Label Free Poly(2,5-dimethoxyaniline)–Multi-Walled Carbon Nanotubes Impedimetric Immunosensor for Fumonisin B1 Detection
Abstract
:1. Introduction
2. Results and Discussion
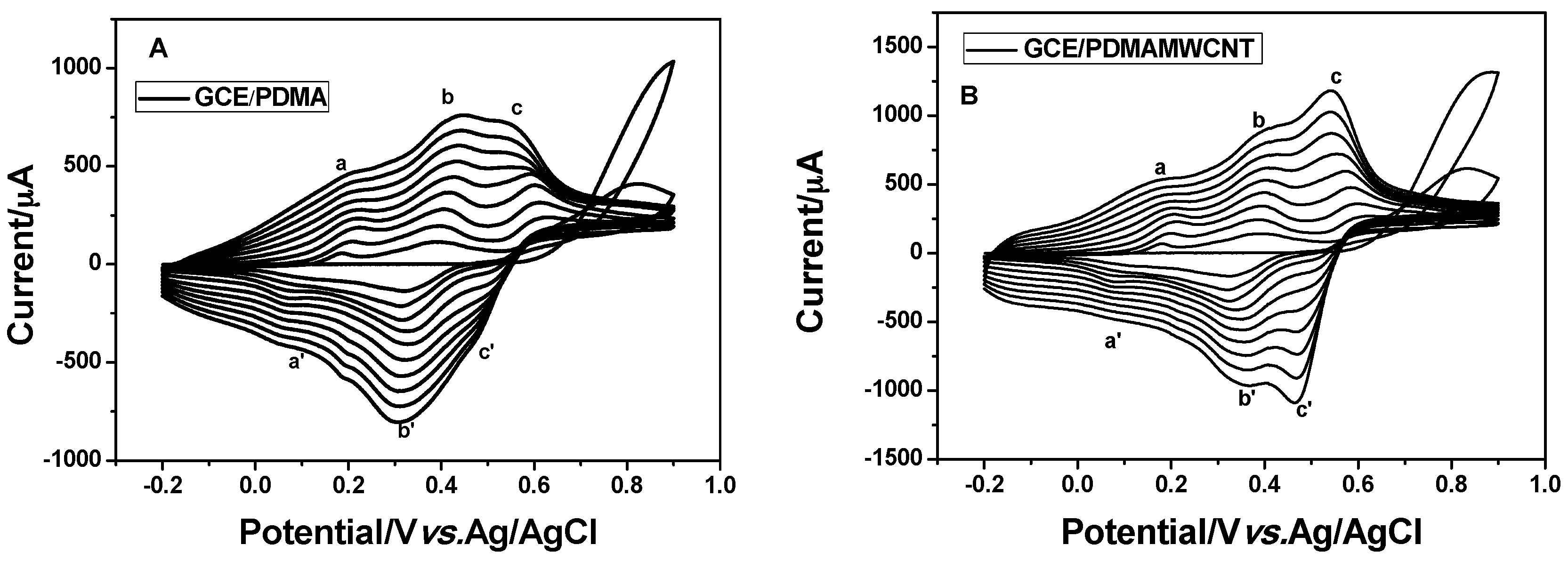
2.1. Electrochemical Synthesis of the PDMA-MWCNT Composite
2.2. Characterization of the PDMA-MWCNT Composite
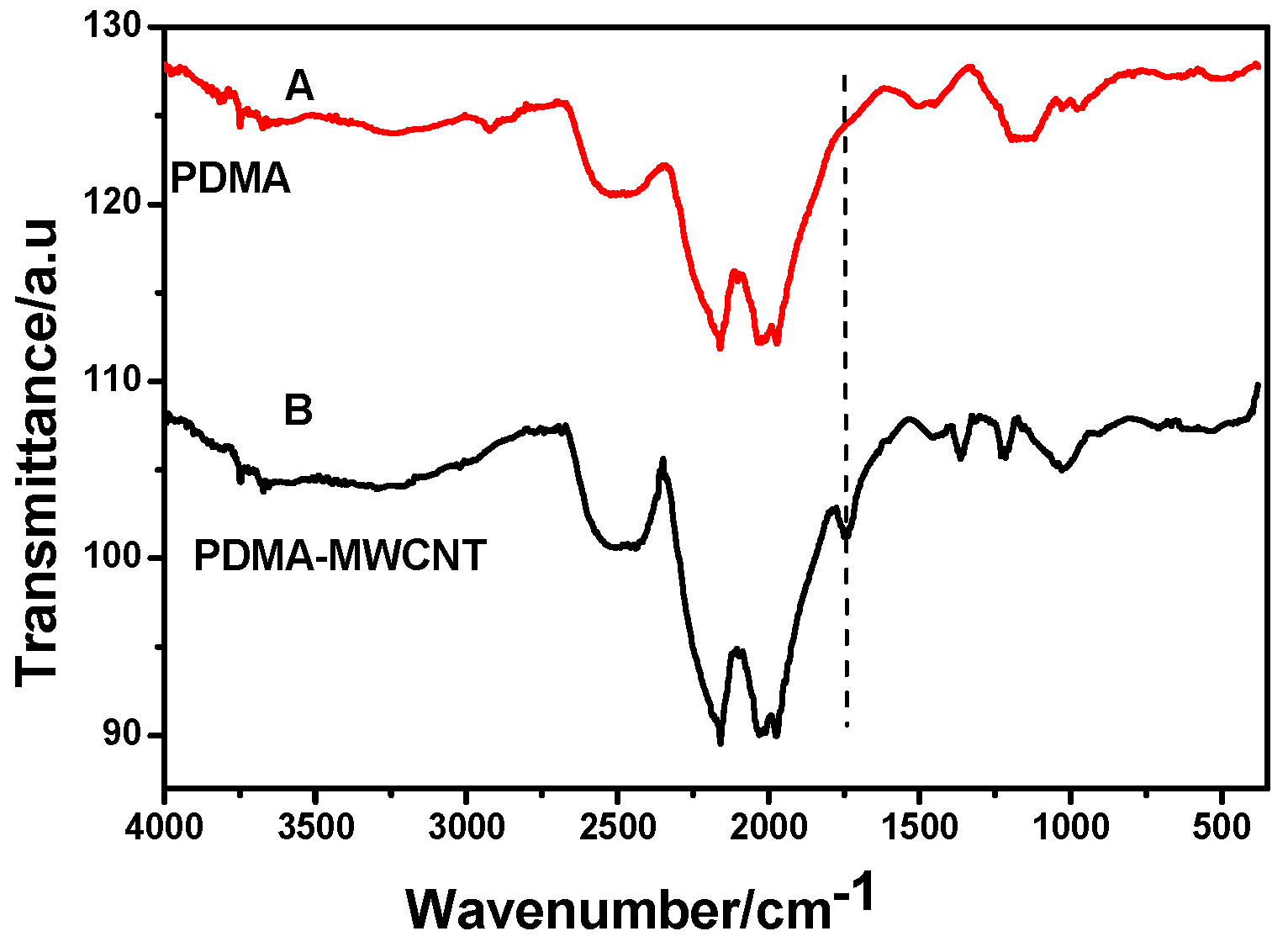
2.2.1. Fourier Transform Infrared Spectroscopy
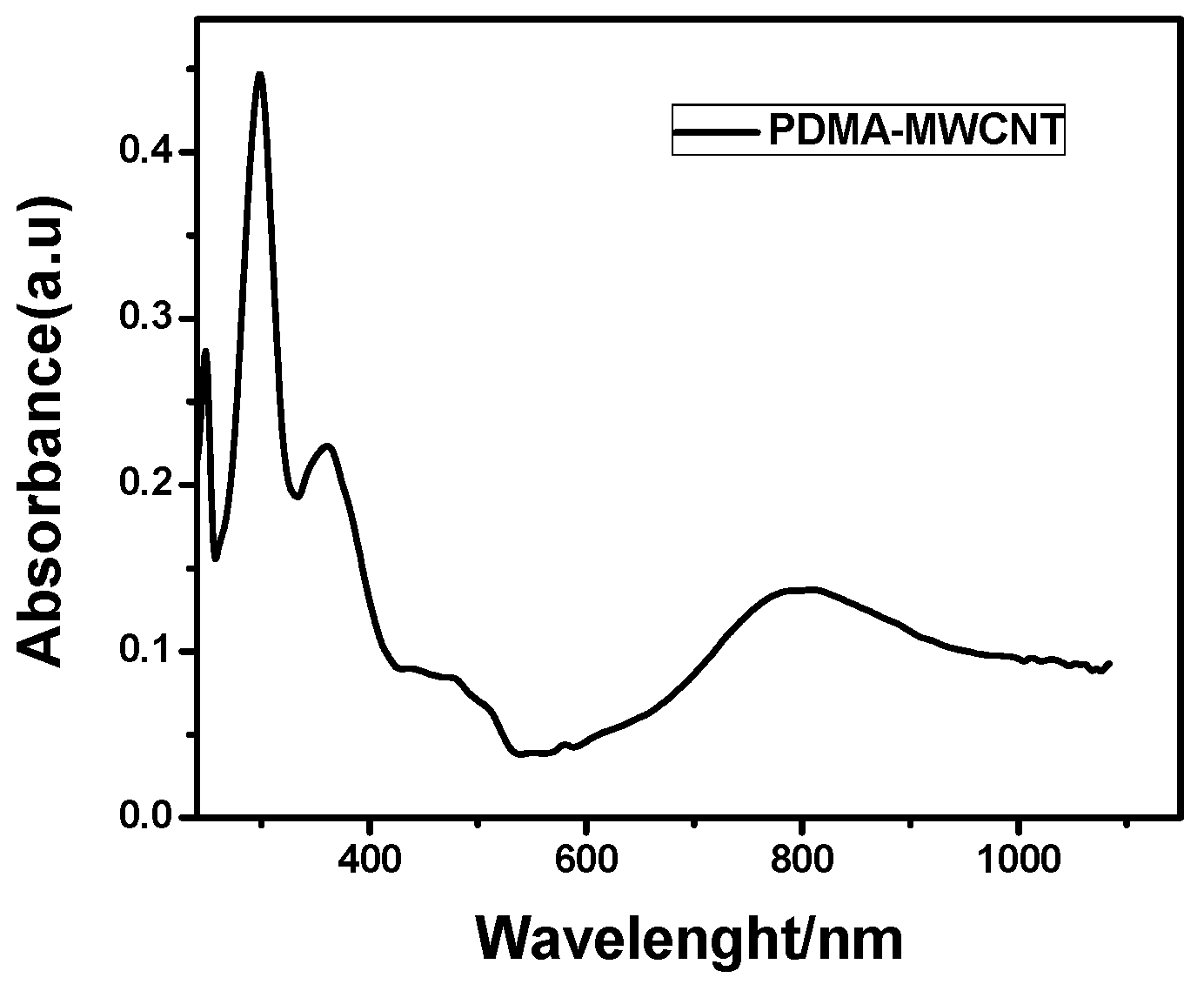
2.2.2. Ultraviolet-Visible Spectroscopy
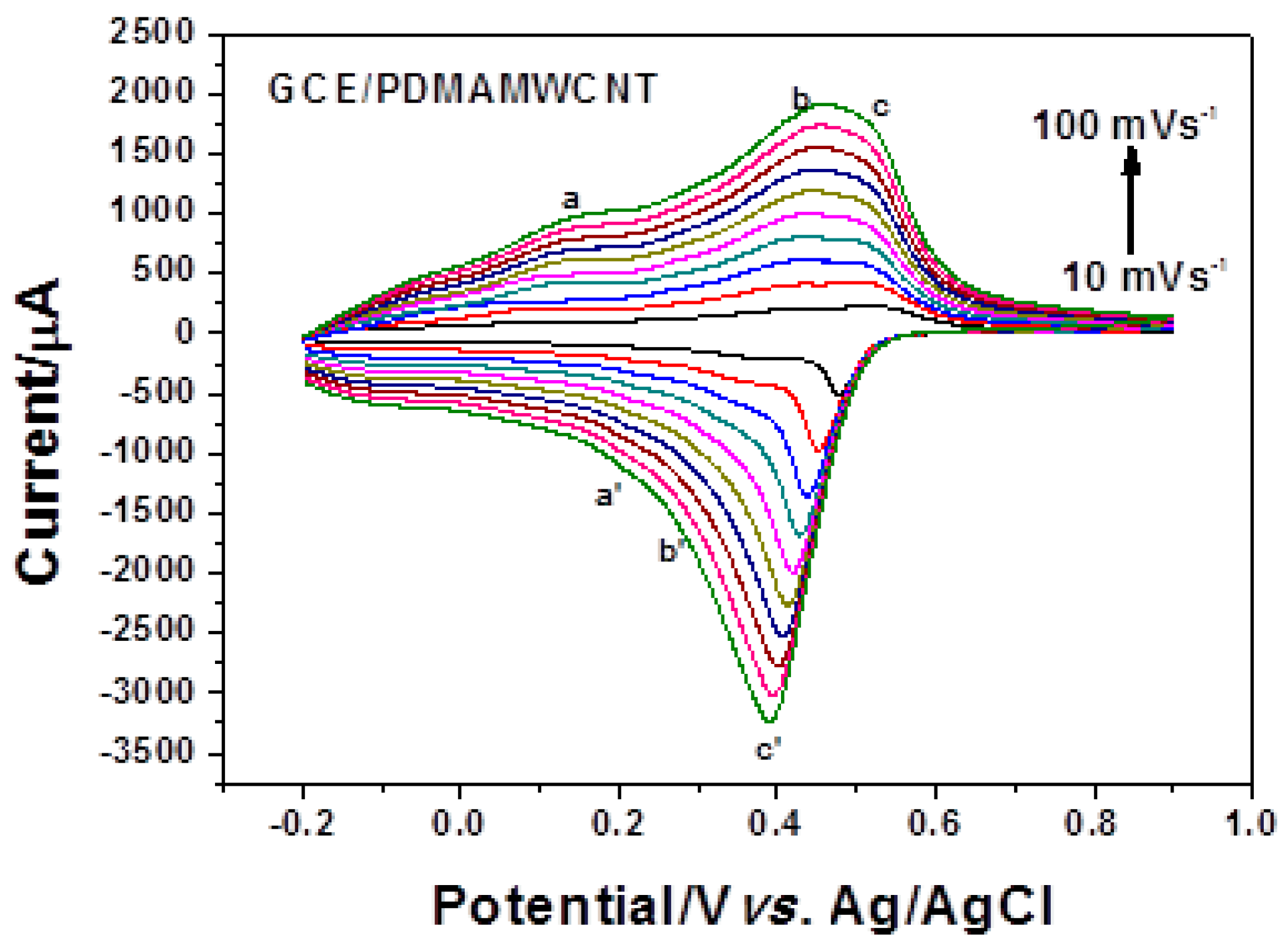
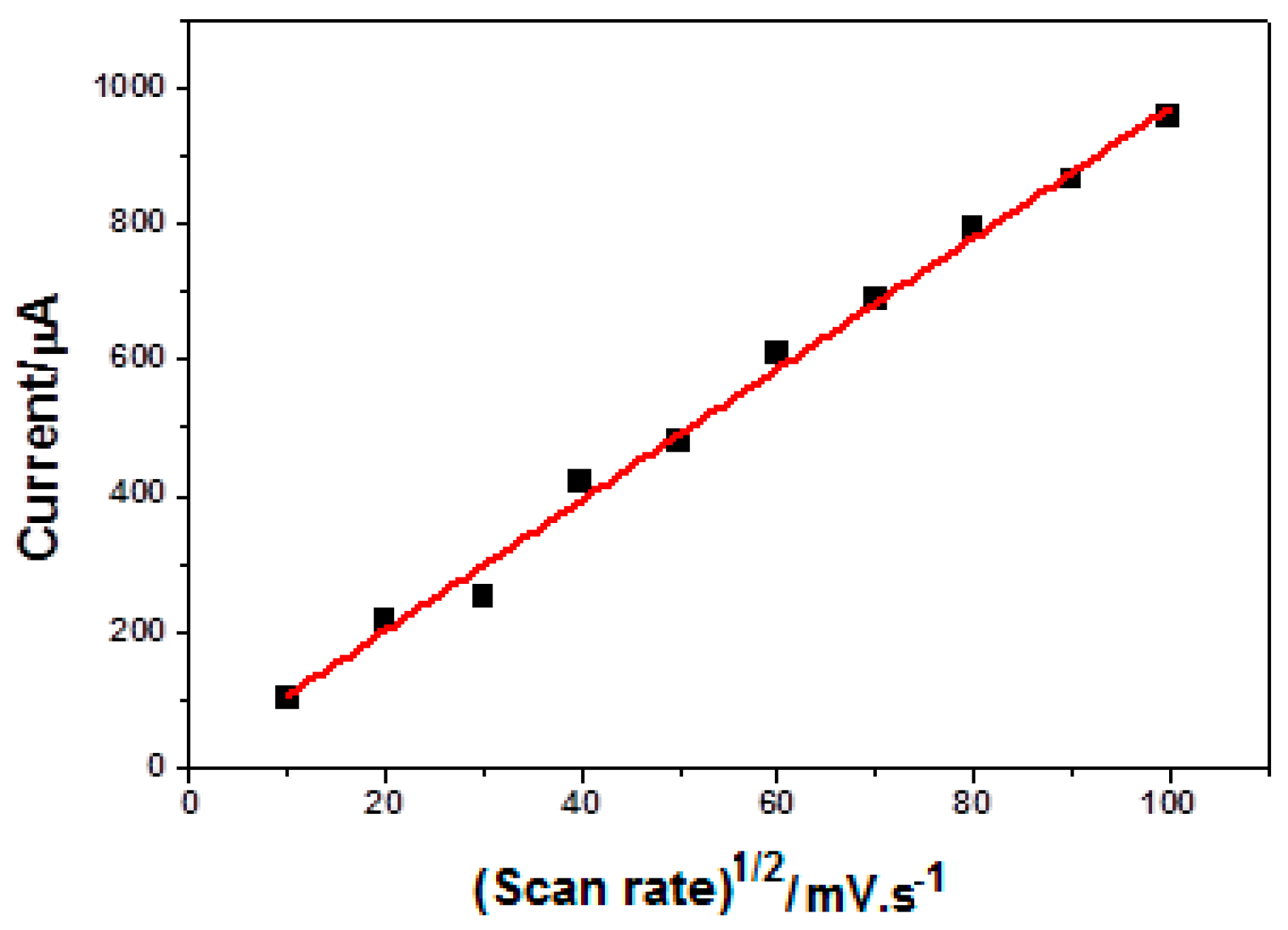
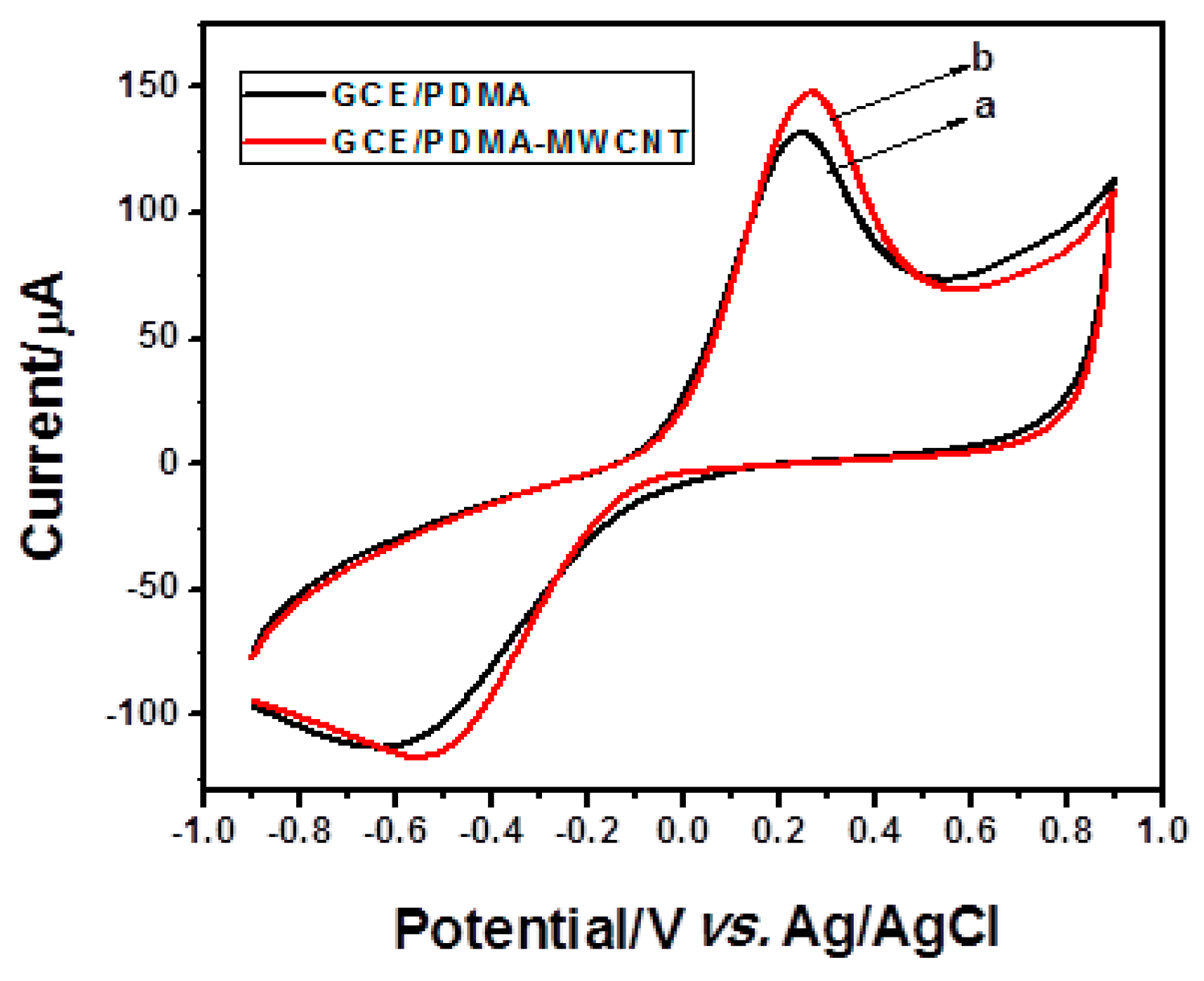
2.2.3. Electrochemical Characterization
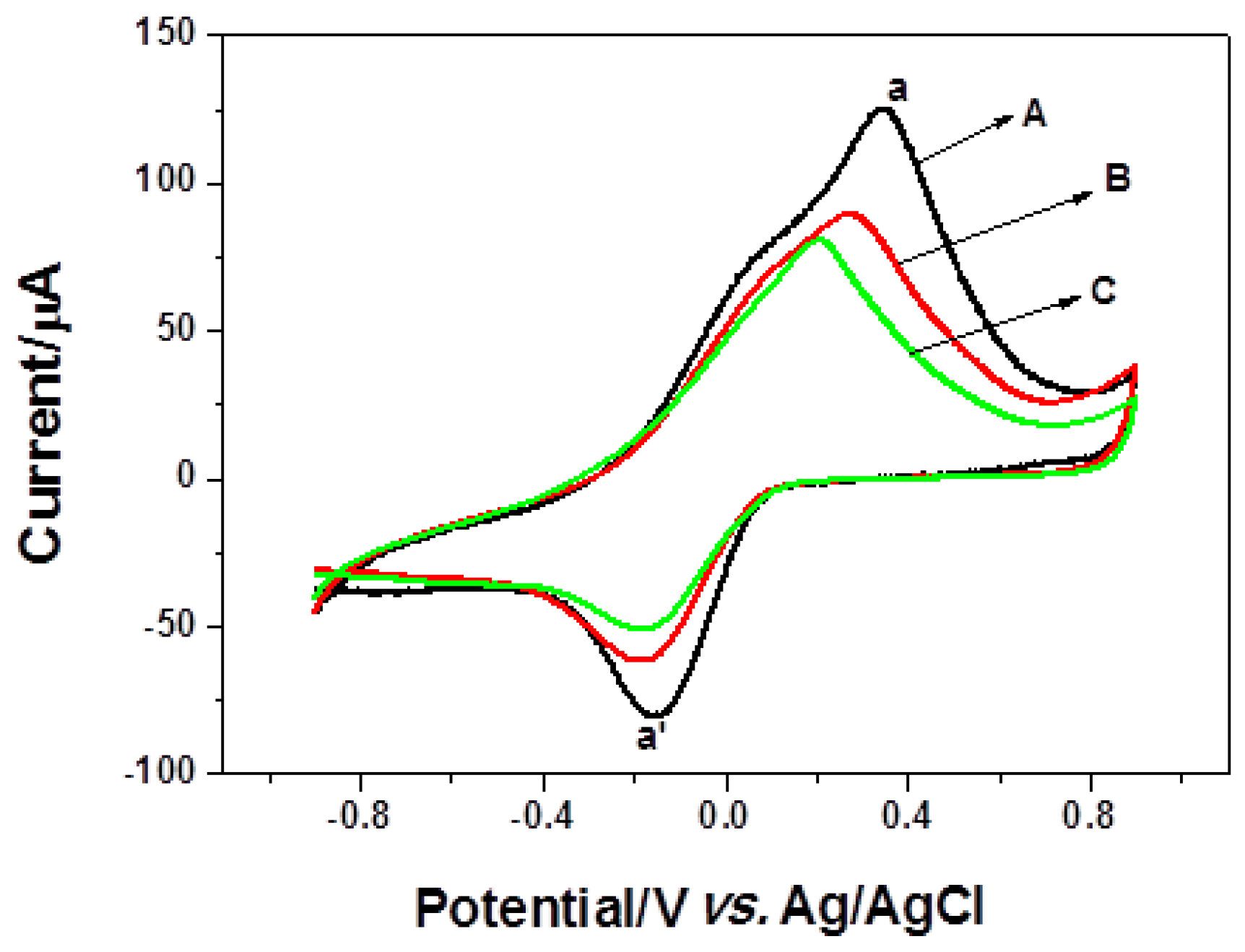
2.3. Immobilization of the Antibody and Characterization of the Immunosensor
2.4. Performance of the Immunosensor
2.5. Stability and Repeatability of the Immunosensor
2.6. Analysis of Certified Corn Reference Material
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Instrumentation
3.3. Electrochemical Synthesis of Poly(2,5-dimethoxyaniline) Multi-Walled Carbon Nanotube Composite-Modified Glassy Carbon Electrode
3.4. Fabrication of the Immunosensor
3.5. Extraction of Fumonisins from Certified Corn Reference Materials
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 6, 497–516. [Google Scholar] [CrossRef]
- Josephs, R.D.; Derbyshire, M.; Stroka, J.; Emons, H.; Anklam, E. Trichothecenes: Reference materials and method validation. Toxicol. Lett. 2004, 153, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.S.; Brasel, J.M. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef]
- Papp, E.; Otta, K.H.; Záray, G.; Mincsovics, E. Liquid chromatographic determination of aflatoxins. Microchem. J. 2002, 73, 39–46. [Google Scholar] [CrossRef]
- Adams, M.; Motarjemi, Y. Basic Food Safety for Health Workers; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- Richard, J.L.; Payne, G.A.; Desjardin, A.E.; Maragos, C.; Norred, W.P.; Pestka, J.J.; Phillips, T.D.; Van Egmond, H.P.; Vardon, P.J.; Whitaker, T.B.; et al. Mycotoxins Risks in Plant, Animal and Human Systems; CAST Task Force Report 139; Council for Agricultural Science and Technology: Ames, IA, USA, 2003. [Google Scholar]
- Yazar, S.; Omurtag, G.Z. Fumonisins, trichothecenes and zearalenone in cereals. Int. J. Mol. Sci. 2008, 9, 2062–2090. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.M.; González, L.; Catalá, A.I. Mechanism of action of sphingolipids and their metabolites in the toxicity of fumonisin B1. Prog. Lipid Res. 2005, 44, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Osorio, A.G.; Silveira, I.C.L.; Bueno, V.L.; Bergmann, C.P. H2SO4/HNO3/HCl functionalization and its effect on dispersion of carbon nanotubes in aqueous media. Appl. Surf. Sci. 2008, 255, 2485–2489. [Google Scholar] [CrossRef]
- Desjardins, A.E.; Manandhar, H.K.; Plattner, R.D.; Manandhar, G.G.; Poling, S.M.; Maragos, C.M. Fusarium species from Nepalese rice and production of mycotoxins and gibberellic acid by selected species. Appl. Environ. Microbiol. 2000, 66, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Severino, L.; Luongo, D.; Bergamo, P.; Lucisano, A.; Rossi, M. Mycotoxins nivalenol and deoxynivalenol differentially modulate cytokine mRNA expression in Jurkat T cells. Cytokine 2006, 36, 75–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rheeder, J.P.; Marasas, W.F.O.; Thiel, P.G.; Sydenham, E.W.; Shephard, G.S.; van Schalkwyk, D.J. Fusarium moniliforme and fumonisins in corn in relation to human esophageal cancer in Transkei. Phytopathology 1992, 82, 353–357. [Google Scholar] [CrossRef]
- Wang, S.; Quan, Y.; Lee, N.; Kennedy, I.R. Rapid determination of fumonisin B1 in food samples by enzyme-linked immunosorbent assay and colloidal gold immunoassay. J. Agric. Food Chem. 2006, 54, 2491–2495. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-Y.; Zheng, Y.T.; Zhang, H.B.; He, C.H.; Wu, W.D.; Zhang, H.B. DNA electrochemical aptasensor for detecting fumonisins B1 based on graphene and thionine nanocomposite. Electroanalysis 2015, 27, 1097–1103. [Google Scholar] [CrossRef]
- Masikini, M.; Mailu, S.; Tsegaye, A.; Njomo, N.; Molapo, K.; Ikpo, C.; Sunday, C.; Rassie, C.; Wilson, L.; Baker, P.; et al. A fumonisins immunosensor based on polyanilino-carbon nanotubes doped with palladium telluride quantum dots. Sensors 2015, 15, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, X.; Zhang, X.; Qing, Y.; Luo, M.; Liu, X.; Li, C.; Li, Y.; Xia, H.; Qiu, J. A highly sensitive electrochemical immunosensor for fumonisin B1 detection in corn using single-walled carbon nanotubes/chitosan. Electroanalysis 2015, 27, 2679–2687. [Google Scholar] [CrossRef]
- Gajendran, P.; Saraswathi, R. Polyaniline-carbon nanotube composites. Pure Appl. Chem. 2008, 80, 2377–2395. [Google Scholar] [CrossRef]
- Gajendran, P.; Saraswathi, R. Enhanced electrochemical growth and redox characteristics of poly(o-phenylenediamine) on a carbon nanotube modified glassy carbon electrode and its application in the electrocatalytic reduction of oxygen. J. Phys. Chem. C 2007, 111, 11320–11328. [Google Scholar] [CrossRef]
- Klinke, C.; Chen, J.; Afzali, A.; Avouris, P. Charge transfer induced polarity switching in carbon nanotube transistors. Nano Lett. 2005, 5, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Morrin, A.; Ngamna, O.; Killard, A.J.; Moulton, S.E.; Smyth, M.R.; Wallace, G.G. An amperometric enzyme biosensor fabricated from polyaniline nanoparticles. Electroanalysis 2005, 17, 423–430. [Google Scholar] [CrossRef]
- Hong, S.Y.; Jung, Y.M.; Kim, S.B.; Park, S.M. Electrochemistry of conductive polymers. 34. Two-dimensional correlation analysis of real-time spectroelectrochemical data for aniline polymerization. J. Phys. Chem. B 2005, 109, 3844–3850. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Yuan, R.; Chai, Y.; Li, W.; Zhong, X.; Zhang, Y. In situ chemo-synthesized multi-wall carbon nanotube-conductive polyaniline nanocomposites: Characterization and application for a glucose amperometric biosensor. Talanta 2011, 85, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Shahadat, M.; Nabi, S.A.; Bushra, R.; Raeissi, A.S.; Umar, K.; Ansari, M.O. Synthesis, characterization, photolytic degradation, electrical conductivity and applications of a nanocomposite adsorbent for the treatment of pollutants. RSC Adv. 2012, 2, 7207–7220. [Google Scholar] [CrossRef]
- Salvatierra, R.V.; Oliveira, M.M.; Zarbin, A.J.G. One-pot synthesis and processing of transparent, conducting, and freestanding carbon nanotubes/polyaniline composite films. Chem. Mater. 2010, 22, 5222–5234. [Google Scholar] [CrossRef]
- Nekrasov, A.A.; Ivanov, V.F.; Vannikov, A.V. Analysis of the structure of polyaniline absorption spectra based on spectroelectrochemical data. J. Electroanal. Chem. 2000, 482, 11–17. [Google Scholar] [CrossRef]
- Klink, M.J.; Iwuoha, E.I.; Ebenso, E.E. Electrochemical properties of nanotubes and nanomicelles from novel polyaniline and derivative. Int. J. Electrochem. Sci. 2012, 7, 3031–3046. [Google Scholar]
- Njomo, N.; Waryo, T.; Masikini, M.; Ikpo, C.O.; Mailu, S.; Tovide, O.; Ross, N.; Williams, A.; Matinise, N.; Sunday, C.E.; et al. Graphenated tantalum(IV) oxide and poly(4-styrene sulphonic acid)-doped polyaniline nanocomposite as cathode material in an electrochemical capacitor. Electrochim. Acta 2014, 128, 226–237. [Google Scholar] [CrossRef]
- Shreepathi, S.; Holze, R. Spectroelectrochemical investigations of soluble polyaniline synthesized via new inverse emulsion pathway. Chem. Mater. 2005, 17, 4078–4085. [Google Scholar] [CrossRef]
- Mavundla, S.E.; Malgas, G.F.; Baker, P.; Iwuoha, E.I. Synthesis and characterization of novel nanophase hexagonal poly(2,5-dimethoxyaniline). Electroanalysis 2008, 20, 2347–2353. [Google Scholar] [CrossRef]
- Iwuoha, E.I.; Mavundla, S.E.; Somerset, V.S.; Petrik, L.F.; Klink, M.J.; Sekota, M.; Bakers, P. Electrochemical and spectroscopic properties of fly ash-polyaniline matrix nanorod composites. Microchim. Acta 2006, 155, 453–458. [Google Scholar] [CrossRef]
- Mathebe, N.G.R.; Morrin, A.; Iwuoha, E.I. Electrochemistry and scanning electron microscopy of polyaniline/peroxidase-based biosensor. Talanta 2004, 64, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Klink, M.J.; Iwuoha, E.I.; Ebenso, E.E. The electro-catalytic and redox-mediator effects of nanostructured PDMA-PSA modified-electrodes as phenol derivative sensors. Int. J. Electrochem. Sci. 2011, 6, 2429–2442. [Google Scholar]
- Zhang, Z.; Wan, M. Composite films of nanostructured polyaniline with poly(vinyl alcohol). Synth. Met. 2002, 128, 83–89. [Google Scholar] [CrossRef]
- Hemant, D.; Chetna, D.; Malhotra, B.D.; Sen, P. Polyaniline-CdS quantum dots composite for mediator free biosensing. J. Biosens. Bioelectron. 2011, 3, 1–9. [Google Scholar]
- Joseph, H.O.O.; Omotayo, A.A.; Nicolette, H.; Everlyne, A.S.; Nazeem, J.; Tesfaye, T.W.; Rachel, F.N.; Priscilla, G.L.B.; Iwuoha, I.E. Electrochemical immunosensor based on polythionine/gold nanoparticles for the determination of aflatoxin B1. Sensors 2008, 8, 8262–8274. [Google Scholar]
- Munkombwe, M. Electrochemical Ochratoxin A Immunosensors Based on Polyaniline Nanocomposites Templated with Amine- and Sulphate-Functionalised Polystyrene Latex Beads. Ph.D. Thesis, University of the Western Cape, Capetown, South Africa, 2010. [Google Scholar]
- Abdul Kadir, M.K.; Tothill, I.E. Development of an electrochemical immunosensor for fumonisins detection in foods. Toxins 2010, 2, 382–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jodra, A.; López, M.Á.; Escarpa, A. Disposable and reliable electrochemical magnetoimmunosensor for Fumonisins simplified determination in maize-based foodstuffs. Biosens. Bioelectron. 2015, 64, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Ezquerra, A.; Vidal, J.C.; Bonel, L.; Castillo, J.R. A validated multi-channel electrochemical immunoassay for rapid fumonisin B1 determination in cereal samples. Anal. Methods 2015, 7, 3742–3749. [Google Scholar] [CrossRef]
- Ndungu, P.; Nechaev, A.; Khotseng, L.; Onyegebule, N.; Davids, W.; Mohammed, R.; Vaivars, G.; Bladegroen, B.; Linkov, V. Carbon nanomaterials synthesized using liquid petroleum gas: Analysis toward applications in hydrogen storage and production. Int. J. Hydrog. Energy 2008, 33, 3102–3106. [Google Scholar] [CrossRef]




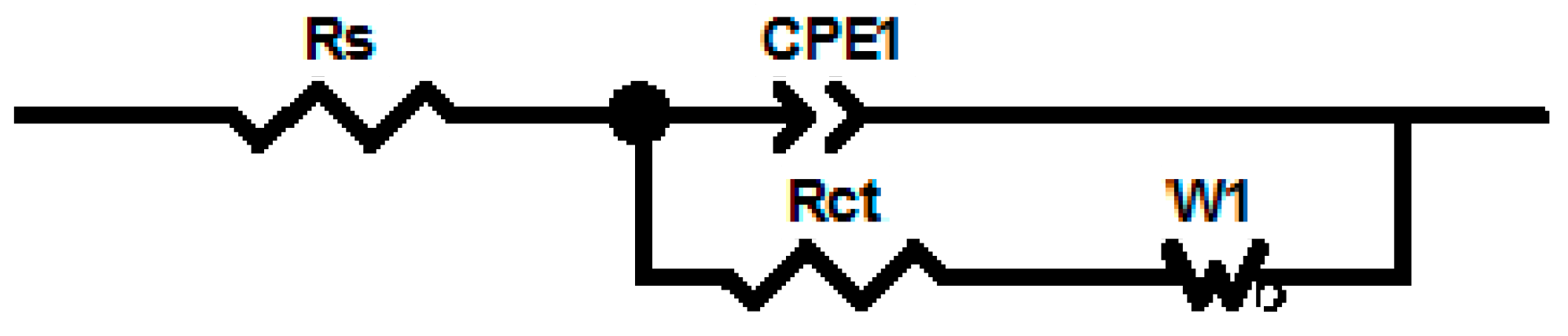
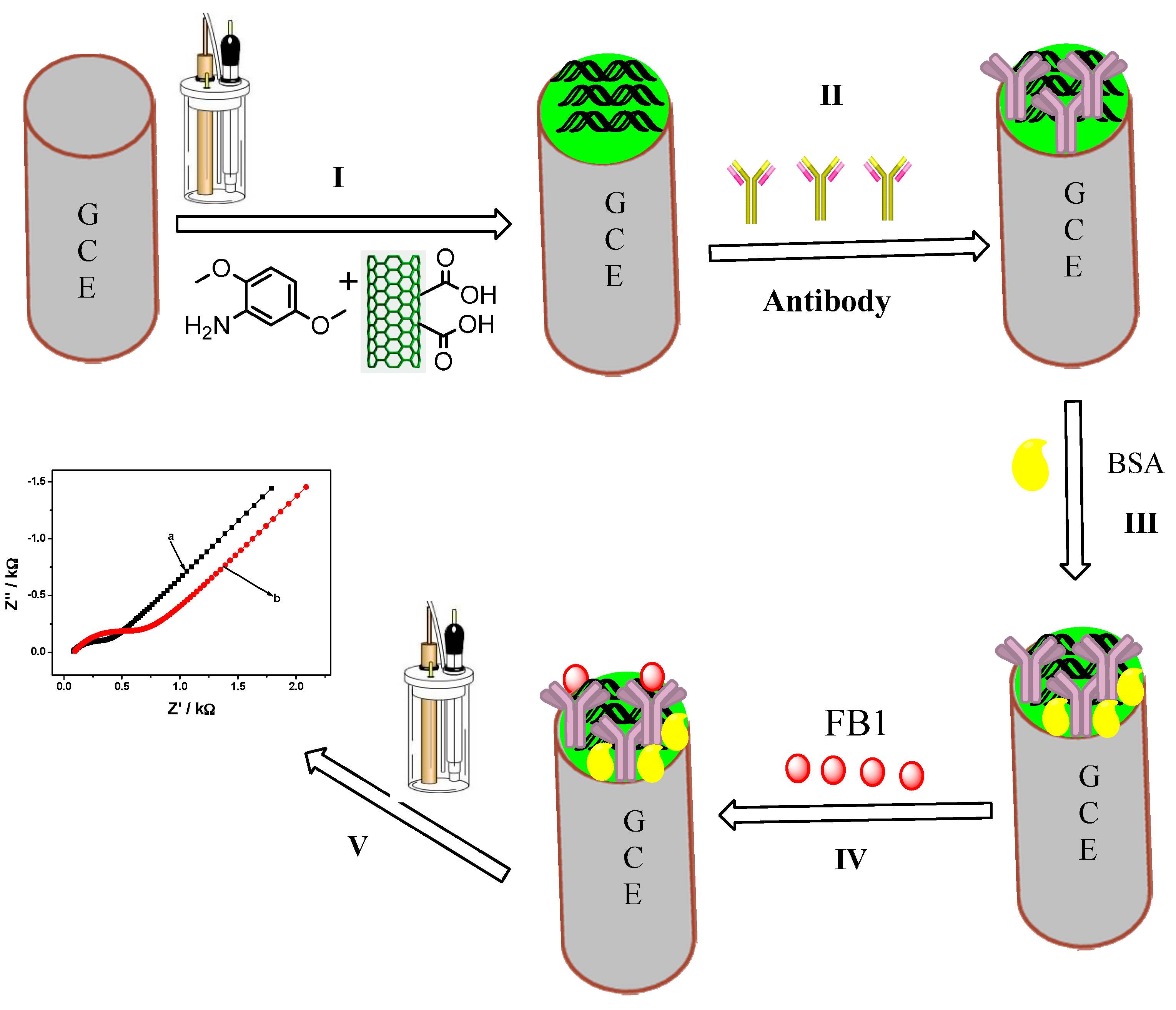
| ELEMENT | GCE/PDMA-MWCNT | GCE/PDMA-MWCNT/Anti-Fms |
|---|---|---|
| Solution resistance (ohm) | 128.6 | 132.1 |
| Charge transfer resistance (ohm) | 1129 | 1214 |
| Constant phase element (F) | 4.228 × 10−6 | 3.6204 × 10−6 |
| Immunosensor | Range of Linearity (ng·L−1) | Detection Limit (pg·L−1) |
|---|---|---|
| GCE/PDMA-MWCNT/anti-Fms (this work) | 7 to 49 | 3.8 |
| Gold screen-printed electrode (SPGE) [37] | 1 to 1 × 106 | 5 × 106 |
| GCE/Au nanoparticles (AuNPs) and graphene/thionine nanocomposite (GSTH) [14] | 1 to 1 × 106 | 1 × 10−3 |
| Magnetoimmunosensor involving magnetic beads and disposable carbon screen-printed electrode (CSPE) [38] | 0 to 5 × 103 | 0.33 × 106 |
| Modified magnetic beads on screen-printed electrode [39] | 0 to 54 × 103 | 0.58 ± 0.05 × 106 |
| Single-walled carbon nanotubes (SWCNT)/chitosan-modified GCE [16] | 1 to 1 × 106 | 2 × 10−3 |
| Commodity | Mycotoxins | Immunosensor (ppm) | Vendor (ppm) | ELISA (ppm) |
|---|---|---|---|---|
| Corn | FB1 + FB2 + FB3 | 0.014 ± 0.00057 | - | 1.18 |
| Corn | FB1 | 0.014 ± 0.00153 | 0.01 | 0.88 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masikini, M.; Williams, A.R.; Sunday, C.E.; Waryo, T.T.; Nxusani, E.; Wilson, L.; Qakala, S.; Bilibana, M.; Douman, S.; Jonnas, A.; et al. Label Free Poly(2,5-dimethoxyaniline)–Multi-Walled Carbon Nanotubes Impedimetric Immunosensor for Fumonisin B1 Detection. Materials 2016, 9, 273. https://doi.org/10.3390/ma9040273
Masikini M, Williams AR, Sunday CE, Waryo TT, Nxusani E, Wilson L, Qakala S, Bilibana M, Douman S, Jonnas A, et al. Label Free Poly(2,5-dimethoxyaniline)–Multi-Walled Carbon Nanotubes Impedimetric Immunosensor for Fumonisin B1 Detection. Materials. 2016; 9(4):273. https://doi.org/10.3390/ma9040273
Chicago/Turabian StyleMasikini, Milua, Avril R. Williams, Christopher E. Sunday, Tesfaye T. Waryo, Ezo Nxusani, Lindsay Wilson, Sinazo Qakala, Mawethu Bilibana, Samantha Douman, Anovuyo Jonnas, and et al. 2016. "Label Free Poly(2,5-dimethoxyaniline)–Multi-Walled Carbon Nanotubes Impedimetric Immunosensor for Fumonisin B1 Detection" Materials 9, no. 4: 273. https://doi.org/10.3390/ma9040273
APA StyleMasikini, M., Williams, A. R., Sunday, C. E., Waryo, T. T., Nxusani, E., Wilson, L., Qakala, S., Bilibana, M., Douman, S., Jonnas, A., Baker, P. G. L., & Iwuoha, E. I. (2016). Label Free Poly(2,5-dimethoxyaniline)–Multi-Walled Carbon Nanotubes Impedimetric Immunosensor for Fumonisin B1 Detection. Materials, 9(4), 273. https://doi.org/10.3390/ma9040273






