Evaluation of Functionalized Porous Titanium Implants for Enhancing Angiogenesis in Vitro
Abstract
:1. Introduction
2. Results
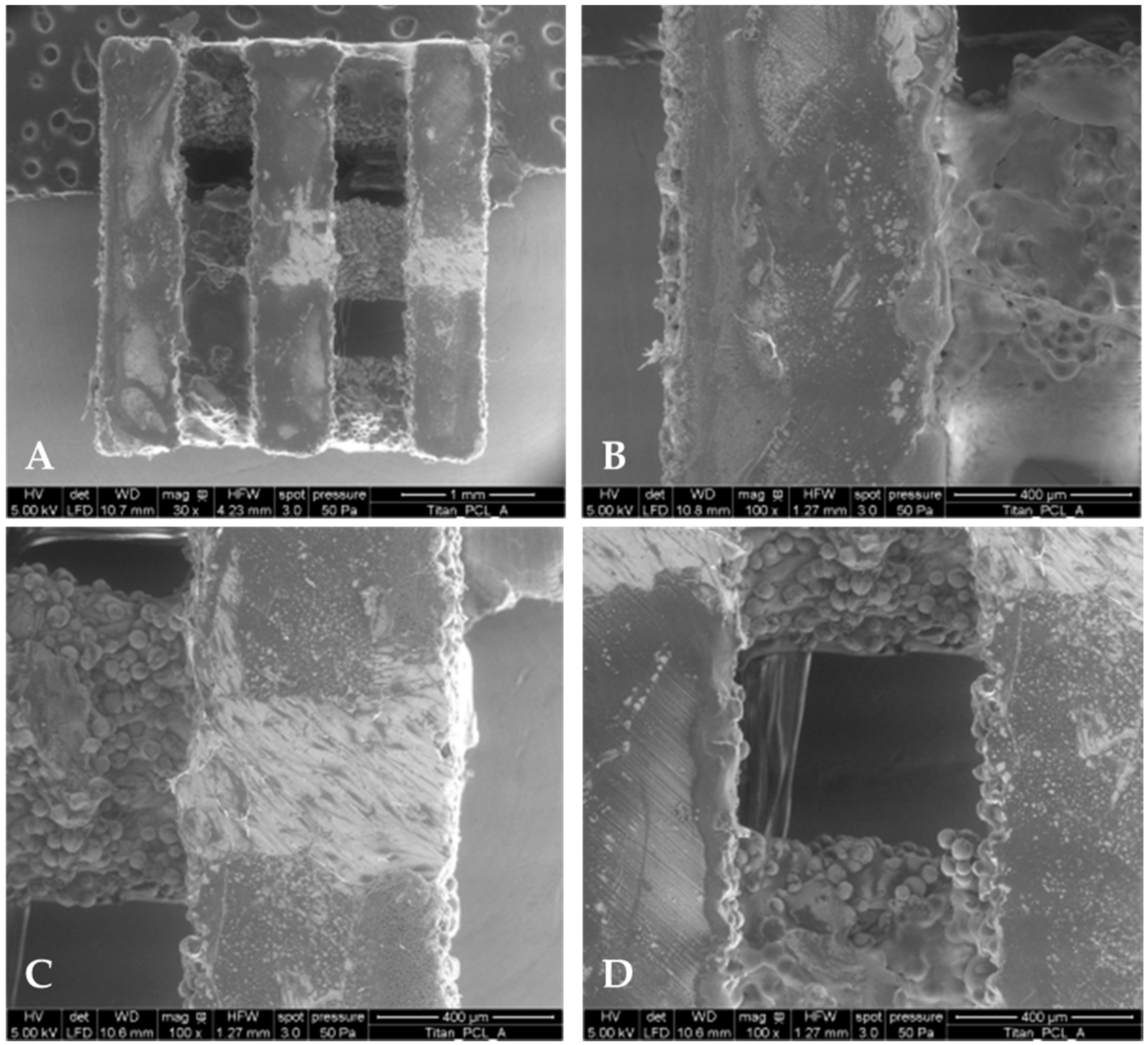
2.1. PCL Coating Thickness and Mass
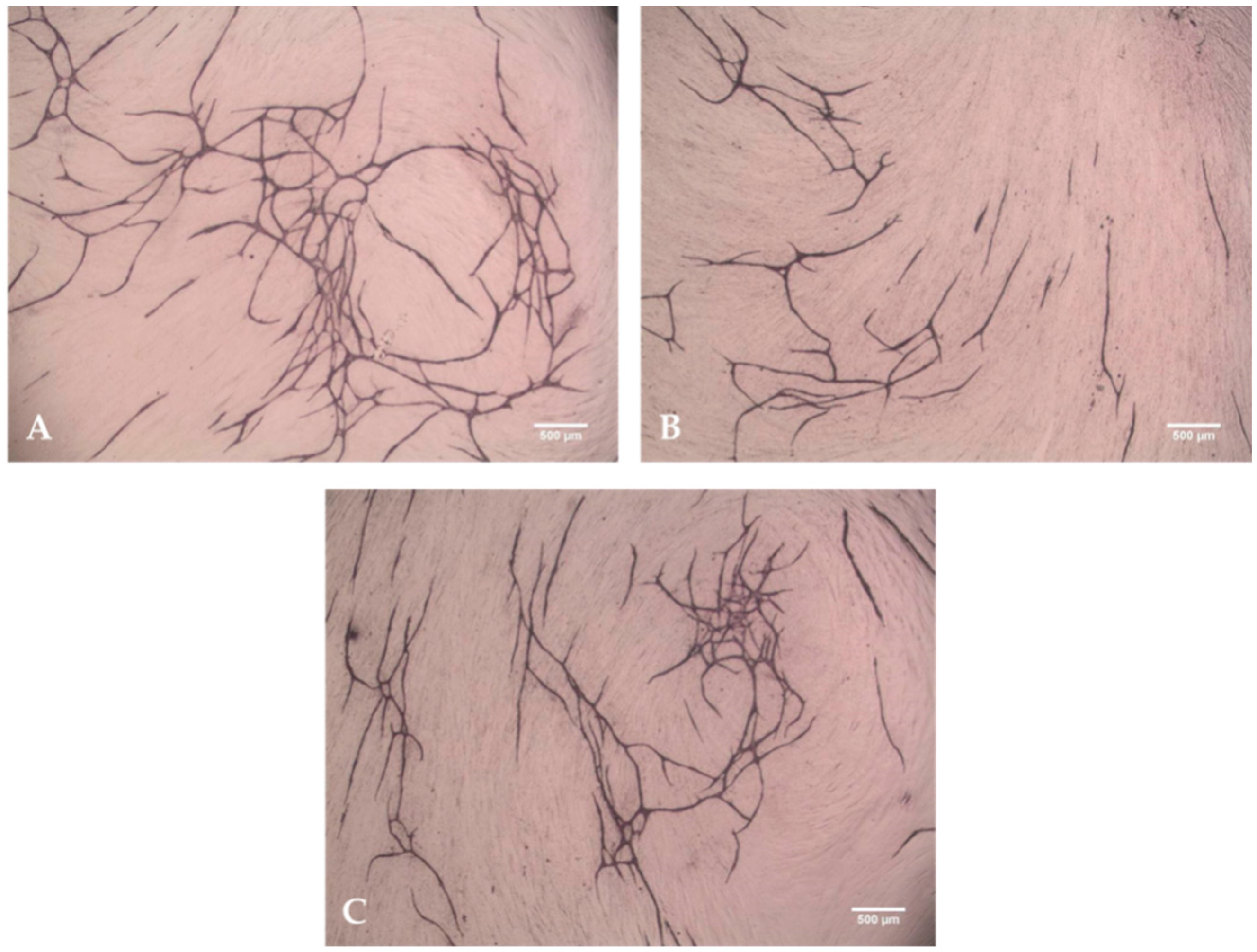
2.2. Migration Assay
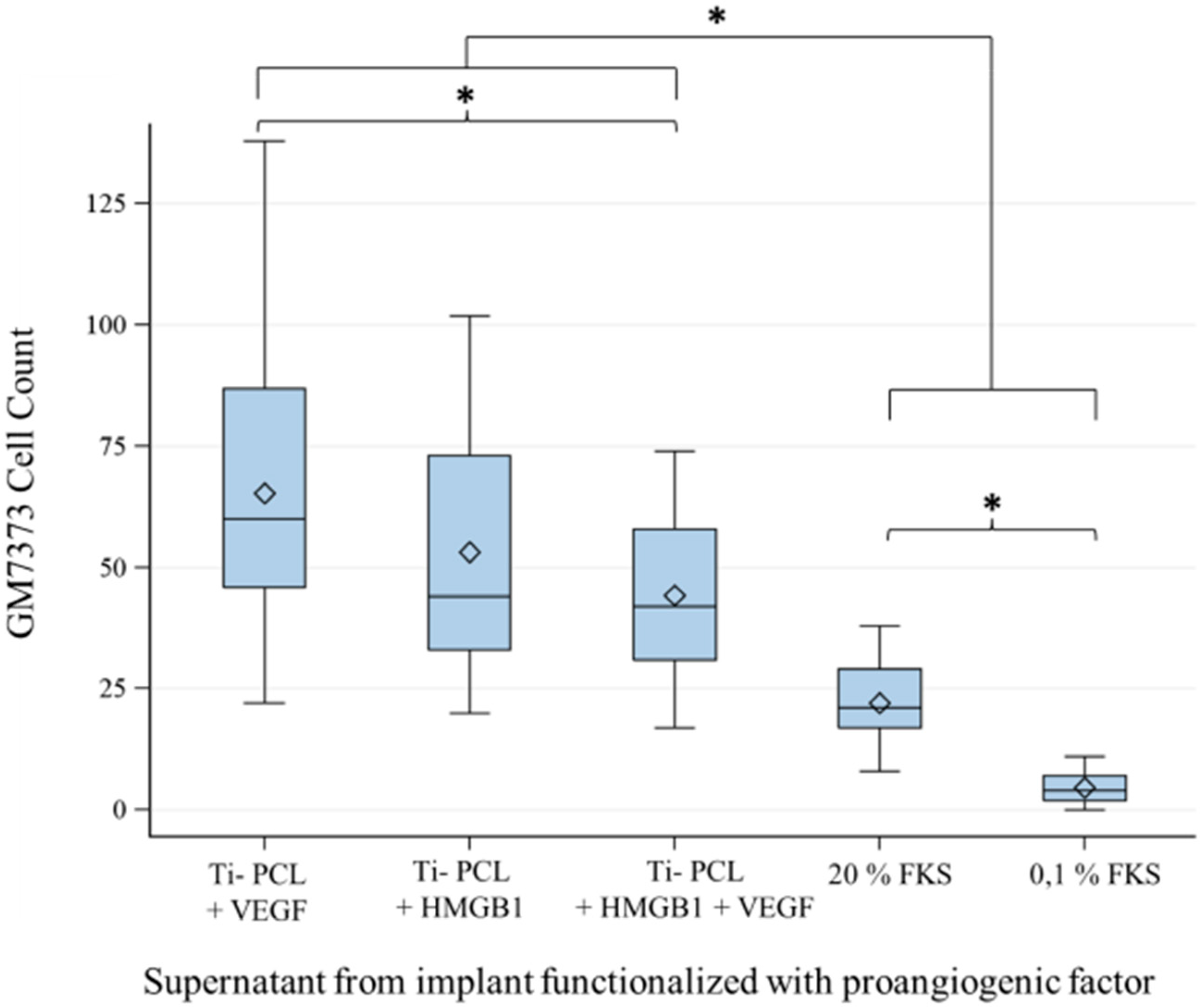
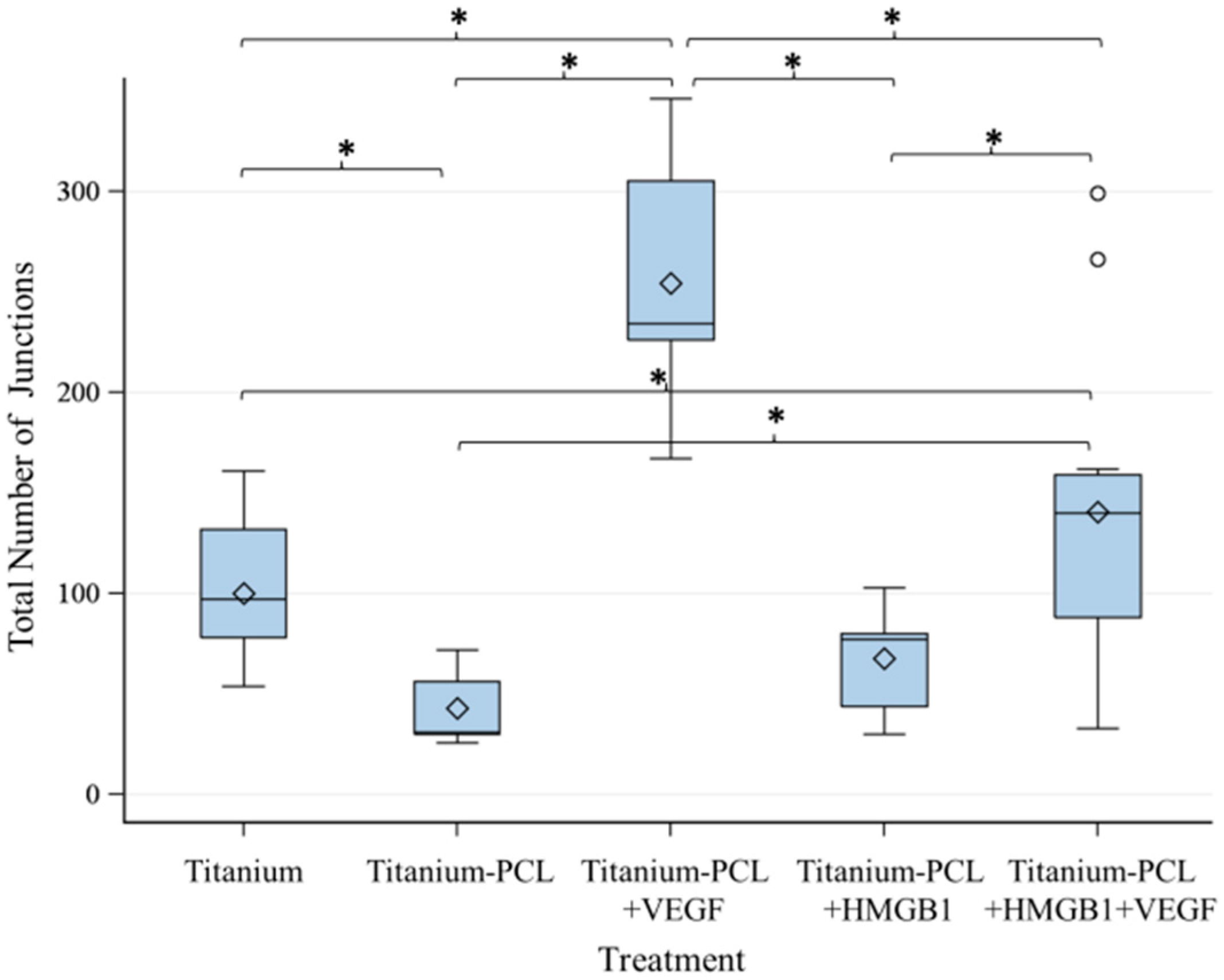
2.3. Angiogenesis Assay with Functionalized Titanium Implants
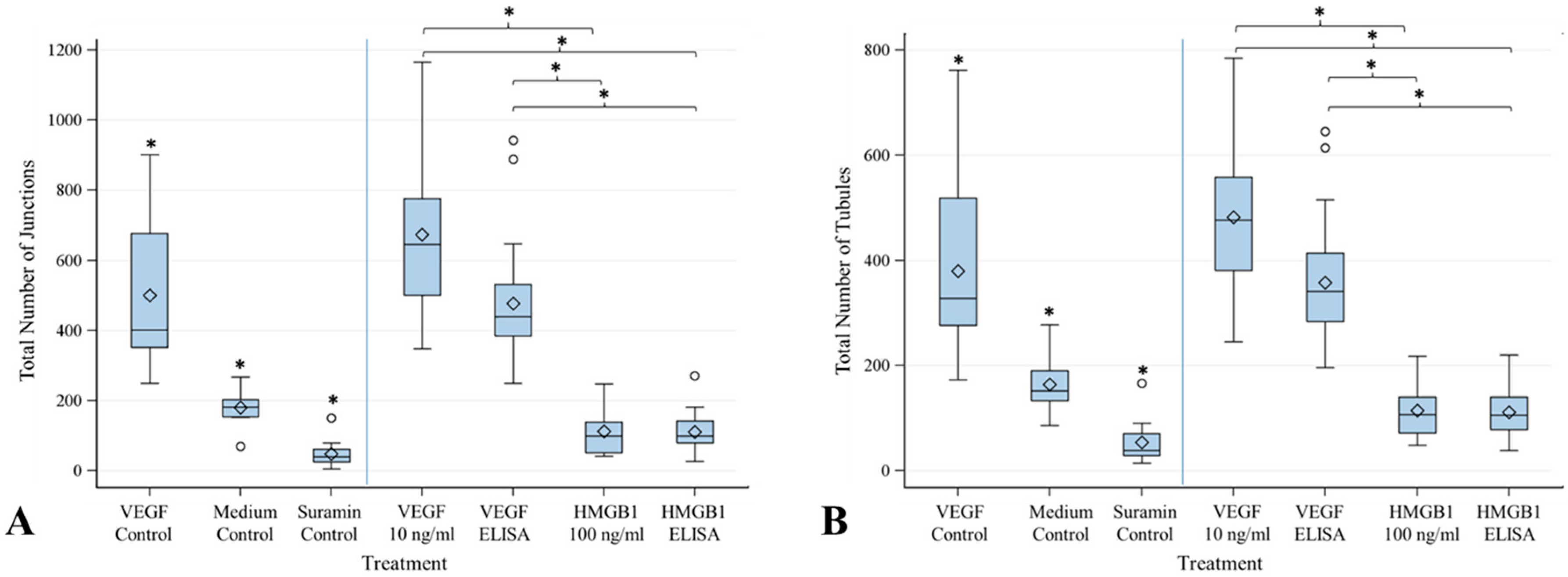
2.4. Angiogenesis Assay with Cytokines HMGB1 and VEGF
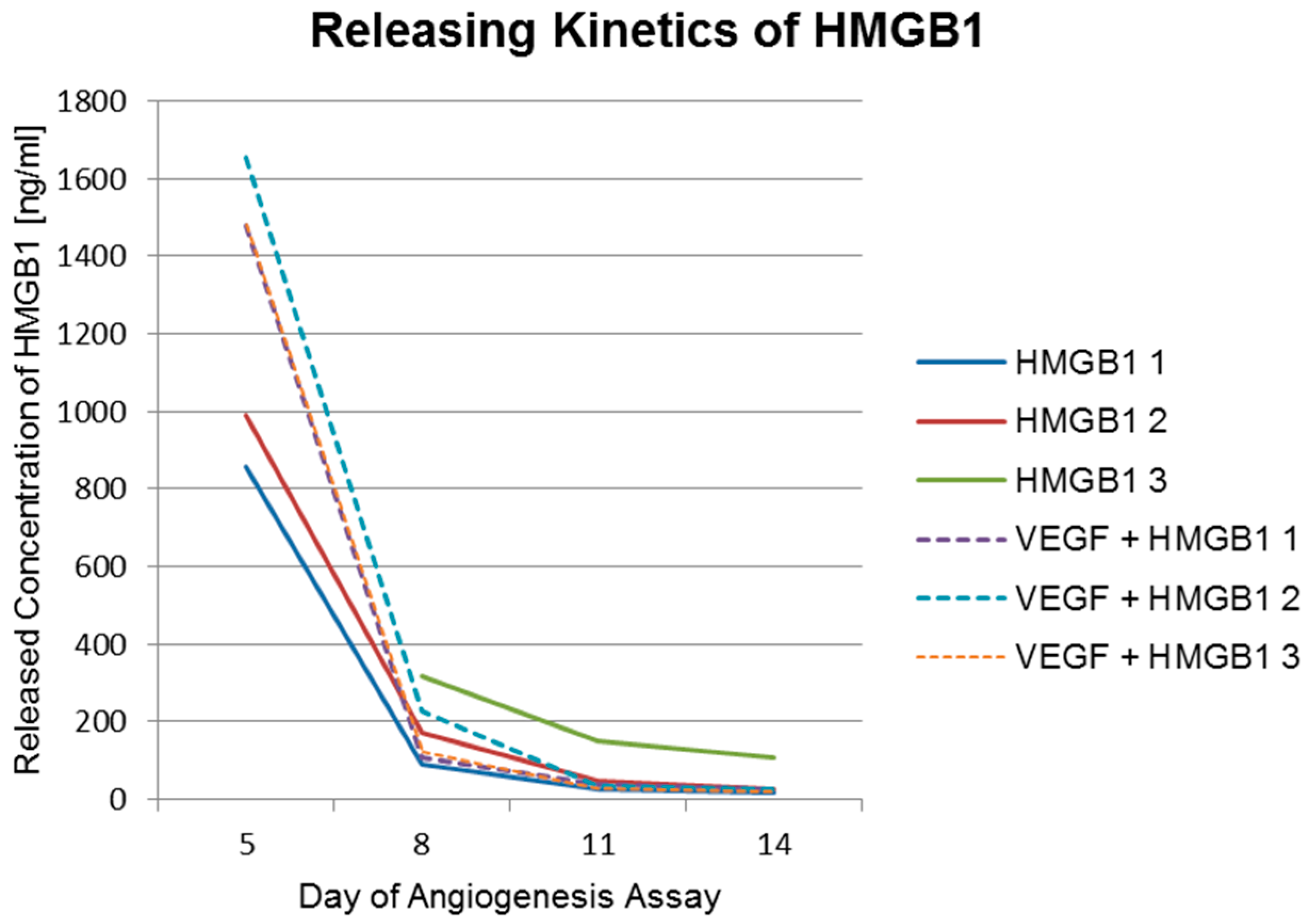
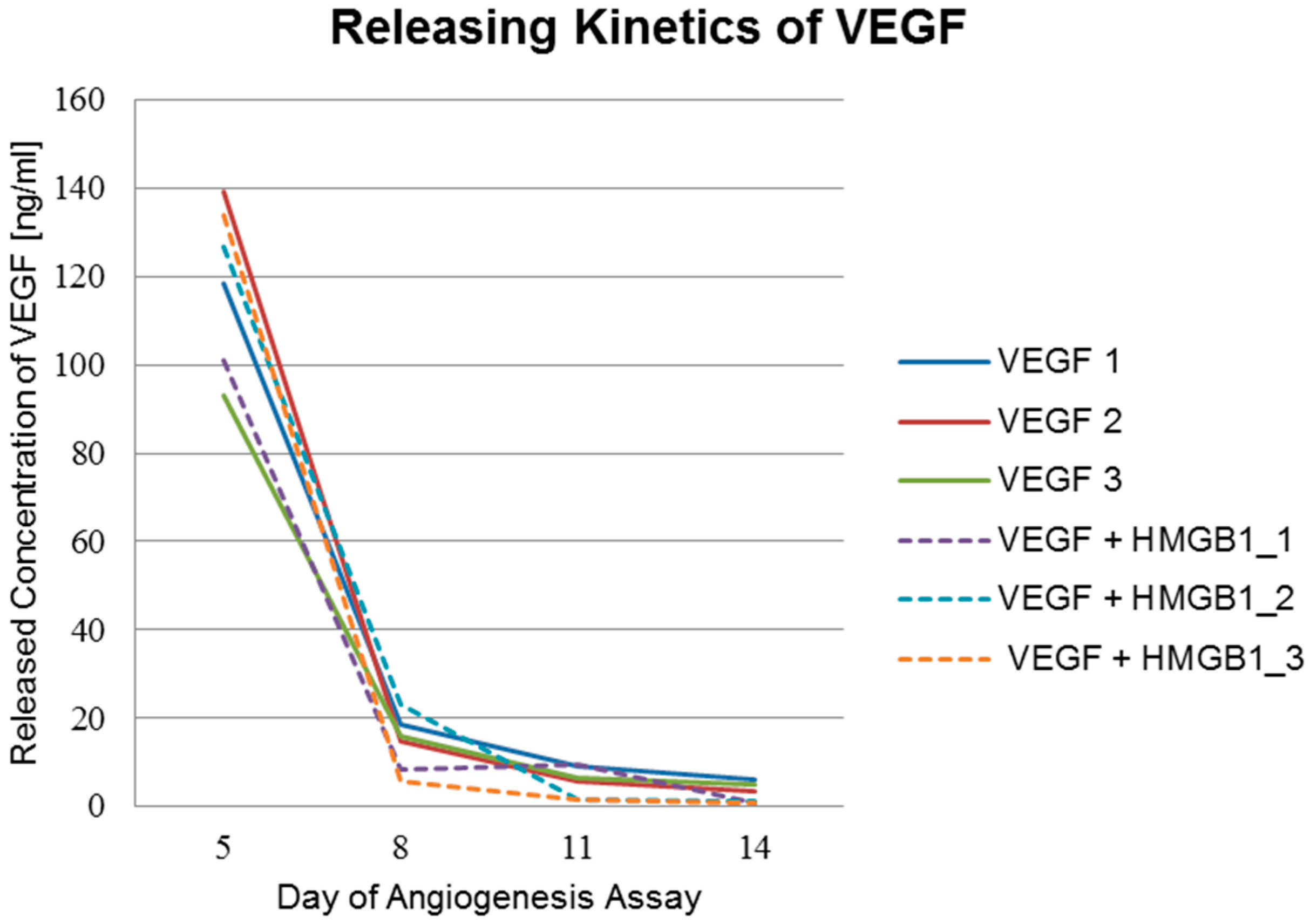
2.5. Factor Releasing Amounts of Functionalized Titanium Implants
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. PCL Coating of Titanium Implants and Characterization
4.3. Incorporation of VEGF and HMGB1 into Titanium Implants
4.4. Migration Assay of GM7373 on Functionalized Titanium PCL Implants
4.5. Angiogenesis Assay with Functionalized Titanium PCL Implants
4.6. Angiogenesis Assay with Cytokines VEGF and HMGB1
4.7. Factor Releasing Kinetics of Functionalized Titanium Implants
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Saran, U.; Gemini Piperni, S.; Chatterjee, S. Role of angiogenesis in bone repair. Arch. Biochem. Biophys. 2014, 561, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Carano, R.A.; Filvaroff, E.H. Angiogenesis and bone repair. Drug Discov. Today 2003, 8, 980–989. [Google Scholar] [CrossRef]
- Kanczler, J.M.; Oreffo, R.O. Osteogenesis and angiogenesis: The potential for engineering bone. Eur. Cells Mater. 2008, 15, 100–114. [Google Scholar]
- Einhorn, T.A. Enhancement of fracture-healing. J. Bone Jt. Surg. Am. Vol. 1995, 77, 940–956. [Google Scholar]
- Guo, J.; Meng, Z.; Chen, G.; Xie, D.; Chen, Y.; Wang, H.; Tang, W.; Liu, L.; Jing, W.; Long, J.; et al. Restoration of critical-size defects in the rabbit mandible using porous nanohydroxyapatite-polyamide scaffolds. Tissue Eng. Part A 2012, 18, 1239–1252. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, C.; Rentsch, B.; Breier, A.; Spekl, K.; Jung, R.; Manthey, S.; Scharnweber, D.; Zwipp, H.; Biewener, A. Long-bone critical-size defects treated with tissue-engineered polycaprolactone-co-lactide scaffolds: A pilot study on rats. J. Biomed. Mater. Res. Part A 2010, 95, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Lv, K.; Zhang, W.; Zhang, X.; Jiang, X.; Zhang, F. The healing of critical-size calvarial bone defects in rat with rhPDGF-BB, BMSCs, and β-TCP scaffolds. J. Mater. Sci. Mater. Med. 2012, 23, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Gellrich, N.C.; Held, U.; Schoen, R.; Pailing, T.; Schramm, A.; Bormann, K.H. Alveolar zygomatic buttress: A new donor site for limited preimplant augmentation procedures. J. Oral Maxillofac. Surg. 2007, 65, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Goulet, J.A.; Senunas, L.E.; DeSilva, G.L.; Greenfield, M.L. Autogenous iliac crest bone graft. Complications and functional assessment. Clin. Orthop. Relat. Res. 1997, 339, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Silber, J.S.; Anderson, D.G.; Daffner, S.D.; Brislin, B.T.; Leland, J.M.; Hilibrand, A.S.; Vaccaro, A.R.; Albert, T.J. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine 2003, 28, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Li, S.H.; Van Blitterswijk, C.A.; de Groot, K. Cancellous bone from porous Ti6Al4V by multiple coating technique. J. Mater. Sci. Mater. Med. 2006, 17, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Van der Stok, J.; Van der Jagt, O.P.; Amin Yavari, S.; De Haas, M.F.; Waarsing, J.H.; Jahr, H.; Van Lieshout, E.M.; Patka, P.; Verhaar, J.A.; Zadpoor, A.A.; et al. Selective laser melting-produced porous titanium scaffolds regenerate bone in critical size cortical bone defects. J. Orthop. Res. 2013, 31, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Janssen, L.M.; van Osch, G.J.; Li, J.P.; Kops, N.; de Groot, K.; Von den Hoff, J.W.; Feenstra, L.; Hardillo, J.A. Tracheal reconstruction: Mucosal survival on porous titanium. Arch. Otolaryngol. Head Neck Surg. 2009, 135, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Li, C.; Zhu, Z.; Liu, C.S. Measurement of the dynamic Young’s modulus of porous titanium and Ti6Al4V. J. Mater. Sci. 2007, 42, 7348–7353. [Google Scholar] [CrossRef]
- Van der Stok, J.; Wang, H.; Amin Yavari, S.; Siebelt, M.; Sandker, M.; Waarsing, J.H.; Verhaar, J.A.; Jahr, H.; Zadpoor, A.A.; Leeuwenburgh, S.C.; et al. Enhanced bone regeneration of cortical segmental bone defects using porous titanium scaffolds incorporated with colloidal gelatin gels for time- and dose-controlled delivery of dual growth factors. Tissue Eng. Part A 2013, 19, 2605–2614. [Google Scholar] [CrossRef] [PubMed]
- Roland, L.; Grau, M.; Matena, J.; Teske, M.; Gieseke, M.; Kampmann, A.; Beyerbach, M.; Murua Escobar, H.; Haferkamp, H.; Gellrich, N.C.; et al. Poly-ε-caprolactone coated and functionalized porous titanium and magnesium implants for enhancing angiogenesis in critically sized bone defects. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Geiger, M.; Li, R.H.; Friess, W. Collagen sponges for bone regeneration with rhBMP-2. Adv. Drug Deliv. Rev. 2003, 55, 1613–1629. [Google Scholar] [CrossRef] [PubMed]
- Lindhorst, D.; Tavassol, F.; von See, C.; Schumann, P.; Laschke, M.W.; Harder, Y.; Bormann, K.H.; Essig, H.; Kokemuller, H.; Kampmann, A.; et al. Effects of VEGF loading on scaffold-confined vascularization. J. Biomed. Mater. Res. Part A 2010, 95, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Koenig, G.; Charpiot, A.; Debry, C.; Voegel, J.-C.; Lavalle, P.; Vautier, D. VEGF-functionalized polyelectrolyte multilayers as proangiogenic prosthetic coatings. Adv. Funct. Mater. 2008, 18, 1767–1775. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Lam, C.X.; Hutmacher, D.W.; Schantz, J.T.; Woodruff, M.A.; Teoh, S.H. Evaluation of polycaprolactone scaffold degradation for 6 months in vitro and in vivo. J. Biomed. Mater. Res. Part A 2009, 90, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, C.; Zonefrati, R.; Galli, G.; Puppi, D.; Pirosa, A.; Chiellini, F.; Martelli, F.S.; Tanini, A.; Brandi, M.L. In vitro behavior of human adipose tissue-derived stem cells on poly(ε-caprolactone) film for bone tissue engineering applications. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, A.A.; Song, S.J.; Susanto, E.; Chuan, P.; Lam, C.X.; Woodruff, M.A.; Hutmacher, D.W.; Cool, S.M. The stimulation of healing within a rat calvarial defect by mPCL-TCP/collagen scaffolds loaded with rhBMP-2. Biomaterials 2009, 30, 2479–2488. [Google Scholar] [CrossRef] [PubMed]
- Breier, G.; Risau, W. The role of vascular endothelial growth factor in blood vessel formation. Trends Cell Biol. 1996, 6, 454–456. [Google Scholar] [CrossRef]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef] [PubMed]
- Schlueter, C.; Weber, H.; Meyer, B.; Rogalla, P.; Roser, K.; Hauke, S.; Bullerdiek, J. Angiogenetic signaling through hypoxia: HMGB1: An angiogenetic switch molecule. Am. J. Pathol. 2005, 166, 1259–1263. [Google Scholar] [CrossRef]
- Van Beijnum, J.R.; Buurman, W.A.; Griffioen, A.W. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis 2008, 11, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Mitola, S.; Belleri, M.; Urbinati, C.; Coltrini, D.; Sparatore, B.; Pedrazzi, M.; Melloni, E.; Presta, M. Cutting edge: Extracellular high mobility group box-1 protein is a proangiogenic cytokine. J. Immunol. 2006, 176, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Matena, J.; Petersen, S.; Gieseke, M.; Kampmann, A.; Teske, M.; Beyerbach, M.; Murua Escobar, H.; Haferkamp, H.; Gellrich, N.C.; Nolte, I. SLM produced porous titanium implant improvements for enhanced vascularization and osteoblast seeding. Int. J. Mol. Sci. 2015, 16, 7478–7492. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, A.; Blood, D.C.; del Toro, G.; Canet, A.; Lee, D.C.; Qu, W.; Tanji, N.; Lu, Y.; Lalla, E.; Fu, C.; et al. Blockade of rage-amphoterin signalling suppresses tumour growth and metastases. Nature 2000, 405, 354–360. [Google Scholar] [PubMed]
- Flege, C.; Vogt, F.; Hoges, S.; Jauer, L.; Borinski, M.; Schulte, V.A.; Hoffmann, R.; Poprawe, R.; Meiners, W.; Jobmann, M.; et al. Development and characterization of a coronary polylactic acid stent prototype generated by selective laser melting. J. Mater. Sci. Mater. Med. 2013, 24, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Vroman, L.; Adams, A.L. Findings with the recording ellipsometer suggesting rapid exchange of specific plasma proteins at liquid/solid interfaces. Surf. Sci. 1969, 16, 438–446. [Google Scholar] [CrossRef]
- Vroman, L.; Adams, A.L. Identification of rapid changes at plasma-solid interfaces. J. Biomed. Mater. Res. 1969, 3, 43–67. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H. Protein adsorption on polymer particles. In Encyclopedia of Surface and Colloid Science; Somasundaran, P., Ed.; CRC Press: Boca Raton, FL, USA, 2004; Volume 5, pp. 519–523. [Google Scholar]
- Hirsh, S.L.; McKenzie, D.R.; Nosworthy, N.J.; Denman, J.A.; Sezerman, O.U.; Bilek, M.M. The vroman effect: Competitive protein exchange with dynamic multilayer protein aggregates. Colloids Surf. B Biointerfaces 2013, 103, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Matena, J.; Petersen, S.; Gieseke, M.; Teske, M.; Beyerbach, M.; Kampmann, A.; Murua Escobar, H.; Gellrich, N.C.; Haferkamp, H.; Nolte, I. Comparison of selective laser melted titanium and magnesium implants coated with PCL. Int. J. Mol. Sci. 2015, 16, 13287–13301. [Google Scholar] [CrossRef] [PubMed]
- V2a Kit Protocol. Available online: http://www.cellworks.co.uk/v2akit.php (accessed on 1 August 2015).




| Sample | Coating Thickness of PCL (µm) |
|---|---|
| Titanium-PCL | 11.6 ± 6.2 |
| Titanium-PCL + VEGF | 11.4 ± 7.3 |
| Titanium-PCL + HMGB1 | 15.5 ± 10.1 |
| Titanium-PCL + VEGF + HMGB1 | 15.3 ± 7.4 |
| Concentration of HMGB1 (ng/mL) | Day 5 | Day 8 | Day 11 | Day 14 |
|---|---|---|---|---|
| HMGB1_1 | 858 | 89 | 27 | 19 |
| HMGB1_2 | 991 | 173 | 49 | 28 |
| HMGB1_3 | >1678 | 316 | 152 | 107 |
| VEGF + HMGB1_1 | 1477 | 110 | 41 | 26 |
| VEGF + HMGB1_2 | 1655 | 228 | 37 | 27 |
| VEGF + HMGB1_3 | 1479 | 121 | 29 | 19 |
| Concentration of VEGF (ng/mL) | Day 5 | Day 8 | Day 11 | Day 14 |
|---|---|---|---|---|
| VEGF_1 | 118 | 19 | 9 | 6 |
| VEGF_2 | 139 | 15 | 6 | 3 |
| VEGF_3 | 93 | 16 | 7 | 5 |
| VEGF + HMGB1_1 | 101 | 8 | 9 | 1 |
| VEGF + HMGB1_2 | 127 | 23 | 2 | 1 |
| VEGF + HMGB1_3 | 134 | 6 | 2 | 1 |
| Points in Time | VEGF (ng/mL) | HMGB1 (ng/mL) |
|---|---|---|
| Concentration added at day 2 | 117 | 924 |
| Concentration added at day 5 | 16 | 130 |
| Concentration added at day 8 | 7 | 76 |
| Concentration added at day 11 | 5 | 24 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roland, L.; Backhaus, S.; Grau, M.; Matena, J.; Teske, M.; Beyerbach, M.; Murua Escobar, H.; Haferkamp, H.; Gellrich, N.-C.; Nolte, I. Evaluation of Functionalized Porous Titanium Implants for Enhancing Angiogenesis in Vitro. Materials 2016, 9, 304. https://doi.org/10.3390/ma9040304
Roland L, Backhaus S, Grau M, Matena J, Teske M, Beyerbach M, Murua Escobar H, Haferkamp H, Gellrich N-C, Nolte I. Evaluation of Functionalized Porous Titanium Implants for Enhancing Angiogenesis in Vitro. Materials. 2016; 9(4):304. https://doi.org/10.3390/ma9040304
Chicago/Turabian StyleRoland, Laura, Samantha Backhaus, Michael Grau, Julia Matena, Michael Teske, Martin Beyerbach, Hugo Murua Escobar, Heinz Haferkamp, Nils-Claudius Gellrich, and Ingo Nolte. 2016. "Evaluation of Functionalized Porous Titanium Implants for Enhancing Angiogenesis in Vitro" Materials 9, no. 4: 304. https://doi.org/10.3390/ma9040304





