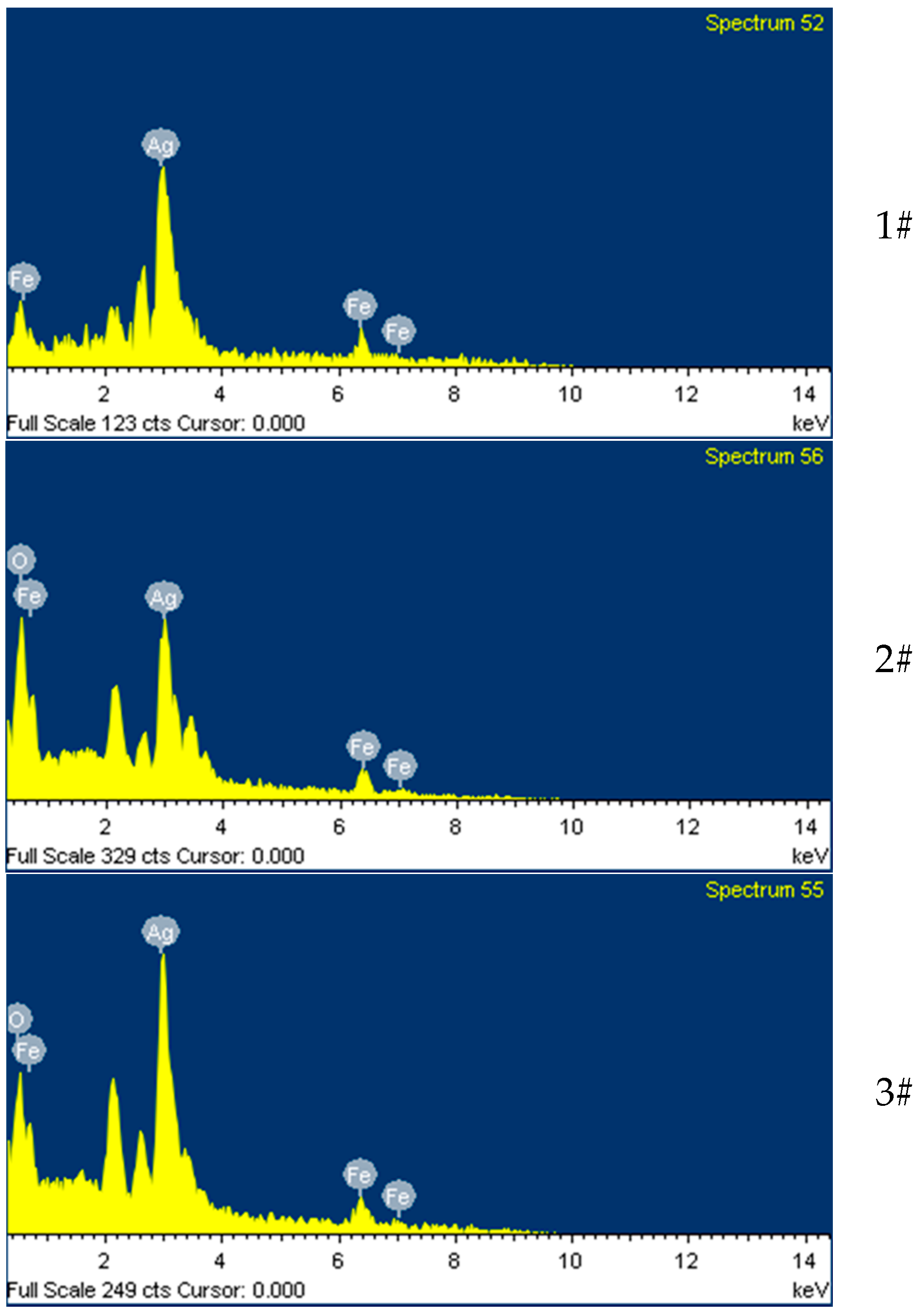
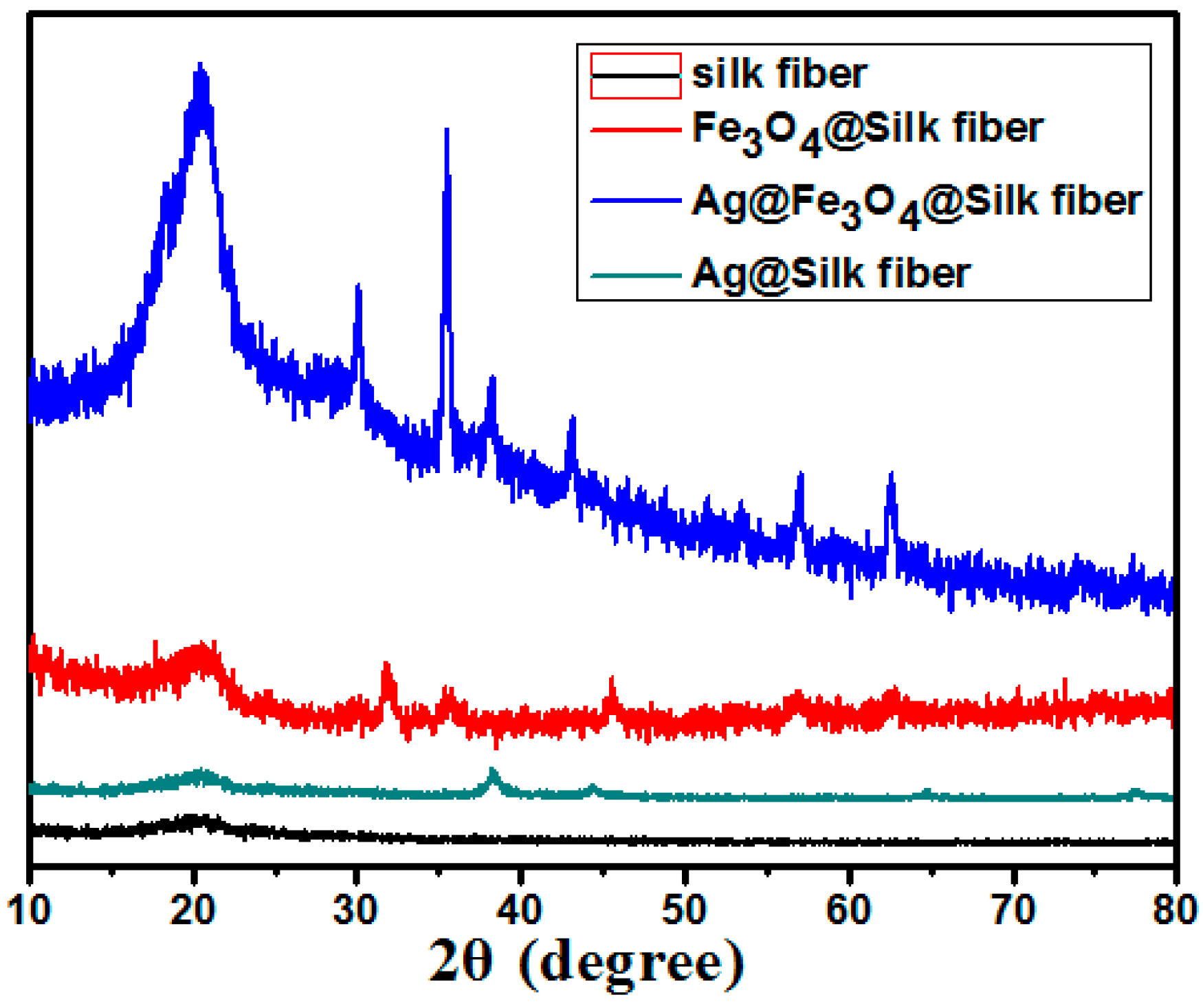
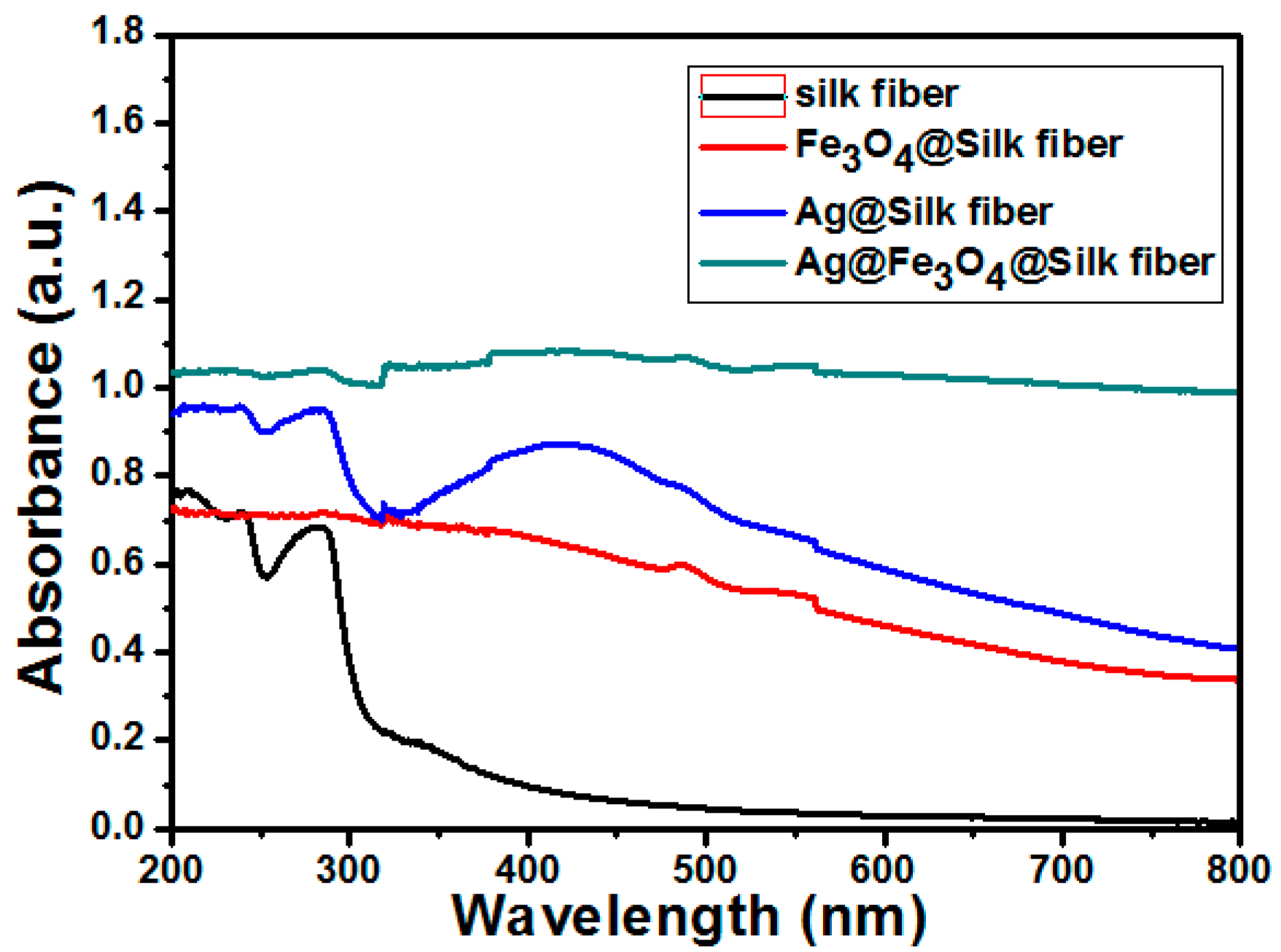
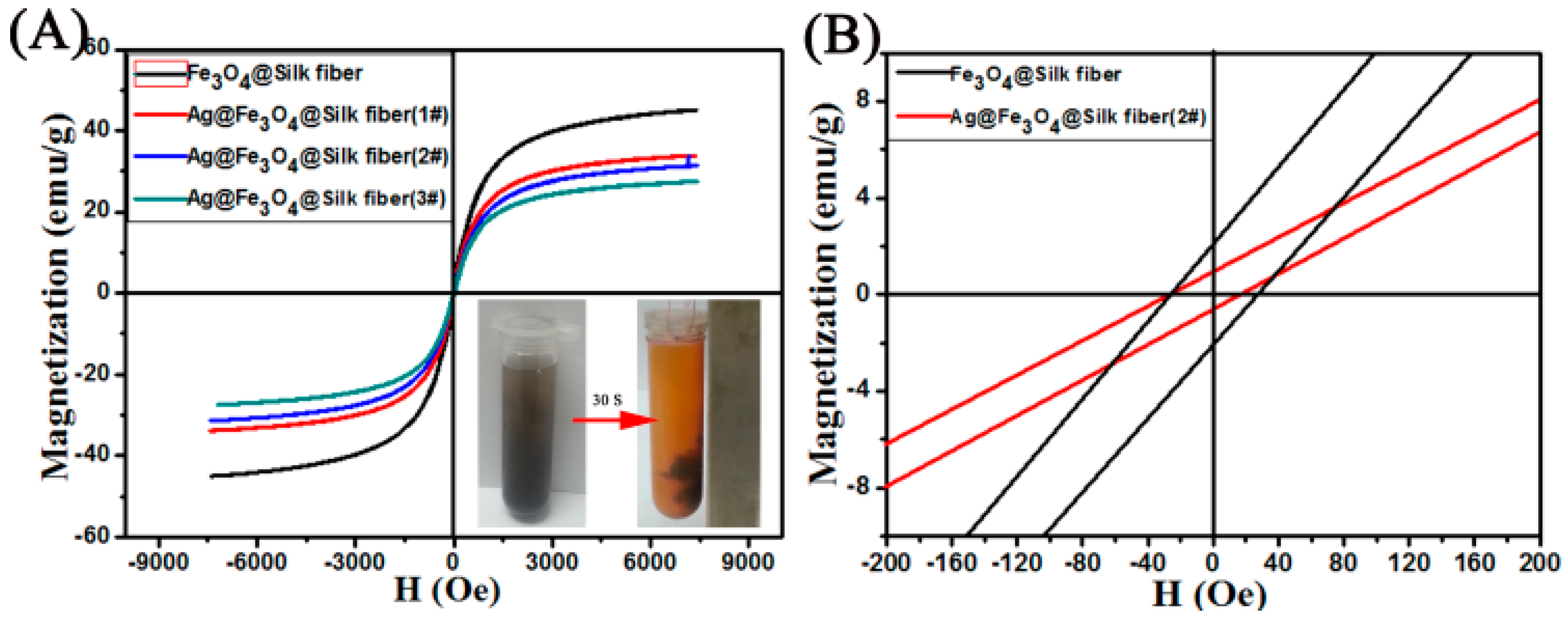
| Materials | Amount of Ag in Ion State (X) (μg/mL) | Total Amount of Ag (Y) (μg/mL) | Y Minus X (μg/mL) |
|---|---|---|---|
| silk fibers | 0 | 0 | 0 |
| Ag–Fe3O4–silk fibers (1#) | 3.2 | 7.6 | 4.4 |
| Ag–Fe3O4–silk fibers (2#) | 4.7 | 10.2 | 5.5 |
| Ag–Fe3O4–silk fibers (3#) | 5.3 | 14.8 | 9.5 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Yin, G.; Yi, Z.; Duan, T. Silk Fiber as the Support and Reductant for the Facile Synthesis of Ag–Fe3O4 Nanocomposites and Its Antibacterial Properties. Materials 2016, 9, 501. https://doi.org/10.3390/ma9070501
Liu X, Yin G, Yi Z, Duan T. Silk Fiber as the Support and Reductant for the Facile Synthesis of Ag–Fe3O4 Nanocomposites and Its Antibacterial Properties. Materials. 2016; 9(7):501. https://doi.org/10.3390/ma9070501
Chicago/Turabian StyleLiu, Xiaonan, Guangfu Yin, Zao Yi, and Tao Duan. 2016. "Silk Fiber as the Support and Reductant for the Facile Synthesis of Ag–Fe3O4 Nanocomposites and Its Antibacterial Properties" Materials 9, no. 7: 501. https://doi.org/10.3390/ma9070501






