Functionalization of Cellulose Nanocrystals in Choline Lactate Ionic Liquid
Abstract
:1. Introduction
2. Results and Discussion
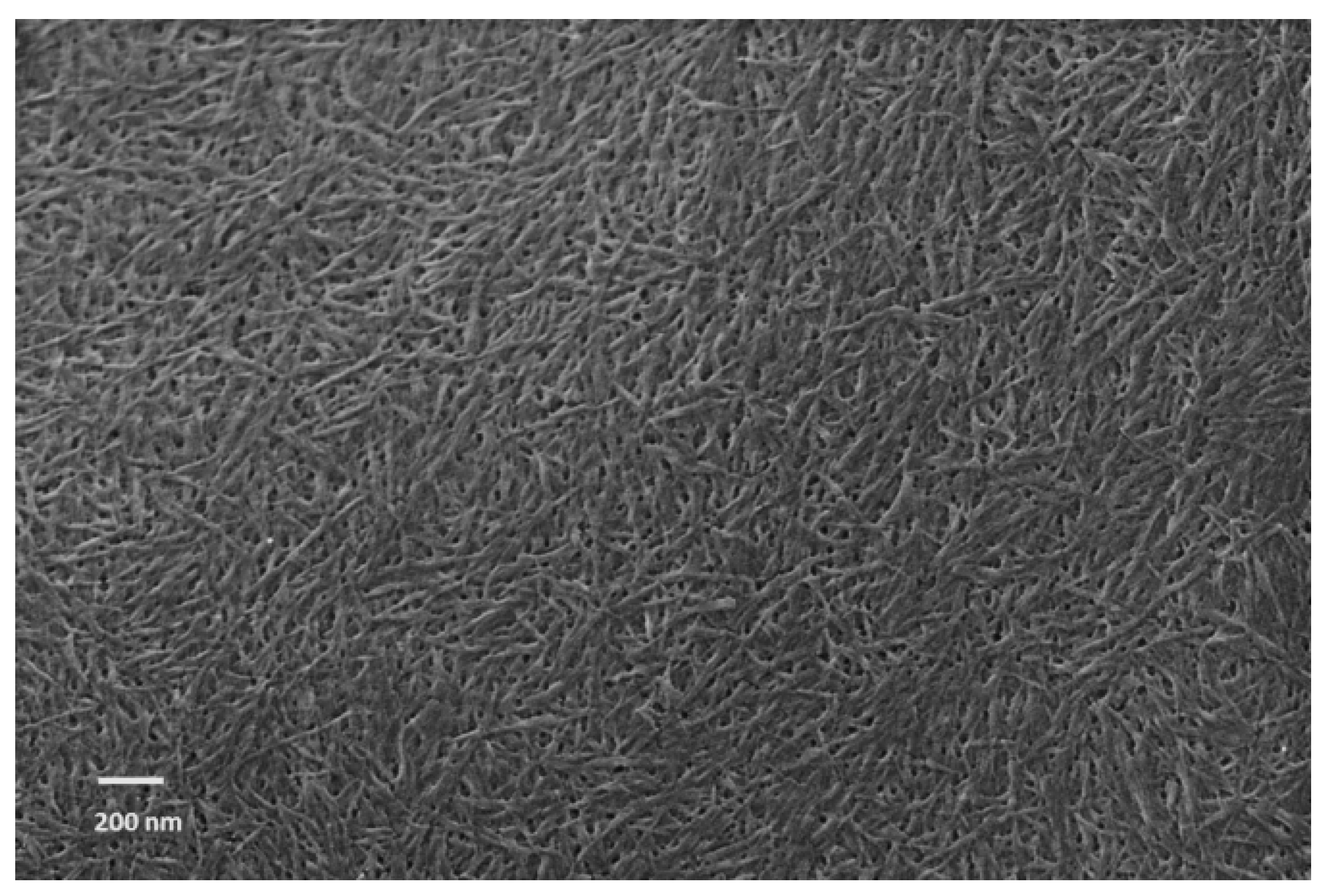
2.1. Preparation of CNCs
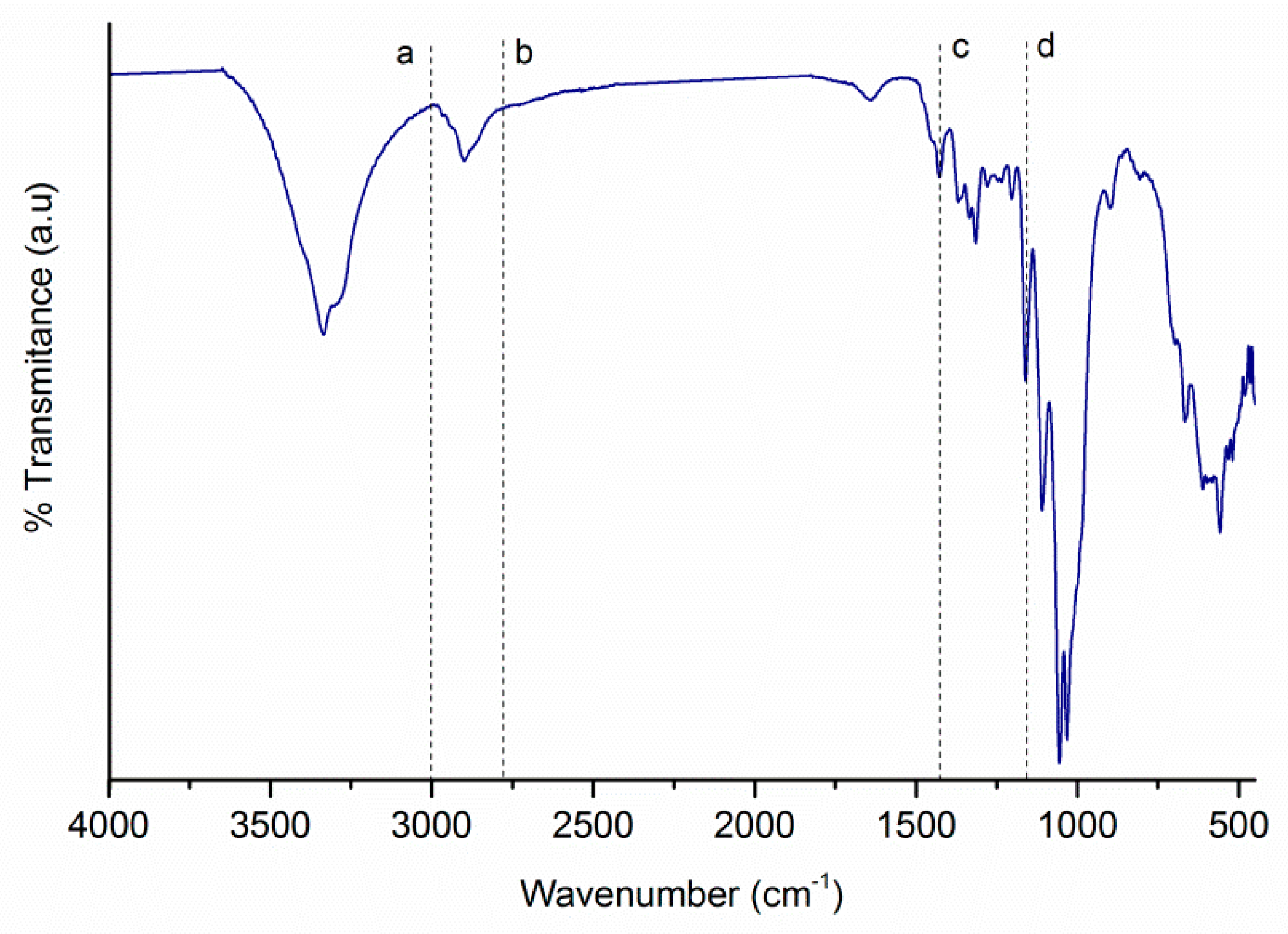
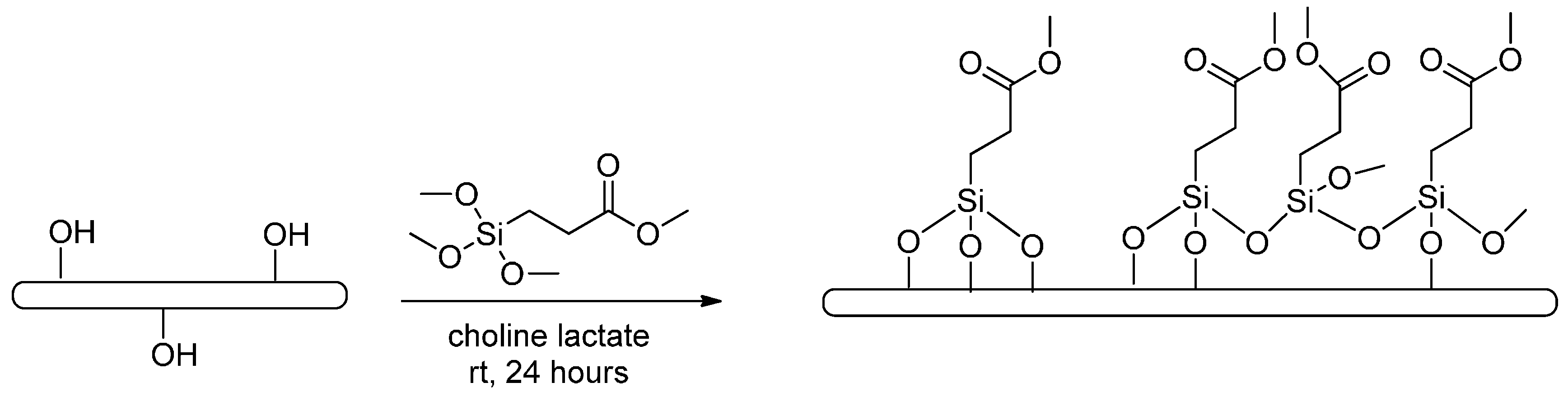
2.2. Functionalization of CNCs
2.3. Preparation of PLA Nanocomposites
2.3.1. Morphological Characterization
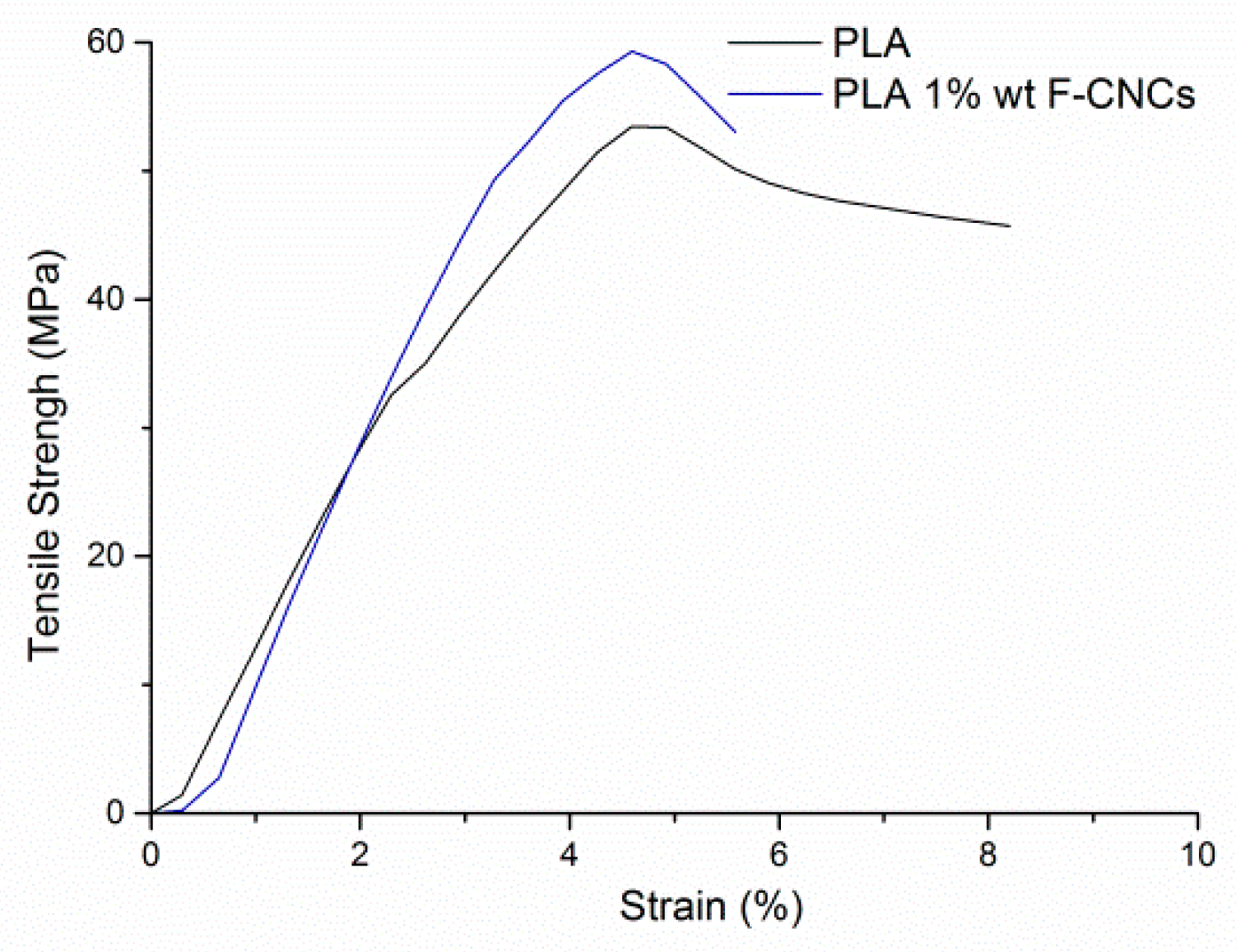
2.3.2. Mechanical Characterization
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Choline Lactate
3.3. Functionalization of CNCs
3.3.1. Functionalization of Sulfated CNCs
3.3.2. CNCs in Aqueous Dispersion
3.3.3. Desulfated CNCs
3.4. Preparation of F-CNCs/PLA Nanocomposites
3.5. Characterization Techniques
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tingaut, P.; Zimmermann, T.; Sèbe, G. Cellulose nanocrystals and microfibrillated cellulose as building blocks for the design of hierarchical functional materials. J. Mater. Chem. 2012, 22, 20105–20111. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Dong, S.; Hirani, A.; Lee, Y.W. Cellulose nanocrystals for drug delivery. In Polysaccharide Materials: Performance by Design; Edgar, K.J., Heinze, T., Buchanan, C.H., Eds.; American Chemical Society: Washington, DC, USA, 2009; pp. 81–91. [Google Scholar]
- Lam, E.; Male, K.B.; Chong, J.H.; Leung, A.C.N.; Luong, J.H.T. Applications of functionalized and nanoparticle-modified nanocrystalline cellulose. Trends Biotechnol. 2012, 30, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lu, C.; Zhou, Z.; Yuan, G.; Xiong, R.; Zhang, X. Green synthesis and formation mechanism of cellulose nanocrystal-supported gold nanoparticles with enhanced catalytic performance. Environ. Sci. Nano. 2014, 1, 71–79. [Google Scholar] [CrossRef]
- Chen, L.; Cao, W.; Quinlan, P.J.; Berry, R.M.; Tam, K.C. Sustainable catalysts from gold-loaded polyamidoamine dendrimer-cellulose nanocrystals. ACS Sustain. Chem. Eng. 2015, 3, 978–985. [Google Scholar] [CrossRef]
- Liu, J.; Plog, A.; Groszewicz, P.; Zhao, L.; Xu, Y.; Breitzke, H.; Stark, A.; Hoffmann, R.; Gutmann, T.; Zhang, K.; et al. Design of a heterogeneous catalyst based on cellulose nanocrystals for cyclopropanation: Synthesis and solid-state NMR characterization. Chem. Eur. J. 2015, 21, 12414–12420. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Berry, R.M.; Tam, K.C. Stimuli-responsive cellulose nanocrystals for surfactant-free oil harvesting. Biomacromolecules 2016, 17, 1748–1756. [Google Scholar] [CrossRef] [PubMed]
- Bawa, P.; Pillay, V.; Choonara, Y.E.; Du Toit, L. Stimuli-responsive polymers and their applications in drug delivery. Biomed. Mater. 2009, 4, 22001–22016. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, W.; Plog, A.; Xu, Y.; Buntkowsky, G.; Gutmann, T.; Zhang, K. Multi-responsive cellulose nanocrystal–rhodamine conjugates: An advanced structure study by solid state dynamic nuclear polarization (DNP) NMR. Phys. Chem. Chem. Phys. 2014, 16, 26322–26329. [Google Scholar] [CrossRef] [PubMed]
- Nogi, M.; Iwamoto, S.; Nakagaito, A.N.; Yano, H. Optically transparent nanofiber paper. Adv. Mater. 2009, 21, 1595–1598. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Sajeevkumar, V.A.; Ramana, K.V.; Sabapathy, S.N.; Siddaramaiah. Augmented properties of PVA hybrid nanocomposites containing cellulose nanocrystals and silver nanoparticles. J. Mater. Chem. 2012, 22, 22433–22439. [Google Scholar]
- Mathew, A.P.; Dufresne, A. Morphological investigation of nanocomposites from sorbitol plasticized starch and tunicin whiskers. Biomacromolecules 2002, 3, 609–617. [Google Scholar] [CrossRef] [PubMed]
- De Mesquita, J.P.; Donnici, C.L.; Pereira, F.V. Biobased nanocomposites from layer-by-Layer assembly of cellulose nanowhiskers with chitosan. Biomacromolecules 2010, 11, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Bendahou, A.; Habibi, Y.; Kaddami, H.; Dufresne, A. Physico-chemical characterization of palm from phoenix dactylifera–L, preparation of cellulose whiskers and natural rubber–based nanocomposites. J. Biobased Mater. Biol. 2009, 3, 81–90. [Google Scholar] [CrossRef]
- Robles, E.; Urruzola, I.; Labidi, J.; Serrano, L. Surface-modified nano-cellulose as reinforcement in poly(lactic acid) to conform new composites. Ind. Crop Prod. 2015, 71, 44–53. [Google Scholar] [CrossRef]
- Pei, A.; Zhou, Q.; Berglund, L.A. Functionalized cellulose nanocrystals as biobased nucleation agents in poly(l-lactide) (PLLA)—Crystallization and mechanical property effects. Compos. Sci. Technol. 2010, 70, 815–821. [Google Scholar] [CrossRef]
- Sullivan, E.M.; Moon, R.J.; Kalaitzidou, K. Processing and characterization of cellulose nanocrystals/polylactic acid nanocomposite films. Materials 2015, 8, 8106–8116. [Google Scholar] [CrossRef]
- Peng, B.L.; Dhar, N.; Liu, H.L.; Tam, K.C. Chemistry and applications of nanocrystalline cellulose and its derivatives: A nanotechnology perspective. Can. J. Chem. Eng. 2011, 89, 1191–1206. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Bhat, A.H.; Ireana Yusra, A.F. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Eyley, S.; Thielemans, W. Surface modification of cellulose nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Wang, X.; Heim, L.O.; Breitzke, H.; Buntkowsky, G.; Zhang, K. Superhydrophobic surfaces from surface-hydrophobized cellulose fibers with stearyl groups. Cellulose 2015, 22, 289–299. [Google Scholar] [CrossRef]
- Goussé, C.; Chanzy, H.; Cerrada, M.L.; Fleury, E. Surface silylation of cellulose microfibrils: Preparation and rheological properties. Polymer 2004, 45, 1569–1575. [Google Scholar] [CrossRef]
- Belgacem, M.N.; Gandini, A. Surface modification of cellulose fibre. In Monomers, Polymers and Composites from Renewable Resources, 1st ed.; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 385–400. [Google Scholar]
- Xie, Y.; Hill, C.A.S.; Xiao, Z.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. Part A 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- De los Ríos, A.P.; Irabien, A.; Hollmann, F.; Hernández Fernández, F.J. Ionic liquids: Green solvents for chemical processing. J. Chem. 2013, 13, 1–2. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, J.; Wu, X.; Guo, B.; Zhang, L.; Liu, F. Interface engineering toward promoting silanization by ionic liquid for high-performance rubber/silica composites. Ind. Eng. Chem. Res. 2015, 54, 10747–10756. [Google Scholar] [CrossRef]
- Tadesse, H.; Luque, R. Advances on biomass pretreatment using ionic liquids: An overview. Energy Environ. Sci. 2011, 4, 3913–3929. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, N.; Matsushita, Y.; Hanaki, M.; Nakashima, K.; Narita, M.; Goto, M.; Takahashi, H. Enzymatic in situ saccharification of cellulose in aqueous-ionic liquid media. Biotechnol. Lett. 2008, 30, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Paulechka, Y.U.; Kabo, G.J.; Blokhin, A.V.; Shaplov, A.S.; Lozinskaya, E.I.; Vygodskii, Y.S. Thermodynamic properties of 1-alkyl-3-methylimidazolium bromide ionic liquids. J. Chem. Thermodyn. 2007, 39, 158–166. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Legido, J.L.; Rodríguez, A. Physical properties of ionic liquids based on 1-alkyl-3-methylimidazolium cation and hexafluorophosphate as anion and temperature dependence. J. Chem. Thermodyn. 2007, 39, 1168–1175. [Google Scholar] [CrossRef]
- Bose, S.; Armstrong, D.W.; Petrich, J.W. Enzyme-catalyzed hydrolysis of cellulose in ionic liquids: A green approach toward the production of biofuels. J. Phys. Chem. B 2010, 114, 8221–8227. [Google Scholar] [CrossRef] [PubMed]
- Chaumont, A.; Wipff, G. Solvation of “big” spherical solutes in room temperature ionic liquids and at their aqueous interface: A molecular dynamics simulation study. J. Mol. Liq. 2007, 131–132, 36–47. [Google Scholar] [CrossRef]
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic liquids and cellulose: Dissolution, chemical modification and preparation of new cellulosic materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.Y.; Abd Hamid, S.B.; Lai, C.W. Preparation of high crystallinity cellulose nanocrystals (CNCs) by ionic liquid solvolysis. Biomass Bioenergy 2015, 81, 584–591. [Google Scholar] [CrossRef]
- Isik, M.; Gracia, R.; Kollnus, L.C.; Tomé, L.C.; Marrucho, I.M.; Mecerreyes, D. Cholinium lactate methacrylate: Ionic liquid monomer for cellulose composites and biocompatible ion gels. Macromol. Symp. 2014, 342, 21–24. [Google Scholar] [CrossRef]
- Liebert, T.; Heinze, T. Interaction of ionic liquids with polysaccharides. Solvents an reaction media for the modification of cellulose. Bioresources 2008, 3, 576–601. [Google Scholar]
- Heinze, T.; Schwikal, K.; Barthel, S. Ionic liquids as reaction medium in cellulose functionalization. Macromol. Biosci. 2005, 5, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Guillory, X.; Chopin, N.; Weiss, P.; Colliec-Jouault, S.; Le Bideau, J. Development of an injectable extracellular matrix for regenerative medicine by silanization of a cellulose derivative in an ionic liquid medium. In Proceedings of the 24th Interdisciplinary Research Conference on Injectable Osteoarticular Biomaterials and Bone Augmentation Procedures, Nantes, France, 5–7 May 2014.
- Montes, S.; Carrasco, P.; Ruiz, V.; Cabañero, G.; Grande, H.J.; Labidi, J.; Odriozola, I. Synergistic reinforcement of poly(vinyl alcohol) nanocomposites with cellulose nanocrystal-stabilized graphene. Compos. Sci. Technol. 2015, 117, 26–31. [Google Scholar] [CrossRef]
- Yang, W.; Dominici, F.; Fortunati, E.; Kenny, J.M.; Puglia, D. Melt free radical grafting of glycidyl methacrylate (GMA) onto fully biodegradable poly(lactic) acid films: Effect of cellulose nanocrystals and a masterbatch process. RSC Adv. 2015, 5, 32350–32357. [Google Scholar] [CrossRef]
- John, M.J.; Anandjiwala, R.; Oksman, K.; Mathew, A.P. Melt-spun polylactic acid fibers: Effect of cellulose nanowhiskers on processing and properties. J. Appl. Polym. Sci. 2013, 127, 274–281. [Google Scholar] [CrossRef]
- Lokanathan, A.R.; Ahsan Uddin, K.M.; Rojas, O.J.; Laine, J. Cellulose nanocrystal-mediated synthesis of silver nanoparticles: Role of sulfate groups in nucleation phenomena. Biomacromolecules 2014, 15, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Abitbol, T.; Kloser, E.; Gray, D.G. Estimation of the surface sulfur content of cellulose nanocrystals prepared by sulfuric acid hydrolysis. Cellulose 2013, 20, 785–794. [Google Scholar] [CrossRef]




| Sample | % S |
|---|---|
| Sulfated CNCs | 0.53 ± 0.03 |
| Desulfated CNCs | 0.04 ± 0.0001 |
| F-CNCs | CNC Pretreatment | CNC:Silane Molar Ratio | IL:CNC Ratio w/w | Reaction Time (h) | TGA Residue (%) |
|---|---|---|---|---|---|
| F1 | Desulfated | 1:10 | 55:1 | 24 | 45.48 |
| F2 | Desulfated | 1:1.25 | 55:1 | 24 | 27.13 |
| F3 | Desulfated | 1:0.6 | 55:1 | 4.5 | No Functionalization |
| F4 | Desulfated | 1:0.6 | 55:1 | 24 | 11.68 |
| F5 | Desulfated | 1:0.6 | 0.81:8.1 Ethanol | 24 | No Functionalization |
| F6 | Sulfated | 1:0.6 | 55:1 | 24 | 20.13 |
| F7 | Aqueous dispersion | 1:0.6 | 55:1 | 24 | 12.09 |
| Material | E Modulus (MPa) | σ (MPa) | ε at Break (%) |
|---|---|---|---|
| PLA | 1780 ± 48 | 52.5 ± 4.2 | 7. 28 ± 0.41 |
| PLA/1 wt % F-CNCs | 1815 ± 176 | 54.7 ± 5.1 | 5.30 ± 0.31 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montes, S.; Azcune, I.; Cabañero, G.; Grande, H.-J.; Odriozola, I.; Labidi, J. Functionalization of Cellulose Nanocrystals in Choline Lactate Ionic Liquid. Materials 2016, 9, 499. https://doi.org/10.3390/ma9070499
Montes S, Azcune I, Cabañero G, Grande H-J, Odriozola I, Labidi J. Functionalization of Cellulose Nanocrystals in Choline Lactate Ionic Liquid. Materials. 2016; 9(7):499. https://doi.org/10.3390/ma9070499
Chicago/Turabian StyleMontes, Sarah, Itxaso Azcune, Germán Cabañero, Hans-Jürgen Grande, Ibon Odriozola, and Jalel Labidi. 2016. "Functionalization of Cellulose Nanocrystals in Choline Lactate Ionic Liquid" Materials 9, no. 7: 499. https://doi.org/10.3390/ma9070499






