Fabrication of Gold-Coated Ultra-Thin Anodic Porous Alumina Substrates for Augmented SERS
Abstract
:1. Introduction
2. Results and Discussion
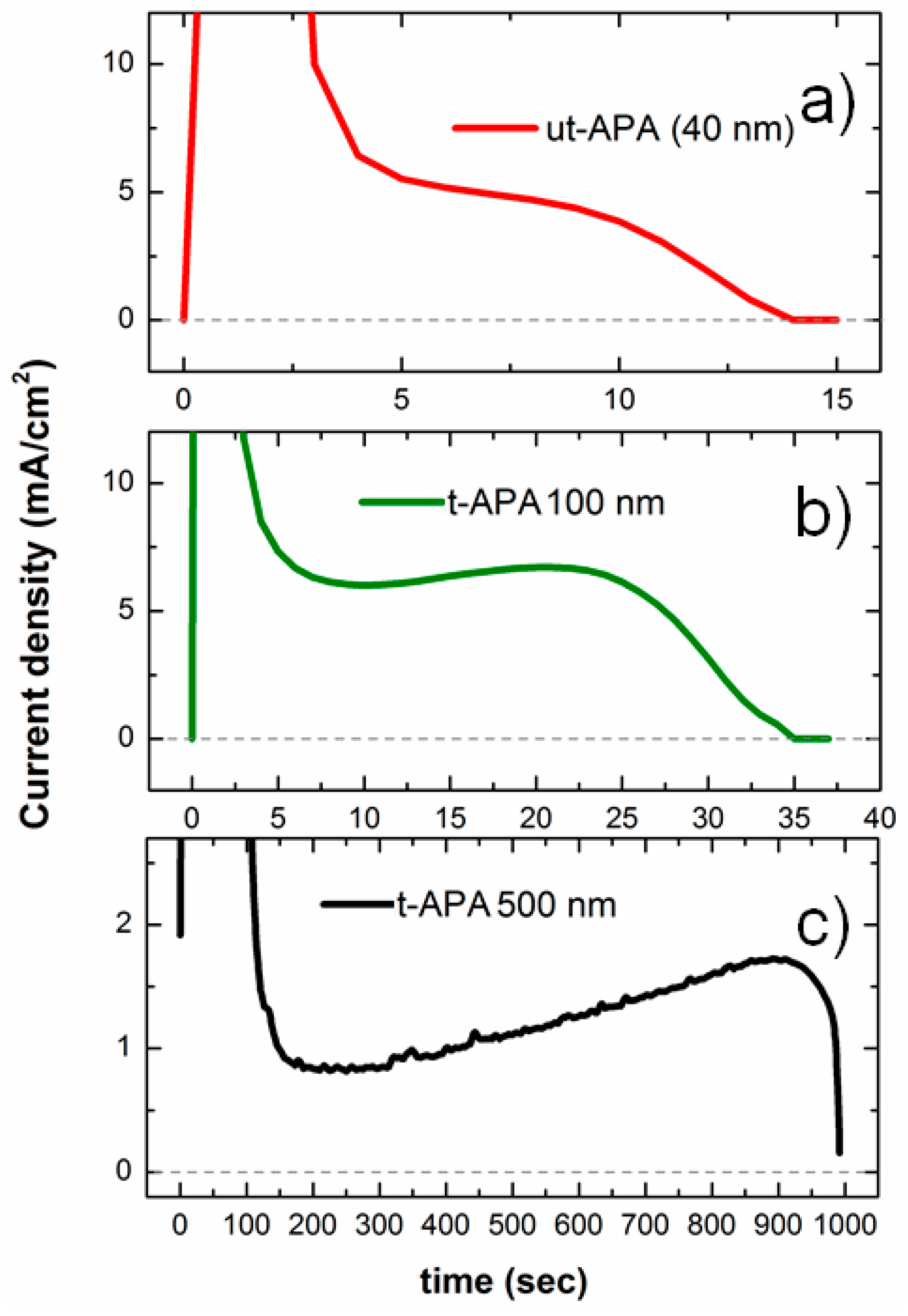
2.1. utAPA‑Au Substrate Preparation
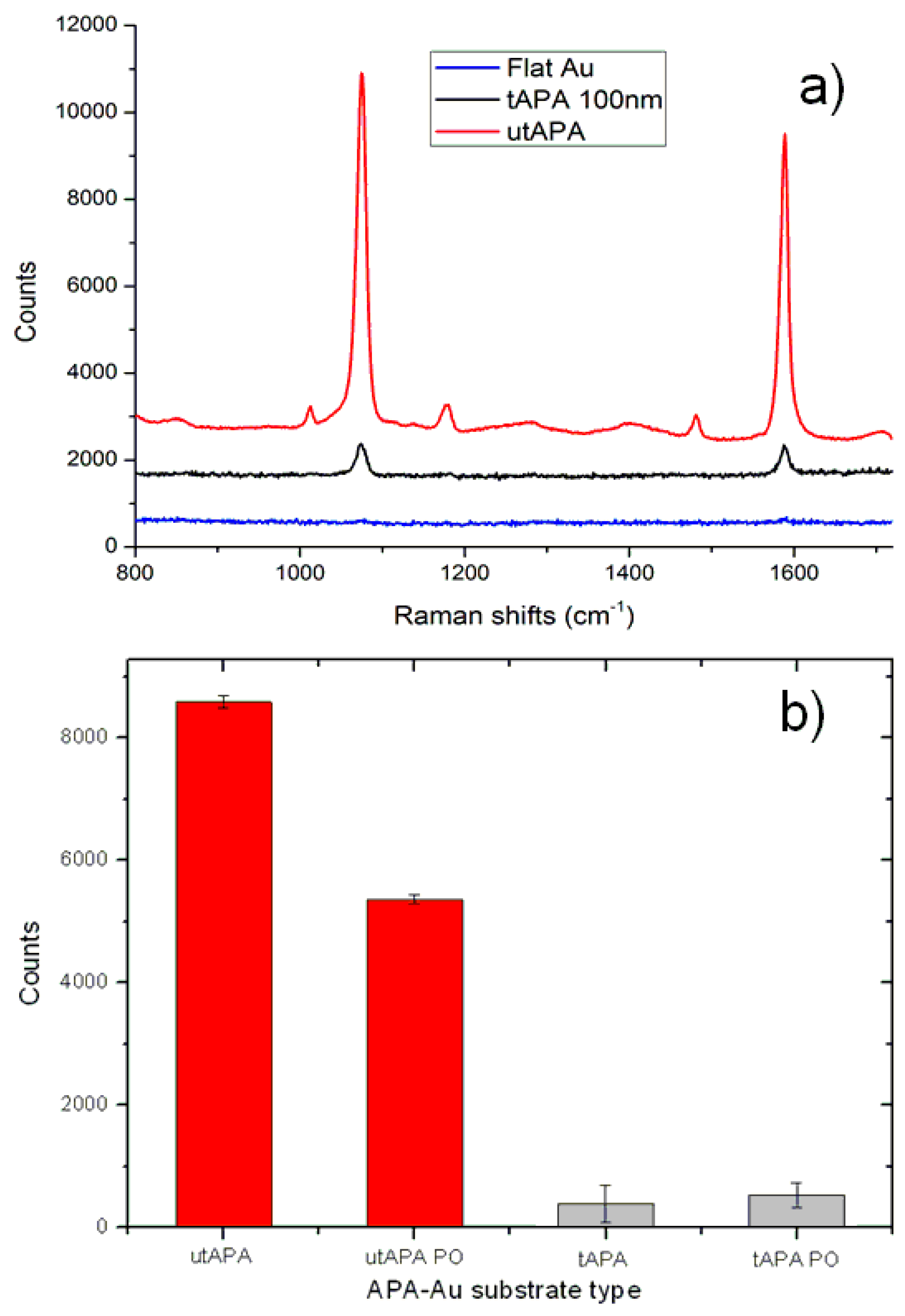
2.2. SERS Measurements
2.3. Numerical Simulations
3. Materials and Methods
3.1. Au‑utAPA Substrate Preparation
3.2. Raman Measurements
3.3. Simulations
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| APA | anodic porous alumina |
| tAPA | thin APA |
| utAPA | ultra-thin APA |
| Al | aluminum |
| Au | gold |
| Si | silicon |
| FIT | finite integration technique |
| SERS | surface-enhanced Raman scattering |
References
- Shingubara, S. Fabrication of nanomaterials using porous alumina templates. J. Nanopart. Res. 2003, 5, 17–30. [Google Scholar] [CrossRef]
- Sulka, G.D. Highly Ordered Anodic Porous Alumina Formation by Self-organized Anodizing; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Q.; Wang, X.Y.; Su, X.G. Fluorescence resonance energy transfer between two quantum dots with immunocomplexes of antigen and antibody as a bridge. Luminescence 2007, 22, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Luo, Y.; Wehrspohn, R.B.; Hillebrand, R.; Schilling, J.; Gösele, U. Perfect two-dimensional porous alumina photonic crystals with duplex oxide layers. J. Appl. Phys. 2003, 94, 4757–4762. [Google Scholar] [CrossRef]
- Masuda, H.; Yamada, M.; Matsumoto, F.; Yokoyama, S.; Mashiko, S.; Nakao, M.; Nishio, K. Lasing from Two-Dimensional Photonic Crystals Using Anodic Porous Alumina. Adv. Mater. 2006, 18, 213–216. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Shackleton, R.; Mamun, S.I.; Fawcett, D. Significance of novel bioinorganic anodic aluminum oxide nanoscaffolds for promoting cellular response. Nanotechnol. Sci. Appl. 2011, 4, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Toccafondi, C.; Dante, S.; Reverberi, A.P.; Salerno, M. Biomedical Applications of Anodic Porous Alumina. Curr. Nanosci. 2015, 11, 572–580. [Google Scholar] [CrossRef]
- Gultepe, E.; Nagesha, D.; Sridhar, S.; Amiji, M. Nanoporous inorganic membranes or coatings for sustained drug delivery in implantable devices. Adv. Drug Deliv. Rev. 2010, 62, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Sinn Aw, M.; Bariana, M.; Kumeria, T.; Wang, Y.; Losic, D. Drug-releasing implants: Current progress, challenges and perspectives. J. Mater. Chem. B 2014, 2, 6157–6182. [Google Scholar] [CrossRef]
- Toccafondi, C.; Thorat, S.; La Rocca, R.; Scarpellini, A.; Salerno, M.; Dante, S.; Das, G. Multifunctional substrates of thin porous alumina for cell biosensors. J. Mater. Sci. Mater. Med. 2014, 25, 2411–2420. [Google Scholar] [CrossRef] [PubMed]
- Toccafondi, C.; La Rocca, R.; Scarpellini, A.; Salerno, M.; Das, G.; Dante, S. Thin nanoporous alumina-based SERS platform for single cell sensing. Appl. Surf. Sci. 2015, 351, 738–745. [Google Scholar] [CrossRef]
- Schlücker, S. Surface-Enhanced Raman Spectroscopy: Concepts and Chemical Applications. Angew. Chem. Int. Ed. 2014, 53, 4756–4795. [Google Scholar] [CrossRef] [PubMed]
- Terekhov, S.N.; Kachan, S.M.; Panarin, A.Y.; Mojzes, P. Surface-enhanced Raman scattering on silvered porous alumina templates: Role of multipolar surface plasmon resonant modes. Phys. Chem. Chem. Phys. 2015, 17, 31780–31789. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.J.; Chumanov, G. Silver Coated Porous Alumina as a New Substrate for Surface-Enhanced Raman Scattering. Appl. Spectrosc. 2001, 55, 1695–1700. [Google Scholar] [CrossRef]
- Chen, J.; Feng, S.; Gao, F.; Grant, E.; Xu, J.; Wang, S.; Huang, Q.; Lu, X. Fabrication of SERS-Active Substrates using Silver Nanofilm-Coated Porous Anodic Aluminum Oxide for Detection of Antibiotics. J. Food Sci. 2015, 80, N834–N840. [Google Scholar] [CrossRef] [PubMed]
- Fleger, Y.; Rosenbluh, M. Surface Plasmons and Surface Enhanced Raman Spectra of Aggregated and Alloyed Gold-Silver Nanoparticles. Optics 2009. [Google Scholar] [CrossRef]
- Pazos-Perez, N.; Ni, W.; Schweikart, A.; Alvarez-Puebla, R.A.; Fery, A.; Liz-Marzan, L.M. Highly uniform SERS substrates formed by wrinkle-confined drying of gold colloids. Chem. Sci. 2010, 1, 174–178. [Google Scholar] [CrossRef]
- Das, G.; Patra, N.; Gopalakrishnan, A.; Zaccaria, R.P.; Toma, A.; Thorat, S.; Di Fabrizio, E.; Diaspro, A.; Salerno, M. Fabrication of large-area ordered and reproducible nanostructures for SERS biosensor application. Analyst 2012, 137, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Kassu, A.; Iii, C.F.; Sharma, A.; Kim, W.; Guo, J. Effect of Pore Size and Film Thickness on Gold-Coated Nanoporous Anodic Aluminum Oxide Substrates for Surface-Enhanced Raman Scattering Sensor. Sensors 2015, 15, 29924–29937. [Google Scholar] [CrossRef] [PubMed]
- Kokonou, M.; Nassiopoulou, A.G.; Giannakopoulos, K.P. Ultra-thin porous anodic alumina films with self-ordered cylindrical vertical pores on a p-type silicon substrate. Nanotechnology 2005, 16, 103–106. [Google Scholar] [CrossRef]
- Diggle, J.; Downie, T.; Goulding, C. Anodic Oxide Films on Aluminum. Chem. Rev. 1969, 69, 365–405. [Google Scholar] [CrossRef]
- Lee, W.; Ji, R.; Gosele, U.; Nielsch, K. Fast fabrication of long-range ordered porous alumina membranes by hard anodization. Nat. Mater. 2006, 5, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.; Patra, N.; Cingolani, R. Use of Ionic Liquid in Fabrication, Characterization, and Processing of Anodic Porous Alumina. Nanoscale Res. Lett. 2009, 4, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Kokonou, M.; Nassiopoulou, A.G.; Giannakopoulos, K.P.; Travlos, A.; Stoica, T.; Kennou, S. Growth and characterization of high density stoichiometric SiO2 dot arrays on Si through an anodic porous alumina template. Nanotechnology 2006, 17, 2146–2151. [Google Scholar] [CrossRef]
- Hillebrand, R.; Hergert, W. Scaling properties of a tetragonal photonic crystal design having a large complete bandgap. Photonics Nanostruct. Fundam. Appl. 2004, 2, 33–39. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Nowak-stępniowska, A.; Michalska-domańska, M.; Norek, M.; Czujko, T.; Bojar, Z. Fabrication and geometric characterization of highly-ordered hexagonally arranged arrays of nanoporous anodic alumina. Polish J. Chem. Technol. 2013, 16, 63–69. [Google Scholar] [CrossRef]
- Toccafondi, C.; Stępniowski, W.J.; Leoncini, M.; Salerno, M. Advanced morphological analysis of patterns of thin anodic porous alumina. Mater. Charact. 2014, 94, 26–36. [Google Scholar] [CrossRef]
- Michota, A.; Bukowska, J. Surface-enhanced Raman scattering (SERS) of 4-mercaptobenzoic acid on silver and gold substrates. J. Raman Spectrosc. 2003, 34, 21–25. [Google Scholar] [CrossRef]
- Wang, A.; Kong, X. Review of Recent Progress of Plasmonic Materials and Nano-Structures for Surface-Enhanced Raman Scattering. Materials 2015, 8, 3024–3052. [Google Scholar] [CrossRef] [PubMed]
- Weiland, T. A Discretization Method for the Solution of Maxwell’s Equations for Six-Component Fields. AEUe Int. J. Electron. Commun. 1977, 31, 116–120. [Google Scholar]



| APA Type | Protocol | V (V) | D (nm) | σ (1010 cm−2) | d (nm) | w (nm) | w/d | w/D |
|---|---|---|---|---|---|---|---|---|
| tAPA 500 nm | Phosphoric acid | 110 | 247 | 0.21 | 66 | 181 | 2.74 | 0.73 |
| tAPA 500 nm | Phosphoric acid + pore opening | 110 | 234 | 0.23 | 157 | 77 | 0.49 | 0.33 |
| tAPA 100 nm | Oxalic acid | 40 | 94 | 1.44 | 21 | 73 | 3.48 | 0.78 |
| tAPA 100 nm | Oxalic acid + pore opening | 40 | 103 | 1.20 | 82 | 21 | 0.26 | 0.20 |
| utAPA | Sulfuric acid + pore opening | 20 | 41 | 7.6 | 13 | 28 | 2.15 | 0.68 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toccafondi, C.; Proietti Zaccaria, R.; Dante, S.; Salerno, M. Fabrication of Gold-Coated Ultra-Thin Anodic Porous Alumina Substrates for Augmented SERS. Materials 2016, 9, 403. https://doi.org/10.3390/ma9060403
Toccafondi C, Proietti Zaccaria R, Dante S, Salerno M. Fabrication of Gold-Coated Ultra-Thin Anodic Porous Alumina Substrates for Augmented SERS. Materials. 2016; 9(6):403. https://doi.org/10.3390/ma9060403
Chicago/Turabian StyleToccafondi, Chiara, Remo Proietti Zaccaria, Silvia Dante, and Marco Salerno. 2016. "Fabrication of Gold-Coated Ultra-Thin Anodic Porous Alumina Substrates for Augmented SERS" Materials 9, no. 6: 403. https://doi.org/10.3390/ma9060403
APA StyleToccafondi, C., Proietti Zaccaria, R., Dante, S., & Salerno, M. (2016). Fabrication of Gold-Coated Ultra-Thin Anodic Porous Alumina Substrates for Augmented SERS. Materials, 9(6), 403. https://doi.org/10.3390/ma9060403








