Polymer Nanocomposites—A Comparison between Carbon Nanotubes, Graphene, and Clay as Nanofillers
Abstract
:1. Introduction
2. Considerations for Developing Nanocomposites
3. Interfacial Interactions between Filler and Polymer
4. Nanocomposite Processing
5. Properties of Nanocomposites
- Reinforcement: While it is well know that nanofillers increase the mechanical properties of composites, it is widely recognized that the excellent properties of nanofillers have yet to be realized. This is particularly true at higher volume fraction. Using the Halpin-Tsai [235] equation, for an aspect ratio of 1000 for 1D nanotubes or 2D platelets, a modulus enhancement by a factor of six is predicted at 0.01 filler volume fraction. Molecular simulations predict similar reinforcing potentials for aspect ratio of approximately 100 [236]. Relative improvements in modulus are expected to be much higher than the increase in tensile strength. Strain at break generally decreases with nanofiller loading. For nanotubes, properties generally increase with increasing nanotube content at low volume fractions but then decrease at higher fractions due to issues related to dispersions and agglomeration. It has also been reported that higher surface area leads to better reinforcement [237], except for single walled nanotubes which the authors attributed to poor dispersion. Even with improved adhesion and dispersion in the polymer matrix, the nanotubes remain randomly dispersed. Attempts have been made to align nanotubes to increase reinforcement. Alignment techniques include melt drawing [9], polymer stretching [148,238,239], alternating-current electric field [240,241,242,243], surface acoustic waves [244], direct-current electric field [241,242,243,245] and magnetic fields [246,247,248]. Studies [9,238,239,249,250,251] have shown that in composites where the nanotubes were aligned, a significant increase in the modulus was obtained over non-aligned composites. However, alignment of nanotubes in the composite also caused anisotropy—with improvement in the perpendicular direction being significantly less [252]. The use of magnetic field as a technique to align nanotubes gave conflicting results on modulus enhancement [246]. There is a great body of literature that covers various aspects of mechanical property enhancements of different polymer systems for various types of nanotubes. These have been summarized in several excellent reviews [54,111] and monographs [25]. The mechanical properties of selected nanotube polymer compositions are shown in Table 1. It is difficult to compare the results as the type of nanotube, the method of processing, the type of functional groups, the aspect ratio, and the type of polymer all affect the properties.As in the case of nanotubes, modulus of graphene filled nanocomposites increased with loading fraction (Table 2). Results indicate that the strength of the interface is critical to the enhancement of the mechanical properties. Modulus increase is more pronounced for elastomeric matrices due to their lower intrinsic modulus. Studies have shown that the mechanical reinforcement of graphene is superior over fillers such as carbon black or single wall nanotube [88]. For epoxy polymers, functionalized graphene sheets have better fracture toughness, fracture energy stiffness, strength, and fatigue resistance at lower loading fractions as compared to nanotubes. [24,253]. Tensile strength has generally also been shown to increase with increase in graphene content, though there are instances when tensile strength decreased [254]. Elongation generally decreased or remained the same. As with nanotube based composites, the improvement in mechanical properties observed falls well short of that predicted theoretically.The mechanical properties of nanoclay mirrortrends are seen in nanotube polymer composites or graphene polymer. Addition increases modulus and tensile strength but decreases elongation at break. However, with clay, the relative reinforcement [2] for a given volume percent of filler in the composite is significantly lower than for graphene composite [88] or carbon nanotube based composite [54]. The tensile modulus of a polymeric material has been shown to be significantly improved when nanocomposites are formed with either pristine or organically modified clays. For nylon 6, tensile strength increased by 42% and modulus by 90% [255,256]. Interestingly, the stiffness increases with the increasing molecular weight of the matrix at any given loading, even though all the moduli of the neat nylon 6 are quite similar [257]. Similarly, the increase in strength relative to the neat matrix for the high molecular weight composite is nearly double compared to that of the low molecular weight composite. While nylon 6 interacts with silicate surface through hydrogen bonding, nancomposites with polyolefins require modification of both polymer and clay. For PP-clay composition, the modulus increases with clay content untill 3 wt% of clay, after which with an increase in clay content the modulus shows minimal improvement [109]. When MA functional groups are incorporated in the PP the stresses are much more effectively transferred from the polymer matrix to the inorganic filler, and thus a higher increase in Young’s modulus was observed. For maleic anhydride functionalized LLDPE, modulus increase is higher with MMT with two alkyl tails [100]. The addition of LLDPE-g-MA is advantageous with clay content of greater than 2.5 wt%.Mechanical properties of thin films developed using LBL have shown promise in terms of mechanical reinforcement. Mamedov [233] used poly(ethyleneamine) (PEI) as the polycation and acid modified SWNT or poly(acrylic acid) (PAA) as the polyanion. The film was heated and cross-linked using glutaraldehyde. The tensile strength of a 40-layer PEI/SWNT/PAA film was reported to be 220 ± 40 MPa while that of PEI/PAA (without CNT) film of the same number of layers was 9 MPa. The modulus of the composite containing SWNT was 35 GPa. These values are better than any known engineering plastics or are obtained by blending with carbon fillers and are similar to those of ceramics. Hu [258] reported linear increase in modulus of silk fibrion graphene thin film with increasing graphene oxide. Kulkarni et al. [259] observed 500% increase in break energy, eight fold increase in modulus, 120% increase in modulus, and increase in ultimate strain in LBL of negatively charged graphene oxide in a polyelectrolyte multilayer. The measured modulus exceeds that predicted by mathematical models. PDDA/MTM multilayers have high strength, flexibility, and resistance to crack propagation [260]. Free standing films of 50, 100, 200 bilayers of PDDA/MMT displayed tensile strength of ~100 MPa and E ~ 11 GPa—the increase was 10× and 50× over virgin polymer [261]. PVA/MMT films covalently cross-linked using glutaraldehyde [234], displayed tensile strength of 400 MPa and E = 106 GPa.
- Electrical Conductivity: The excellent electrical conductivity of CNT’s and graphene can be exploited to make traditionally insulating polymer matrices into electrically conductive materials for various applications in conductive adhesives, antistatic coatings, and films. There is a critical loading (percolation threshold) when the composite transitions from an insulator to a conductor due to the formation of a continuous conducting network. Below the percolating threshold, the electrical properties are dominated by the dielectric matrix and hence the composite is non-conductive as the fillers do not form a continuous network for electrons to flow. Beyond the percolation threshold, a small increase in loading will result in a significant increase in conductivity. As filler content increases, the fillers begin to form a contact with each other. At percolation threshold, conduction paths are created in the insulating matrix causing an increase in conduction. However, beyond a certain level of filler concentration a plateau in conductivity is reached. Factors affecting percolation threshold include aspect ratio, functionalization, processing, polymer type, dispersion etc. [53]. For example, it has been reported [292] that nanocomposites prepared by in situ polymerization showed significant increase in electrical conductivity compared to melt blending. There are no clear trends regarding the type of polymer and its effect on conductivity, although electrically conducting polymers have higher conductivities in the case of CNTs. According to theoretical prediction [293], rod-like structures percolate at one half the volume fraction of disk-like structures. However, there are examples that indicate that graphene has a lower electrical percolation threshold than CNTs [294]. Contradicting results have been published concerning the effect of aspect ratio on the percolation threshold [295,296]. Percolation threshold becomes greater as particles are aligned parallel. In general, SWNT composites have lower conductivities than MWNT composites due to the high contact resistance in SWNT composites because of smaller diameter. Using a similar processing method it was observed that the conductivity of graphene PS films had conductivity several orders of magnitude lower than films with CNTs [297]. For aligned CNT/epoxy composites, the electrical percolation threshold was 0.0025 wt% [298,299]. In comparison, the lowest percolation threshold for graphene based composite was 0.19 wt% for PS solvent blended with isocyanate-treated GO [69,87]. Electric conductivity of CNT and graphene based composites are summarized in several review [54,84,88,300].
- Thermal Conductivity—The thermal conductivity of carbon nanotubes has been estimated to be in the range between 650 and 10,000 W/mK. The thermal conductivity of a typical polymer ranges between 0.3 and 0.4 W/mK. It was anticipated that nanotube based composites would experience a significant increase in thermal conductivity (similar to electrical conductivity enhancements). In reality the increase has been rather modest (typically less than 1 W/mK). The reason can be attributed to large resistance to heat transfer at the nanotube-polymer interface. Functionalized nanotubes gave higher thermal conductivity than unfunctionalized tubes indicating that higher dispersion aids conductivity [301]. There have been few studies relating to the thermal conductivity of graphene/polymer composites. The most improvement in the thermal conductivity of graphene based nanocomposites were those where the nanocomposites were produced via in situ polymerization using chemically modified graphene [92,302]. Since thermal conductivity of composites increases linearly with filler content, a 20 fold increase was obtained by loading the composite with nanofiller [303].
- Thermal Stability: Polymers have a high thermal expansion coefficient when compared to metals. The addition of fillers like clay or nanotubes of graphene reduces the thermal expansion of polymers by constraining the movement of a significant volume of polymer chains because of their interaction with the filler. Graphite has a positive thermal expansion coefficient and when incorporated into polymers does not reduce the expansion of polymers [6]. However, incorporating reduced GO or SWNTs into resins displayed the effect of reduced thermal expansion [304]. Graphene oxide or single walled carbon nanotube have a negative thermal expansion coefficient and hence, composites containing GO or SWNT increase the thermal stability by decreasing the coefficient of expansion. Liu et al. [305] reported that the degradation temperature of PS increased from 400 to 450 °C when impregnated with graphene sheets. Thermal stabilities of graphene/PMMA were reported to be higher than that of PMMA [285]. Clay nanocomposite samples prepared using injection molding displayed anisotropy; the expansion coefficient in the flow direction was lower than in the perpendicular direction [127,306] and this difference was attributed to the orientation of platelets in the respective direction. Similar results were observed when MMT was aligned using magnetic or electric field [307,308].
- Glass Transition Temperature: Fillers can be an impediment to the motion of polymer chains due to the interfacial interaction between the polymer and the filler. Studies have confirmed that both Tg and the breadth of the transition can be affected by nanofillers [309,310]. Factors that influence Tg include sample thickness [311,312], sample preparation and measurement [313], nanoparticle dimension [309,310,314], and chemical structure of the polymers [315]. The interaction of the filler with the surface will determine the degree of change of Tg. Surfaces that interact strongly with the polymer causes an increase in Tg [316,317] relative to the bulk. The unadsorbed material can have the same or lower Tg than the bulk depending on the nature of the adsorbed layer. Even hydrogen bonds at the polymer-substrate interface can increase Tg relative to bulk values [313,317]. Graphene platelets with higher aspect ratio, higher surface roughness, and which are well dispersed in the polymer lead to a composite with higher Tg. Liao [313] observed that solvent and melt blending processes lead to insignificant changes in the Tg of polymer-graphene or polymer-GO composites, while in situ polymerization with unmodified graphene or solvent blending with chemically modified graphene or GO causes an increase in Tg. The authors attributed this to the covalent bonding between the graphene and the polymer. The type of polymer also affects how and whether the Tg increases, decreases or remains unchanged. For example, nanospherical silica showed an increase in Tg with PVP, decrease in Tg with PMMA, while Tg was unchanged with PS [318]. Mixing a polymer and nanotube can increase or decrease Tg depending on the surface functionalization of nanotubes. However, polyimide mixed using a dispersion-reaction scheme with non-functionalized depressed Tg, with acid-functionalized, and amine-functionalized tubes, elevated Tg [319]. Another important effect due to nanotube polymer interaction is the amount of material participating in the glass transition [320]. Also, nanotube dimension does not affect polymer/nanotube interaction. However, nanotubes can also affect the growth rate of crystals as they alter chain mobility and provide impediment to growth [110]. Higher Tg in some exfoliated and intercalated polymer clay nanocomposites has been attributed to the large interlayer distance between the clay platelets and the strong polymer—filler interactions that exist in the system [321,322,323]. When the interlayer distance is less than the characteristic length of polymer chains for relaxation, Tg is either depressed or absent [125,324].
- Barrier and Membrane Separation Properties—Composites containing fillers with large aspect ratio can impede and alter the diffusion path of penetrating molecules. Well dispersed fillers create a tortuous path for permeants to travel. A decrease in gas permeability, that is independent of the type of gas [99] was observed for clay reinforced composites. A 1%-loading of clay in PET showed a two fold reduction in O2 permeability. Messersmith and Giannelis [325] reported that the water vapor permeability of PCL clay nanocomposites decreased over neat PCL. Permeability was also observed to decrease as the aspect ratio of the platelets increased. Defect-free graphene sheets are impermeable to gas molecules [15], and hence, graphene polymer composites films can be used as protective elements in electronics and fuel cells that are sensitive to the presence of gases such as oxygen and moisture [123,326]. GO conjugated polymer nanocomposite films have been shown to significantly reduce oxygen and carbon dioxide permeation [326,327]. Graphene polyimide composite films have been reported to display high moisture barrier properties [328]. It has been reported that modified GO reduced the permeability of thermoplastic polyurethane more than modified MMT platelet layers at similar loadings.
- Flammability Resistance: Polymers will burn easily compared to metals or ceramics. Above a certain temperature a polymer decomposes releasing gaseous products that react with the oxygen in the air and burn. Well dispersed nanoplatelet/nanotube in the polymeric matrix capable of forming a continuous network, form a protective layer on the surface. This protective layer which acts as a heat shield, in turn, prevents the gaseous degradation products from diffusing through it and reacting with the oxygen in the air. Nanofiller impregnated polymer shows a significant reduction in the maximum heat release [329] compared to neat polymer, though the total heat release remained unchanged. Aspect ratio of dispersed silicate layers and the processing method have a strong effect on the fire-retardant properties [330,331]. Both clays and nanotubes have been investigated as flame retardants. Nanotubes have been reported to be more effective retardants [29,332] over clay. However, because of the heat localization due to the high thermal conductivity and low specific heat, time to ignition is lower with nanotube addition. Poorly dispersed nanofillers or low concentration of fillers result in the formation of a discontinuous network leading to much poorer flame resistance [29].
6. Future Outlook
- It has been well documented that dispersion of nanofillers is critical in achieving properties of nanocomposites. However, many of the processing techniques used to manufacture these composites are not economically viable. Solvent processing, LBL assembly, and electrospinning while resulting in better dispersions of nanofiller in the polymer are not cost-effective. Melt processing, the only economically viable processing technique, generally leads to poor dispersion and less than optimal properties.
- The problems associated with mechanical reinforcement of melt processed composites need urgent attention. At higher volume fractions, SWNT remains agglomerated. MWNTs are easy to disperse at much higher loading. There is an increase in interfacial area with decrease in tube diameter. For example, at 0.5 wt% of nanotubes MWNT has 70% of the interfacial area of SWNT [29]. Hence, SWNT lose their intrinsic advantage of higher aspect ratio. Similarly, composites at higher loading do not perform as well as at lower loadings. This puts a ceiling on the magnitude of reinforcement.
- Development and quality of polymer composites with CNT, graphene or clay depend upon a number of factors such as types of CNTs (MWCNT or SWCNT), layers of graphene or clay, purity, length of CNTs, diameter and length of CNT (aspect ratio), loading of nanofillers, dispersion in the matrix, alignment (tough to align graphene or nanoclay), and interaction between the polymer and the nanofiller. However, there are no systematic studies that compare the effect of aspect ratio, nanofiller purity, degree of functionalization, and type of functional group on the properties of the composite. For example, minimal reinforcement is obtained from graphene flakes with an aspect ratio of 1000, while both modulus and strength doubled for graphene with an aspect ratio of 2000 [333].
- Most work has been conducted using a single type of nanofiller in a polymer matrix. Simultaneous incorporation of different nanofillers may significantly enhance the properties of composites. The synergy in properties between multiple nanofillers needs to be investigated. For example, 1D fillers might interfere with the stacking of 2D platelets. Incorporating CNTs into glass fiber composites inhibits crack formations due to the large density of nucleation sites provided by CNT [334].
- Load transfer between nanofiller and polymer has been achieved by both non covalent and covalent modifications with functional groups. Introduction of covalently functional bonds disrupts the π conjugation of CNTs and graphene, leading to a negative effect on the electrical properties of the resulting composites. It has been reported that a combination of non-covalent and covalently fuctionalization on CNT can enhance compatibilizer-polymer interaction leading to better mechanical and electrical properties [335,336]. Hence, it is critical to develop an understanding of the interface between the non-covalently functionalized CNT/graphene and the polymeric matrix to enable simultaneous enhancement of both mechanical and electrical properties of the composites.
- It has been reported that nanofiller can act as nucleating agent and affect polymer crystallinity [113,337,338]. There should be more attempts to correlate the extent of change crystallinity with the mechanical properties of composite. Similarly, the effect of surfactants on Tg of nanoparticles should be careful investigated [313].
7. Summary and Conclusions
Acknowledgments
Conflicts of Interest
References
- Edwards, D.C. Polymer-filler interactions in rubber reinforcement. J. Mater. Sci. 1990, 25, 4175–4185. [Google Scholar] [CrossRef]
- Fornes, T.D.; Paul, D.R. Modeling properties of nylon 6/clay nanocomposites using composite theories. Polymer 2003, 44, 4993–5013. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun'ko, Y.K. Small but strong: A review of the mechanical properties of carbon nanotube-polymer composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Lin, B.; Gelves, G.A.; Haber, J.A.; Sundararaj, U. Electrical, Rheological, and Mechanical Properties of Polystyrene/Copper Nanowire Nanocomposites. Ind. Eng. Chem. Res. 2007, 46, 2481–2487. [Google Scholar] [CrossRef]
- Okada, A.; Usuki, A. Twenty Years of Polymer-Clay Nanocomposites. Macromol. Mater. Eng. 2006, 291, 1449–1476. [Google Scholar] [CrossRef]
- Fasolino, A.; Los, J.H.; Katsnelson, M.I. Intrinsic ripples in graphene. Nat. Mater. 2007, 6, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, A.; Claus, R. Molecular Self-Assembly of TiO2/Polymer Nanocomposite Films. J. Phys. Chem. B 1997, 101, 1385–1388. [Google Scholar] [CrossRef]
- Huang, J.; He, C.; Xiao, Y.; Mya, K.Y.; Dai, J.; Siow, Y.P. Polyimide/POSS nanocomposites: Interfacial interaction, thermal properties and mechanical properties. Polymer 2003, 44, 4491–4499. [Google Scholar] [CrossRef]
- Thostenson, E.K.; Chou, T.W. Aligned multi-walled carbon nanotube-reinforced composites: Processing and mechanical characterization. J. Phys. D Appl. Phys. 2002, 35, L77–L80. [Google Scholar] [CrossRef]
- Wong, E.W.; Sheehan, P.E.; Lieber, C.M. Nanobeam mechanics: Elasticity, strength, and toughness of nanorods and nanotubes. Science 1997, 277, 1971–1975. [Google Scholar] [CrossRef]
- Yu, M.; Dyer, M.J.; Kelly, T.F.; Ruoff, R.S. Strength and breaking mechanism of multiwalled carbon nanotubes under tensile load. Science 2000, 287, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Bunch, J.S.; Alden, J.S.; Zande, A.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Impermeable Atomic Membranes from Graphene Sheets. Nano Lett. 2008, 8, 2458–2462. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Evans, J.R.G. Elastic moduli of clay platelets. Scripta Mater. 2006, 54, 1581–1585. [Google Scholar] [CrossRef]
- Suter, J.L.; Coveney, P.V.; Greenwell, H.C.; Thyveetil, M.-A. Large-scale molecular dynamics study of montmorillonite clay: Emergence of undulatory fluctuations and determination of material properties. J. Phys. Chem. C 2007, 111, 8248–8259. [Google Scholar] [CrossRef]
- Brune, D.A.; Bicerano, J. Micromechanics of nanocomposites: Comparison of tensile and compressive elastic moduli, and prediction of effects of incomplete exfoliation and imperfect alignment on modulus. Polymer 2002, 43, 369–387. [Google Scholar] [CrossRef]
- Ward, I.M.; Sweeney, J. An Introduction to the Mechanical Properties of Solid Polymers; John Wiley: Sussex, UK, 2004. [Google Scholar]
- Nielsen, L.E.; Landel, R.F. Mechanical Properties of Polymers and Composites; Marcel Dekker: New York, NY, USA, 1994. [Google Scholar]
- Hu, K.; Kulkarni, D.D.; Choi, I.; Tsukruk, V.V. Graphene-polymer nanocomposites for structuraland functional applications. Prog. Polym. Sci. 2014, 39, 1934–1972. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer Nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Biswas, M.; Ray, S.S. Recent progress in synthesis and evaluation of polymer-montmorillonite nanocomposites. Adv. Polym. Sci. 2001, 155, 167–221. [Google Scholar]
- Rafiee, M.A.; Rafiee, J.; Wang, Z.; Song, H.; Yu, Z.Z.; Koratkar, N. Enhanced Mechanical Properties of Nanocomposites at Low Graphene Content. ACS Nano 2009, 3, 3884–3890. [Google Scholar] [CrossRef] [PubMed]
- Grady, B.P. Carbon Nanotube-Polymer Composites Manufacture, Properties, and Applications; John Wiley and Sons: New York, NY, USA, 2011. [Google Scholar]
- Das, P.; Jani-Markus, S.; Malho, B.Z.; Klemradt, U.; Walther, A. Facile Access to Large-Scale, Self-Assembled, Nacre-Inspired, High-Performance Materials with Tunable Nanoscale Periodicities. ACS Appl. Mater. Interfaces 2013, 5, 3738–3747. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, P.; Tang, Z.; Shim, B.S.; Kotov, N.A. Counterintuitive Effect of Molecular Strength and Role of Molecular Rigidity on Mechanical Properties of Layer-by-Layer Assembled Nanocomposites. Nano Lett. 2007, 7, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Bitinis, N.; Hernandez, M.; Verdejo, R.; Kenny, J.M.; Lopez-Manchado, M.A. Recent Advances in Clay/Polymer Nanocomposites. Adv. Mater. 2011, 23, 5229–5236. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, T.; Du, F.M.; Douglas, J.F.; Winey, K.I.; Harris, R.H.; Shields, J.R. Nanoparticle networks reduce the flammability of polymer nanocomposites. Nat. Mater. 2005, 4, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Zhou, Y.; Zou, Z. Versatile Graphene-Promoting Photocatalytic Performance of Semiconductors: Basic Principles, Synthesis, Solar Energy Conversion, and Environmental Applications. Adv. Funct. Mater. 2013, 23, 4996–5008. [Google Scholar] [CrossRef]
- Qian, D.; Dickey, E.C.; Andrews, R.; Rantell, T. Load transfer and deformation mechanisms in carbon nanotube-polystyrene composites. Appl. Phys. Lett. 2000, 76, 2868–2870. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Gun’ko, Y.K. Mechanical Reinforcement of Polymers Using Carbon Nanotubes. Adv. Mater. 2006, 18, 637–640. [Google Scholar] [CrossRef]
- Byrne, M.T.; Gun’ko, Y.K. Recent Advances in Research on Carbon Nanotube-Polymer Composites. Adv. Mater. 2010, 22, 1672–1688. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Nicolosi, V.; Rickard, D.; Bergin, S.D.; Aherne, D.; Coleman, J.N. Quantitative Evaluation of Surfactant-stabilized Single-walled Carbon Nanotubes: Dispersion Quality and Its Correlation with Zeta Potential. J. Phys. Chem. C 2008, 112, 10692–10699. [Google Scholar] [CrossRef]
- Amiran, J.; Nicolosi, V.; Bergin, S.D.; Khan, U.; Lyons, P.E.; Coleman, J.N. High quality dispersions of functionalized single walled nanotubes at high concentration. J. Phys. Chem. C 2008, 112, 3519–3524. [Google Scholar] [CrossRef]
- Coleman, J.N. Liquid-Phase Exfoliation of Nanotubes and Graphene. Adv. Funct. Mater. 2009, 19, 3680–3695. [Google Scholar] [CrossRef]
- Vaisman, L.; Wagner, H.D.; Marom, G. The role of surfactants in dispersion of carbon nanotubes. Adv. Colloid Interface Sci. 2006, 128–130, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Tkalya, E.E.; Ghislandi, M.; de With, G.; Koning, C.E. The use of surfactants for dispersing carbon nanotubes and graphene to make conductive nanocomposites. Curr. Opin. Colloid Interface Sci. 2012, 17, 225–232. [Google Scholar] [CrossRef]
- Constanze, L.; Huzil, J.T.; Marina, V.I.; Marianna, F. Non-Covalent Functionalization of Carbon Nanotubes with Surfactants for Pharmaceutical Applications—A Critical Mini-Review. Drug Deliv. Lett. 2011, 1, 45–57. [Google Scholar]
- Zhang, L.; Kiny, V.U.; Peng, H.; Zhu, J.; Lobo, R.F.M.; Margrave, J.L.; Khabashesku, V.N. Sidewall Functionalization of Single-Walled Carbon Nanotubes with Hydroxyl Group-Terminated Moieties. Chem. Mater. 2004, 16, 2055–2061. [Google Scholar] [CrossRef]
- Choi, J.Y.; Han, S.W.; Huh, W.S.; Tan, L.S.; Baek, J.B. In situ grafting of carboxylic acid-terminated hyperbranched poly(ether-ketone) to the surface of carbon nanotubes. Polymer 2007, 48, 4034–4040. [Google Scholar] [CrossRef]
- Pompeo, F.; Resasco, D.E. Water solubilization of single-walled carbon nanotubes by functionalization with glucosamine. Nano Lett. 2002, 2, 369–373. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Shen, Y.; Wang, M.; Li, J. Poly-l-lysine Functionalization of Single-Walled Carbon Nanotubes. J. Phys. Chem. B 2004, 108, 15343–15346. [Google Scholar] [CrossRef]
- Li, X.; Zhan, Q.; Dai, L. Direct Measurements of Interactions between Polypeptides and Carbon Nanotubes. J. Phys. Chem. B 2006, 110, 12621–12625. [Google Scholar] [CrossRef] [PubMed]
- Star, A.; Steuerman, D.W.; Heath, J.R.; Stoddart, J.F. Starched Carbon Nanotubes. Angew. Chem. Int. Ed. 2002, 41, 2508–2512. [Google Scholar] [CrossRef]
- Huang, W.; Fernando, S.; Allard, L.F.; Sun, Y.-P. Solubilization of Single-Walled Carbon Nanotubes with Diamine-Terminated Oligomeric Poly(ethylene Glycol) in Different Functionalization Reactions. Nano Lett. 2003, 3, 565–568. [Google Scholar] [CrossRef]
- Qin, S.; Qin, D.; Ford, W.T.; Herrera, J.E.; Resasco, D.E.; Bachilo, S.M.; Weisman, R.B. Solubilization and Purification of Single-Wall Carbon Nanotubes in Water by in Situ Radical Polymerization of Sodium 4-Styrenesulfonate. Macromolecules 2004, 37, 3965–3967. [Google Scholar] [CrossRef]
- Fernando, K.A.S.; Lin, Y.; Zhou, B.; Grah, M.; Joseph, R.; Allard, L.F.; Sun, Y.-P. Poly(ethylene-co-vinyl alcohol) Functionalized Single-Walled Carbon Nanotubes and Related Nanocomposites. J. Nanosci. Nanotechnol. 2005, 5, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Barber, A.H.; Nuriel, S.; Wagner, H.D. Mechanical Properties of Functionalized Single-Walled Carbon-Nanotube/Poly(vinyl alcohol) Nanocomposites. Adv. Funct. Mater. 2005, 15, 975–980. [Google Scholar] [CrossRef]
- Ausman, K.D.; Piner, R.; Lourie, O.; Ruoff, R.S. Organic Solvent Dispersions of Single-Walled Carbon Nanotubes: Toward Solutions of Pristine Nanotubes. J. Phys. Chem. B 2000, 104, 8911–8915. [Google Scholar] [CrossRef]
- Du, F.; Fischer, J.E.; Winey, K.I. Coagulation method for preparing single-walled carbon nanotube/poly(methyl methacrylate) composites and their modulus, electrical conductivity, and thermal stability. J. Polym. Sci. B Polym. Phys. 2003, 41, 3333–3338. [Google Scholar] [CrossRef]
- Chen, J.; Hamon, M.A.; Hu, H.; Chen, Y.; Rao, A.M.; Eklund, P.C.; Haddon, R.C. Solution Properties of Single-Walled Carbon Nanotubes. Science 1998, 282, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon nanotube-polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci 2010, 35, 357–401. [Google Scholar] [CrossRef]
- Bahr, J.L.; Tour, J.M. Covalent chemistry of single-wall carbon nanotubes. J. Mater. Chem. 2002, 12, 1952–1958. [Google Scholar] [CrossRef]
- Sinnott, S.B. Chemical Functionalization of Carbon Nanotubes. J. Nanosci. Nanotechnol. 2002, 2, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.P.; Fu, K.; Lin, Y.; Huang, W. Functionalized Carbon Nanotubes: Properties and Applications. Acc. Chem. Res. 2002, 35, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, A.; Khan, U.; Nirmalraj, P.N.; Boland, J.; Coleman, J.N. Graphene Dispersion and Exfoliation in Low Boiling Point Solvents. J. Phys. Chem. C 2011, 115, 5422–5428. [Google Scholar] [CrossRef]
- Lotya, M.; King, P.J.; Khan, U.; De, S.; Coleman, J.N. High-Concentration, Surfactant-Stabilized Graphene Dispersions. ACS Nano 2010, 4, 3155–3162. [Google Scholar] [CrossRef] [PubMed]
- Bergin, S.D.; Nicolosi, V.; Cathcart, H.; Rickard, D.; Sun, Z.; Blau, W.J.; Coleman, J.N. Large Populations of Individual Nanotubes in Surfactant-Based Dispersions without the Need for Ultracentrifugation. J. Phys. Chem. C 2008, 112, 972–977. [Google Scholar] [CrossRef]
- Hummers, W.; Offeman, R. Preparation of Graphite Oxide. J. Am. Chem. Soc. 1958, 80, 1339–1339. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielwaski, C.W.; Ruoff, R.S. The Chemistry of Graphene Oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Cheng, H. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of Graphite Oxide Revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villar-Rodil, S.; Martínez-Alonso, A.; Tascón, J.M.D. Graphene Oxide Dispersions in Organic Solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, J.; Zhang, J.; Liu, J.; Yang, X.; Zhao, H. Exfoliated Graphite Oxide Decorated by PDMAEMA Chains and Polymer Particles. Langmuir 2009, 25, 11808–11814. [Google Scholar] [CrossRef] [PubMed]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Fang, M.; Wang, K.; Lu, H.; Yang, Y.; Nutt, S. Single-layer graphene nanosheets with controlled grafting of polymer chains. J. Mater. Chem. 2010, 20, 1982–1992. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Li, D.; Muller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Piner, R.; Chen, X.; Wu, N.; Nguyen, S.; Ruoff, R. Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly(sodium 4-styrenesulfonate). J. Mater. Chem. 2006, 16, 155–158. [Google Scholar] [CrossRef]
- Wang, G.; Yang, J.; Park, J.; Gou, X.; Wang, B.; Liu, H.; Yao, J. Facile Synthesis and Characterization of Graphene Nanosheets. J. Phys. Chem. C 2008, 112, 8192–8195. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Gournis, D.; Petridis, D.; Szabó, T.; Szeri, A.; Dékány, I. Graphite Oxide: Chemical Reduction to Graphite and Surface Modification with Primary Aliphatic Amines and Amino Acids. Langmuir 2003, 19, 6050–6055. [Google Scholar] [CrossRef]
- Wang, X.; Zhi, L.; Müllen, K. Transparent, Conductive Graphene Electrodes for Dye-Sensitized Solar Cells. Nano Lett. 2008, 8, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of Solution-Processed Reduced Graphene Oxide Films as Transparent Conductors. ACS Nano 2008, 8, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Ramesha, G.K.; Sampath, S. Electrochemical Reduction of Oriented Graphene Oxide Films: An in Situ Raman Spectroelectrochemical Study. J. Phys. Chem. C 2009, 113, 7985–7989. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, X.; Zhang, J.; Boey, F.; Zhang, H. Direct Electrochemical Reduction of Single-Layer Graphene Oxide and Subsequent Functionalization with Glucose Oxidase. J. Phys. Chem. C 2009, 113, 14071–14075. [Google Scholar] [CrossRef]
- Williams, G.; Seger, B.; Kamat, P.V. TiO2-Graphene Nanocomposites. UV-Assisted Photocatalytic Reduction of Graphene Oxide. ACS Nano 2208, 2, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Quintana, M.; Vazquez, E.; Prato, M. Organic Functionalization of Graphene in Dispersions. Acc. Chem. Res. 2013, 46, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Criado, A.; Melchionna, M.; Marchesan, S.; Prato, M. The Covalent Functionalization of Graphene on Substrates. Angew. Chem. Int. Ed. 2015, 54, 10734–10750. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.K.; Pumera, M. Covalent chemistry on graphene. Chem. Soc. Rev. 2013, 42, 3222–3233. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Simmons, T.; Shah, R.; Wolfe, C.; Lewis, K.M.; Washington, M.; Nayak, S.K.; Talapatra, S.; Kar, S. Stable Aqueous Dispersions of Noncovalently Functionalized Graphene from Graphite and their Multifunctional High-Performance Applications. Nano Lett. 2010, 10, 4295–4301. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.T.; Zalautdinov, M.; Baldwin, J.W.; Snow, E.S.; Wei, Z.; Seehan, P.; Houston, B.H. Wafer-sacle reduced graphene oxide films for nanomechanical devices. Nano Lett. 2008, 8, 3441–3445. [Google Scholar] [CrossRef] [PubMed]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene polymer composites. Prog. Polym. Sci 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Gomez, M.A.; Martınez, G. Polymeric Modification of Graphene through Esterification of Graphite Oxide and Poly(vinyl alcohol). Macromolecules 2009, 42, 6331–6334. [Google Scholar] [CrossRef]
- Matsuo, Y.; Tahara, K.; Sugie, Y. Structure and Thermal Properties of Poly(ethylene oxide)-intercalated Graphite Oxide. Carbon 1997, 35, 113–120. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/Polymer Nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef]
- Zheng, Q.; Geng, Y.; Wang, S.; Li, Z.; Kim, J. Effects of functional groups on the mechanical and wrinkling properties of graphene sheets. Carbon 2010, 48, 4315–4322. [Google Scholar] [CrossRef]
- Liu, G.; Zhuang, X.; Chen, Y.; Zhang, B.; Zhu, J.; Zhu, C.; Neoh, K.; Kang, E. Bistable electrical switching and electronic memory effect in a solution-processable graphene oxide-donor polymer complex. Appl. Phys. Lett. 2009, 95. [Google Scholar] [CrossRef]
- Fang, M.; Wang, K.; Lu, H.; Yang, Y.; Nutt, S. Covalent polymer functionalization of graphene nanosheets and mechanical properties of composites. J. Mater. Chem. 2009, 19, 7098–7105. [Google Scholar] [CrossRef]
- Vaia, R.A.; Sauer, B.B.; Tse, O.K.; Giannelis, E.P. Relaxations of confined chains in polymer nanocomposites: Glass transition properties of poly(ethylene oxide) intercalated in montmorillonite. J. Polym. Sci. B Polym. Phys. 1997, 35, 59–67. [Google Scholar] [CrossRef]
- Vaia, R.A.; Vasudevan, S.; Krawiec, W.; Scanlon, L.G.; Giannelis, E.P. New polymer electrolyte nanocomposites: Melt intercalation of poly(ethylene oxide) in mica-type silicates. Adv. Mater. 1995, 7, 154–156. [Google Scholar] [CrossRef]
- Wu, J.; Lerner, M.M. Structural, thermal, and electrical characterization of layered nanocomposites derived from sodium-montmorillonite and polyethers. Chem. Mater. 1993, 5, 835–838. [Google Scholar] [CrossRef]
- Aranda, P.; Ruiz-Hitzky, E. Poly(ethylene oxide)-silicate intercalation materials. Chem. Mater. 1992, 4, 1395–1403. [Google Scholar] [CrossRef]
- Fornes, T.D.; Hunter, D.L.; Paul, D.R. Nylon-6 Nanocomposites from Alkylammonium-Modified Clay: The Role of Alkyl Tails on Exfoliation. Macromolecules 2004, 37, 1793–1798. [Google Scholar] [CrossRef]
- Tanaka, G.; Goettler, L.A. Predicting the binding energy for nylon 6,6/clay nanocomposites by molecular modeling. Polymer 2002, 43, 541–553. [Google Scholar] [CrossRef]
- Kojima, Y.; Usuki, A.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigiato, O. Mechanical properties of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1185–1189. [Google Scholar] [CrossRef]
- Hotta, S.; Paul, D.R. Nanocomposites formed from linear low density polyethylene and organoclays. Polymer 2004, 45, 7639–7654. [Google Scholar] [CrossRef]
- Chen, B.; Evans, J.R.G.; Greenwell, H.C.; Boulet, P.; Coveney, P.V.; Bowdenf, A.A.; Whiting, A. A critical appraisal of polymer-clay nanocomposites. Chem. Soc. Rev. 2008, 37, 568–594. [Google Scholar] [CrossRef] [PubMed]
- Faucheu, J.; Gauthier, C.; Chazeau, L.; Cavaillé, J.Y.; Mellon, V.; Lami, E.B. Miniemulsion polymerization for synthesis of structured clay/polymer nanocomposites: Short review and recent advances. Polymer 2010, 51, 6–17. [Google Scholar] [CrossRef]
- Pinnavaia, T.J. Intercalated Clay Catalyst. Science 1983, 220, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Vaia, R.A.; Jandt, K.D.; Kramer, E.J.; Giannelis, E.P. Microstructural Evolution of Melt Intercalated Polymer-Organically Modified Layered Silicates Nanocomposites. Chem. Mater. 1996, 8, 2628–2635. [Google Scholar] [CrossRef]
- Wang, D.; Wilkie, C.A. A stibonium-modified clay and its polystyrene nanocomposite. Polym. Degrad. Stab. 2003, 82, 309–315. [Google Scholar] [CrossRef]
- Zhang, J.; Wilkie, C.A. A carbocation substituted clay and its styrene nanocomposite. Polym. Degrad. Stab. 2004, 83, 301–307. [Google Scholar] [CrossRef]
- Su, S.; Jiang, D.D.; Wilkie, C.A. Poly(methyl methacrylate), polypropylene and polyethylene nanocomposite formation by melt blending using novel polymerically-modified clays. Polym. Degrad. Stab. 2004, 84, 321–331. [Google Scholar] [CrossRef]
- Kim, D.H.; Fasulo, P.D.; Rodgers, W.R.; Paul, D.R. Structure and properties of polypropylene-based nanocomposies: Effect of PP-g-MA to organoclay ratio. Polymer 2007, 48, 5308–5323. [Google Scholar] [CrossRef]
- Manias, E. Polypropylene/montmorillonite nanocomposites. Review of the synthetic routes and materials properties. Chem. Mater. 2001, 13, 3516–3523. [Google Scholar] [CrossRef]
- Grady, B.P. Carbon Nanotube-Polymer Composites Manufacture, Properties, and Applications; John Wiley and Sons: New York, NY, USA, 2011; p. 145. [Google Scholar]
- McClory, C.; Chin, S.J.; McNally, T. Polymer/Carbon Nanotube Composites. Aust. J. Chem. 2009, 62, 762–785. [Google Scholar] [CrossRef]
- Andrews, R.; Weisenberger, M.C. Carbon nanotube polymer composites. Curr. Opin. Solid State Mater. Sci. 2004, 8, 31–37. [Google Scholar] [CrossRef]
- Coleman, J.N.; Cadek, M.; Blake, R.; Nicolosi, V.; Ryan, K.P.; Belton, C.; Fonseca, A.; Nagy, J.B.; Gun'ko, Y.K.; Blau, W.J. High Performance Nanotube-Reinforced Plastics: Understanding the Mechanism of Strength Increase. Adv. Funct. Mater. 2004, 14, 791–798. [Google Scholar] [CrossRef]
- Villmow, T.; Potschke, P.; Pegel, S.; Haussler, L.; Kretzschmar, B. Influence of twin-screw extrusion conditions on the dispersion of multi-walled carbon nanotubes in poly(lactic acid) matrix. Polymer 2008, 49, 3500–3509. [Google Scholar] [CrossRef]
- Wu, D.; Sun, Y.; Zhang, M. Kinetics Study on Melt Compounding of Carbon Nanotube/Polypropylene Nanocomposites. J. Polym. Sci. B Polym. Phys. 2009, 47, 608–618. [Google Scholar] [CrossRef]
- Hong, J.S.; Kim, C. Extension-induced dispersion of multi-walled carbon nanotubes in non-Newtonian fluid. J. Rheol. 2007, 51, 833–850. [Google Scholar] [CrossRef]
- Kim, I.H.; Jeong, Y.G. Polylactide/Exfoliated Graphite Nanocomposites with Enhanced Thermal Stability, Mechanical Modulus, and Electrical Conductivity. J. Polym. Sci. B Polym. Phys. 2010, 48, 850–858. [Google Scholar] [CrossRef]
- Zhang, H.B.; Zheng, W.G.; Yan, Q.; Yang, Y.; Wang, J.W.; Lu, Z.H.; Ji, G.Y.; Yu, Z.Z. Electrically conductive polyethylene terephthalate/graphene nanocomposites prepared by melt compounding. Polymer 2010, 51, 1191–1196. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Fukushima, H.; Drzal, L.T. Multifunctional polypropylene composites produced by incorporation of exfoliated graphite nanoplatelets. Carbon 2007, 45, 1446–1452. [Google Scholar] [CrossRef]
- Weng, W.; Chen, G.; Wu, D. Transport properties of electrically conducting nylon 6/foliated graphite nanocomposites. Polymer 2005, 46, 6250–6257. [Google Scholar] [CrossRef]
- Kim, H.; Macosko, C.W. Processing-property relationship of polycarbonate/graphene composites. Polymer 2009, 50, 3797–3809. [Google Scholar] [CrossRef]
- Chen, G.; Wu, C.; Weng, W.; Wu, D.; Yan, W. Preparation of polystyrene/graphite nanosheet composites. Polymer 2003, 44, 1781–1784. [Google Scholar] [CrossRef]
- Kim, H.; Miura, Y.; Macosko, C.W. Graphene/Polyurethane Nanocomposites for Improved Gas Barrier and Electrical Conductivity. Chem. Mater. 2010, 22, 3441–3450. [Google Scholar] [CrossRef]
- Vaia, R.A.; Giannelis, E.P. Polymer melt intercalation in organically-modified layered silicates: Model predictions and experiment. Macromolecules 1997, 30, 8000–8009. [Google Scholar] [CrossRef]
- Vaia, R.A.; Ishii, H.; Giannelis, E.P. Synthesis and properties of two-dimensional nanostructures by direct intercalation of polymer melts in layered silicates. Chem. Mater. 1993, 5, 1694–1696. [Google Scholar] [CrossRef]
- Ray, S.S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci 2003, 28, 1539–1641. [Google Scholar]
- Yoon, P.J.; Hunter, D.L.; Paul, D.R. Polycarbonate nanocomposites. Part 1. Effect of organoclay structure on morphology and properties. Polymer 2003, 44, 5323–5339. [Google Scholar] [CrossRef]
- Yoon, P.J.; Hunter, D.L.; Paul, D.R. Polycarbonate nanocomposites: Part 2. Degradation and color formation. Polymer 2003, 44, 5341–5354. [Google Scholar] [CrossRef]
- Chen, B.; Evans, J.R.G. Poly(epsilon-caprolactone)-Clay Nanocomposites: Structure and Mechanical Properties. Macromolecules 2006, 39, 747–754. [Google Scholar] [CrossRef]
- Lepoittevin, B.; Devalckenaere, M.; Pantoustier, N.; Alexandre, M.; Kubies, D.; Calberg, C.; Jérôme, R.; Dubois, P. Poly(ε-caprolactone)/clay nanocomposites prepared by melt intercalation: Mechanical, thermal and rheological properties. Polymer 2002, 43, 4017–4023. [Google Scholar] [CrossRef]
- Ray, S.S.; Yamada, K.; Okamoto, M.; Ogami, A.; Ueda, K. New polylactide/layered silicate nanocomposites. 3. High-performance biodegradable materials. Chem. Mater. 2003, 15, 1456–1465. [Google Scholar]
- Ray, S.S.; Maiti, P.; Okamoto, M.; Yamada, K.; Ueda, K. New Polylactide/Layered Silicate Nanocomposites. 1. Preparation, Characterization, and Properties. Macromolecules 2002, 35, 3104–3110. [Google Scholar]
- Maiti, P.; Yamada, K.; Okamoto, M.; Ueda, K.; Okamoto, K. New polylactide/layered silicate nanocomposites: Role of organoclays. Chem. Mater. 2002, 14, 4654–4661. [Google Scholar] [CrossRef]
- Wang, J.H.; Young, T.H.; Lin, D.J.; Sun, M.K.; Huag, H.S.; Cheng, L.P. Preparation of Clay/PMMA Nanocomposites with Intercalated or Exfoliated Structure for Bone Cement Synthesis. Macromol. Mater. Eng. 2006, 291, 661–669. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W.C. Effect of clay modification on the dynamic mechanical and dielectric properties of PMMA nanocomposites via melt blending. Polymer 2013, 12, 128–144. [Google Scholar] [CrossRef]
- Shen, L.; Phang, I.Y.; Chen, L.; Liu, T.; Zeng, K. Nanoindentation and morphological studies on nylon 66 nanocomposites. I. Effect of clay loading. Polymer 2004, 45, 3341–3349. [Google Scholar] [CrossRef]
- Masenelli-Varlot, K.; Reynaud, E.; Vigier, G.; Varlet, J. Mechanical Properties of Clay-Reinforced Polyamide. J. Polym. Sci. B Polym. Phys. 2002, 40, 272–283. [Google Scholar] [CrossRef]
- Cho, J.W.; Paul, D.R. Nylon 6 Nanocomposites by Melt Compounding. Polymer 2001, 42, 1083–1094. [Google Scholar] [CrossRef]
- Stretz, H.A.; Paul, D.R.; Cassidy, P.E. Poly(styrene-co-acrylonitrile)/montmorillonite organoclay mixtures: A model systems for ABS nanocomposites. Polymer 2005, 46, 3818–3830. [Google Scholar] [CrossRef]
- Ma, H.; Tong, L.; Xu, Z.; Fang, Z. Clay network in ABS-graft-MAH nanocomposites: Rheology and flammability. Polym. Degrad. Stab. 2007, 92, 1439–1445. [Google Scholar] [CrossRef]
- Abraham, T.N.; Ratna, D.; Siengchin, S.; Karger-Kocsis, J. Structure and properties of polyethylene oxideorgano clay nanocomposite prepared via melt mixing. Polym. Eng. Sci. 2009, 49, 379–390. [Google Scholar] [CrossRef]
- Choudhary, S.; Sengwa, R.J. Dielectric properties and structures of melt-compounded poly(ethylene oxide)-montmorillonite nanocomposites. J. Appl. Polym. Sci. 2012, 124, 4847–4853. [Google Scholar] [CrossRef]
- Aranda, P.; Mosqueda, E.; Pérez-Cappe, E.; Ruiz-Hitzky, E. Electrical characterization of poly(ethylene oxide)-clay nanocomposites prepared by microwave irradiation. J. Polym. Sci. B Polym. Phys. 2003, 41, 3249–3263. [Google Scholar] [CrossRef]
- Liu, L.; Qi, Z.; Zhu, X. Studies on Nylon 6/Clay Nanocomposites by Melt-Intercalation Process. J. Appl. Polym. Sci. 1999, 71, 1133–1138. [Google Scholar] [CrossRef]
- Kawasumi, M.; Hasegawa, N.; Kato, M.; Usuki, A.; Okada, A. Preparation and Mechanical Properties of Polypropylene-Clay Hybrids. Macromolecules 1997, 30, 6333–6338. [Google Scholar] [CrossRef]
- Dennis, H.R.; Hunter, D.L.; Chang, D.; Kim, S.; White, J.L.; Cho, J.W.; Paul, D.R. Effect of melt processing conditions on the extent of the exfoliation in organoclay-based composites. Polymer 2001, 42, 9513–9522. [Google Scholar] [CrossRef]
- Shaffer, M.S.P.; Windle, A.H. Fabrication and characterization of CNT-PVA composites. Adv. Mater. 1999, 11, 937–941. [Google Scholar] [CrossRef]
- Jin, L.; Bower, C.; Zhou, O. Alignment of carbon nanotubes in a polymer matrix by mechanical stretching. Appl. Phys. Lett. 1998, 73, 1197–1199. [Google Scholar] [CrossRef]
- Safadi, B.; Andrews, R.; Grulke, E.A. Multiwalled carbon nanotube polymer composites: Synthesis and characterization of thin films. J. Appl. Polym. Sci. 2002, 84, 2660–2669. [Google Scholar] [CrossRef]
- Haggenmueller, R.; Fischer, J.E.; Winey, K.I. Single wall carbon nanotube/ polyethylene nanocomposites: Nucleating and templating polyethylene crystallites. Macromolecules 2006, 39, 2964–2971. [Google Scholar] [CrossRef]
- Geng, H.Z.; Rosen, R.; Zheng, B.; Shimoda, H.; Fleming, L.; Liu, J.; Zhou, O. Fabrication and Properties of Composites of Poly(ethylene oxide) and Functionalized Carbon Nanotubes. Adv. Mater. 2002, 14, 1387–1390. [Google Scholar] [CrossRef]
- Jang, J.; Baea, J.; Yoon, S.H. A study on the effect of surface treatment of carbon nanotubes for liquid crystalline epoxide-carbon nanotube composites. J. Mater. Chem. 2003, 13, 676–681. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Stephan, O.; Colliex, C.; Trauth, D. Aligned carbon nanotube arrays formed by cutting a polymer resin-nanotube composite. Science 1994, 265, 1212–1214. [Google Scholar] [CrossRef] [PubMed]
- Špitalský, Z.; Matějka, L.; Šlouf, M.; Konyushenko, E.N.; Kovářová, J.; Zemek, J.; Kotek, J. Modification of carbon nanotubes and its effect on properties of carbon nanotube/epoxy nanocomposites. Polym. Compos. 2009, 30, 1378–1387. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Aranda, P. Polymer-salt intercalation complexes in layer silicates. Adv. Mater. 1990, 2, 545–547. [Google Scholar] [CrossRef]
- Shen, Z.; Simon, G.P.; Cheng, Y.B. Comparison of solution intercalation and melt intercalation of polymer-clay nanocomposites. Polymer 2002, 43, 4251–4260. [Google Scholar] [CrossRef]
- Ogata, N.; Jimenez, G.; Kawai, H.; Ogihara, T. Structure and Thermal/Mechanical Properties of Poly(l-lactide)-Clay Blend. J. Polym. Sci. B Polym. Phys. 1997, 35, 389–396. [Google Scholar] [CrossRef]
- Sur, G.S.; Sun, H.L.; Lee, T.J.; Lyu, S.G.; Mark, J.E. Composites prepared by penetrating poly(ethylene oxide) chains into mesoporous silica. Colloid Polym. Sci. 2003, 281, 1040–1045. [Google Scholar] [CrossRef]
- Carrado, K.A.; Thiyagarajan, P.; Elde, D.L. Polyvinyl alcohol-clay complexes formed by direct synthesis. Clay Clay Miner. 1996, 44, 506–514. [Google Scholar] [CrossRef]
- Chang, J.H.; Park, D.K.; Ihn, K.J. Montmorillonite-Based Nanocomposites of Polybenzoxazole: Synthesis and Characterization (I). J. Polym. Sci. B Polym. Phys. 2001, 39, 471–476. [Google Scholar] [CrossRef]
- Geng, Y.; Liu, M.Y.; Li, J.; Shi, X.M.; Kim, J.K. Effects of surfactant treatment on the mechanical and electrical properties of CNT/epoxy nanocomposites. Compos. A 2008, 39, 1876–1883. [Google Scholar] [CrossRef]
- Ma, P.C.; Kim, J.K.; Tang, B.Z. Effects of silane functionalization on the properties of carbon nanotube/epoxy nanocomposites. Compos. Sci. Technol. 2007, 67, 2965–2972. [Google Scholar] [CrossRef]
- Liu, X.Q.; Chan-Park, M.B. Facile way to disperse single-walled carbon nanotubes using a noncovalent method and their reinforcing effect in poly(methyl methyacrylate) composites. J. Appl. Polym. Sci. 2009, 114, 3414–3419. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Chen, Y.; Wang, Y.; Mitra, S. Microwave-induced rapid nanocomposite using dispersed single-wall carbon nanotubes as the nuclei. J. Mater. Sci. 2009, 44, 1245–1250. [Google Scholar] [CrossRef]
- Trujillo, M.; Arnal, M.L.; Müller, A.J.; Laredo, E.; Bredeau, S.; Bonduel, D.; Dubois, P. Thermal and Morphological Characterization of Nanocomposites Prepared by in-Situ Polymerization of High-Density Polyethylene on Carbon Nanotubes. Macromolecules 2007, 40, 6268–6276. [Google Scholar] [CrossRef]
- Kaminsky, W.; Funck, A. In situ polymerization of olefins with nanoparticles by metallocene-catalysis. Macromol. Symp. 2007, 260, 1–8. [Google Scholar] [CrossRef]
- Kwon, S.M.; Kim, H.S.; Myung, S.J.; Jin, H.J. Poly(methyl methacrylate)/multiwalled carbon nanotube microspheres fabricated via in-situ polymerization. J. Polym. Sci. B Polym. Phys. 2008, 46, 182–189. [Google Scholar] [CrossRef]
- Song, W.H.; Ni, Q.P.; Zheng, Z.; Tian, L.Y.; Wang, X.L. The preparation of biodegradable polyurethane/carbon nanotube composite based on in situ cross-linking. Polym. Adv. Technol. 2009, 20, 327–331. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, H. Comparison of the properties of waterborne polyurethane/multiwalled carbon nanotube and acid-treated multiwalled carbon nanotube composites prepared by in situ polymerization. J. Polym. Sci. A Polym. Chem. 2005, 43, 3973–3985. [Google Scholar] [CrossRef]
- Castro, M.; Lu, J.; Bruzaud, S.; Kumar, B.; Feller, J.F. Carbon nanotube/poly(e-caprolcatone) composite vapor sensors. Carbon 2009, 47, 1930–1942. [Google Scholar] [CrossRef]
- Biedron, T.; Pietrzak, L.; Kubisa, P. Ionic liquid functionalized polylactide by cationic polymerization: Synthesis and stabilization of carbon nanotube suspensions. J. Polym. Sci. A Polym. Chem. 2011, 49, 5239–5244. [Google Scholar] [CrossRef]
- Liu, P.; Gong, K.; Xiao, P. Preparation and Characterization of Poly(vinyl acetate)-Intercalated Graphite Oxide. Carbon 1999, 37, 2073–2075. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, C. In situ Polymerization Approach to Graphene-Reinforced Nylon-6 Composites. Macromolecules 2010, 43, 6716–6723. [Google Scholar] [CrossRef]
- Ding, R.; Hu, Y.; Gui, Z.; Zong, R.; Chen, Z.; Fan, W. Preparation and characterization of polystyrene/graphite oxide nanocomposite by emulsion polymerization. Polym. Degrad. Stab. 2003, 81, 473–476. [Google Scholar] [CrossRef]
- Wang, W.F.; Pan, C.Y. Preparation and characterization of poly(methyl methacrylate)-intercalated graphite oxide/poly(methyl methacrylate) nanocomposite. Polym. Eng. Sci. 2004, 44, 2335–2339. [Google Scholar]
- Furuichi, N.; Kurokawa, Y.; Fujita, K.; Oya, A.; Yasuda, H.; Kiso, M. Preparation and properties of polypropylene reinforced by smectite. J. Mater. Sci. 1996, 31, 4307–4310. [Google Scholar] [CrossRef]
- Tseng, C.R.; Wu, J.Y.; Lee, H.Y.; Chang, F.C. Preparation and crystallization behavior of syndiotactic polystyrene-clay nanocomposites. Polymer 2001, 42, 10063–10070. [Google Scholar] [CrossRef]
- Lee, D.C.; Jang, L.W. Preparation and Characterization of PMMA-Clay Hybrid Composite by Emulsion Polymerization. J. Appl. Polym. Sci. 1996, 61, 1117–1122. [Google Scholar] [CrossRef]
- Noh, M.H.; Lee, D.C. Comparison of characteristics of SAN–MMT nanocomposites prepared by emulsion and solution polymerization. J. Appl. Polym. Sci. 1999, 74, 2811–2819. [Google Scholar] [CrossRef]
- Moraes, R.P.; Santos, A.M.; Oliveira, P.C.; Souza, F.C.T.; Amaral, M.; Valera, T.S.; Demarquette, N.R. Poly(styrene-co-butyl acrylate)-Brazilian Montmorillonite Nanocomposites, Synthesis of Hybrid Latexes via Miniemulsion Polymerization. Macromol. Symp. 2006, 245, 106–115. [Google Scholar] [CrossRef]
- Sun, Q.; Deng, Y.; Wang, Z.L. Synthesis and Characterization of Polystyrene Encapsulated Laponite Composites via Miniemulsion Polymerization. Macromol. Mater. Eng. 2004, 289, 288–295. [Google Scholar] [CrossRef]
- Samakande, A.; Sanderson, R.D.; Hartmann, P.C. Encapsulated clay particles in polystyrene by RAFT mediated miniemulsion polymerization. J. Polym. Sci. A Polym. Chem. 2008, 46, 7114–7126. [Google Scholar] [CrossRef]
- Teo, W.F.; Ramaseshan, R.; Fujihara, K.; Ramakrishna, S. A dynamic liquid support system for continuous electrospun yarn fabrication. Polymer 2007, 48, 3400–3405. [Google Scholar] [CrossRef]
- Ko, F.; Gogotsi, Y.; Ali, A.; Naguib, N.; Ye, H.; Yang, G.L.; Li, C.; Willis, P. Electrospinning of Continuous Carbon Nanotube-Filled Nanofiber Yarns. Adv. Mater. 2003, 15, 1161–1165. [Google Scholar] [CrossRef]
- Yu, J.; Qiu, Y.; Zha, X.; Yu, M.; Yu, J.; Rafique, J.; Yin, J. Production of aligned helical polymer nanofibers by electrospinning. Eur. Polym. J. 2008, 44, 2838–2844. [Google Scholar] [CrossRef]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibers. J. Electrost. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.Y.; Salick, M.R.; Cordie, T.M.; Peng, X.F.; Turng, L.S. Electrospinning thermoplastic polyurethane/graphene oxide scaffolds for small diameter vascular graft applications. Mater. Sci. Eng. C 2015, 49, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.; Dhakshnamoorthy, M.; Jelmy, E.J.; Vasanthakumari, R.; Kothurkar, N.K. Synthesis and characterization of graphene oxide-polyimide nanofiber composites. RSC Adv. 2014, 4, 9743–9749. [Google Scholar] [CrossRef]
- Panzavolta, S.; Bracci, B.; Gualandi, C.; Focarete, M.L.; Treossi, E.; Kouroupis-Agalou, K.; Rubini, K.; Bosia, F.; Brely, L.; Pugno, N.M.; et al. Structural reinforcement and failure analysis in composite nanofibers of graphene oxide and gelatin. Carbon 2014, 78, 566–577. [Google Scholar] [CrossRef]
- Qi, Y.Y.; Tai, Z.X.; Sun, D.F.; Chen, J.T.; Ma, H.B.; Yan, X.B.; Liu, B.; Xue, Q.J. Fabrication and characterization of poly(vinyl alcohol)/graphene oxide nanofibrous biocomposite scaffolds. J. Appl. Polym. Sci. 2013, 127, 1885–1894. [Google Scholar] [CrossRef]
- Barzegar, F.; Bello, A.; Fabiane, M.; Khamlich, S.; Momodu, D.; Taghizadeh, F.; Dangbegnon, J.; Manyala, N. Preparation and characterization of poly(vinyl alcohol)/graphene nanofibers synthesized by electrospinning. J. Phys.Chem. Solids 2015, 77, 139–145. [Google Scholar] [CrossRef]
- Pant, H.R.; Park, C.H.; Tijing, L.D.; Amarjargal, A.; Lee, D.H.; Kim, C.S. Bimodal fiber diameter distributed graphene oxide/nylon-6 composite nanofibrous mats via electrospinning. Colloids Surf. A Physicochem. Eng. Asp. 2012, 407, 121–125. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, J.; Xie, S.; Liu, J.; Xin, Z.; Liu, X.; Belfiore, L.A. Leveling graphene sheets through electrospinning and their conductivity. RSC Adv. 2015, 5, 42174–42177. [Google Scholar] [CrossRef]
- Bao, Q.; Zhang, H.; Yang, J.; Wang, S.; Tang, D.Y.; Jose, R.; Ramakrishna, S.; Lim, C.T.; Loh, K.P. Graphene-Polymer Nanofiber Membrane for Ultrafast Photonics. Adv. Funct. Mater. 2010, 20, 782–791. [Google Scholar] [CrossRef]
- Wang, M.; Yu, J.H.; Hsieh, A.J.; Rutledge, G.C. Effect of tethering chemistry of cationic surfactants on clay exfoliation, electrospinning and diameter of PMMA/clay nanocomposite fibers. Polymer 2010, 51, 6295–6302. [Google Scholar] [CrossRef]
- Islam, M.S.; Yeum, J.H.; Das, A.K. Effect of pullulan/poly(vinyl alcohol) blend system on the montmorillonite structure with property characterization of electrospun pullulan/poly(vinyl alcohol)/montmorillonite nanofibers. J. Colloid Interface Sci. 2012, 368, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Rong, J.; Nie, G.; Qiao, J.; Wang, H.; Wu, D.; Su, Z.; Niu, Z.; Huang, Y. Enhanced orientation of PEO polymer chains induced by nanoclays in electrospun PEO/clay composite nanofibers. Colloid Polym. Sci. 2013, 291, 1541–1546. [Google Scholar] [CrossRef]
- Neppalli, R.; Wanjale, S.; Birajdar, M.; Causin, V. The effect of clay and of electrospinning on the polymorphism, structure and morphology of poly(vinylidene fluoride). Eur. Polym. J. 2013, 49, 90–99. [Google Scholar] [CrossRef]
- Dong, Y.; Haroosh, H.; Bickford, T. Development and characterisation of novel electrospun polylactic acid/tubular clay nanocomposites. J. Mater. Sci. 2011, 46, 6148–6153. [Google Scholar] [CrossRef]
- Kim, G.M.; Michler, G.H.; Ania, F.; Calleja, F.J.B. Temperature dependence of polymorphism in electrospun nanofibres of PA6 and PA6/clay nanocomposite. Polymer 2007, 48, 4814–4823. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Chen, S.; Wachtel, E.; Koga, T.; Sokolov, J.C.; Rafailovich, M.H. Electrospinning of poly(ethylene-co-vinyl acetate)/clay nanocomposite fibers. J. Polym. Sci. B Polym. Phys. 2009, 47, 2501–2508. [Google Scholar] [CrossRef]
- Kim, G.M.; Michler, G.H.; Potschke, P. Deformation processes of ultrahigh porous multiwalled carbon nanotubes/polycarbonate composite fibers prepared by electrospinning. Polymer 2005, 46, 7346–7351. [Google Scholar] [CrossRef]
- Kedem, S.; Schmidt, J.; Paz, Y.; Cohen, Y. Composite Polymer Nanofibers with Carbon Nanotubes and Titanium Dioxide Particles. Langmuir 2005, 21, 5600–5604. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Ge, J.J.; Zeng, J.; Li, Q.; Reneker, D.H.; Greiner, A.; Cheng, S.Z.D. Electrospun Polyacrylonitrile Nanofibers Containing a High Concentration of Well-Aligned Multiwall Carbon Nanotubes. Chem. Mater. 2005, 17, 967–973. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, Y.; Wei, F.; Luo, G.; Qian, W. Elastic deformation of multiwalled carbon nanotubes in electrospun MWCNTs–PEO and MWCNTs–PVA nanofibers. Polymer 2005, 46, 12689–12695. [Google Scholar] [CrossRef]
- Minoo, N.; Tong, L.; Mark, P.S.; Liming, D.; Xungai, W. Electrospun single-walled carbon nanotube/polyvinyl alcohol composite nanofibers: Structure-property relationships. Nanotechnology 2008, 19. [Google Scholar] [CrossRef]
- Dror, Y.; Salalha, W.; Pyckhout-Hintzen, W.; Yarin, A.; Zussman, E.; Cohen, Y. From Carbon Nanotube Dispersion to Composite Nanofibers, in Scattering Methods and the Properties of Polymer Materials; Springer: Berlin, Germnay; Heidelberg, Germnay, 2005; pp. 64–69. [Google Scholar]
- Sundaray, B.; Subramanian, V.; Natarajan, T.S.; Krishnamurthy, K. Electrical conductivity of a single electrospun fiber of poly(methyl methacrylate) and multiwalled carbon nanotube nanocomposite. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Sen, R.; Zhao, B.; Perea, D.; Itkis, M.E.; Hu, H.; Love, J.; Bekyarova, E.; Haddon, R.C. Preparation of Single-Walled Carbon Nanotube Reinforced Polystyrene and Polyurethane Nanofibers and Membranes by Electrospinning. Nano Lett. 2004, 4, 459–464. [Google Scholar] [CrossRef]
- Saeed, K.; Park, S.Y.; Lee, H.J.; Baek, J.B.; Huh, W.S. Preparation of electrospun nanofibers of carbon nanotube/polycaprolactone nanocomposite. Polymer 2006, 47, 8019–8025. [Google Scholar] [CrossRef]
- Pan, C.; Ge, L.Q.; Gu, Z. Fabrication of multi-walled carbon nanotube reinforced polyelectrolyte hollow nanofibers by electrospinning. Compos. Sci. Technol. 2007, 67, 3271–3277. [Google Scholar] [CrossRef]
- Jeong, J.S.; Jeon, S.Y.; Lee, T.Y.; Park, J.H.; Shin, J.H.; Alegaonkar, P.S.; Berdinsky, A.S.; Yoo, J.B. Fabrication of MWNTs/nylon conductive composite nanofibers by electrospinning. Diam. Relat. Mater. 2006, 15, 1839–1843. [Google Scholar] [CrossRef]
- Ayutsede, J.; Gandhi, M.; Sukigara, S.; Ye, H.; Hsu, C.; Gogotsi, Y.; Ko, F. Carbon Nanotube Reinforced Bombyx mori Silk Nanofibers by the Electrospinning Process. Biomacromolecules 2006, 7, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, P.; Shim, B.S.; Kotov, N.A. Polymer/clay and polymer/carbon nanotube hybrid organic-inorganic multilayered composites made by sequential layering of nanometer scale films. Coord. Chem. Rev. 2009, 253, 2835–2851. [Google Scholar] [CrossRef]
- Caruso, F.; Caruso, R.A.; Mohwald, H. Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating. Science 1998, 282, 1111–1114. [Google Scholar] [CrossRef] [PubMed]
- Kleinfeld, E.R.; Ferguson, G.S. Stepwise formation of multilayered nanostructural films from macromolecular precursors. Science 1994, 265, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Kotov, N.A.; Dékány, I.; Fendler, J.H. Ultrathin graphite oxide-polyelectrolyte composites prepared by self-assembly: Transition between conductive and non-conductive states. Adv. Mater. 1996, 8, 637–641. [Google Scholar] [CrossRef]
- Lee, T.; Min, S.H.; Gu, M.; Jung, Y.K.; Lee, W.; Lee, J.U.; Seong, D.G.; Kim, B.S. Layer-by-Layer Assembly for Graphene-Based Multilayer Nanocomposites: Synthesis and Applications. Chem. Mater. 2015, 27, 3785–3796. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.D.; Schmitt, J. Buildup of ultrathin multilayer films by a self-assembly process: III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Films 1992, 210–211, 831–835. [Google Scholar] [CrossRef]
- Picart, C.; Lavalle, P.; Hubert, P.; Cuisinier, F.J.G.; Decher, G.; Schaaf, P.; Voegel, J.C. Buildup Mechanism for Poly(l-lysine)/Hyaluronic Acid Films onto a Solid Surface. Langmuir 2001, 17, 7414–7424. [Google Scholar] [CrossRef]
- Bergbreiter, D.E.; Franchina, J.G.; Sussman, L. Polyvalent Hydrogen-Bonding Functionalization of Ultrathin Hyperbranched Films on Polyethylene and Gold. Macromolecules 2001, 34, 3018–3023. [Google Scholar] [CrossRef]
- Such, G.K.; Johnston, A.P.R.; Caruso, F. Engineered hydrogen-bonded polymer multilayers: From assembly to biomedical applications. Chem. Soc. Rev. 2011, 40, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ma, N.; Chen, Q.; Wang, W.; Wang, L. Halogen Bonding as a New Driving Force for Layer-by-Layer Assembly. Langmuir 2007, 23, 9540–9542. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, Y.; Mitsuishi, M.; Ito, S.; Yamamoto, M. Preparation of the Layer-by-Layer Deposited Ultrathin Film Based on the Charge-Transfer Interaction. Langmuir 1997, 13, 1385–1387. [Google Scholar] [CrossRef]
- Kohli, P.; Blanchard, G.J. Applying Polymer Chemistry to Interfaces: Layer-by-Layer and Spontaneous Growth of Covalently Bound Multilayers. Langmuir 2000, 16, 4655–4661. [Google Scholar] [CrossRef]
- Sun, J.; Wu, T.; Liu, F.; Wang, Z.; Zhang, X.; Shen, J. Covalently Attached Multilayer Assemblies by Sequential Adsorption of Polycationic Diazo-Resins and Polyanionic Poly(acrylic acid). Langmuir 2000, 16, 4620–4624. [Google Scholar] [CrossRef]
- Bergbreiter, D.E.; Chance, B.S. “Click”-Based Covalent Layer-by-Layer Assembly on Polyethylene Using Water-Soluble Polymeric Reagents. Macromolecules 2007, 40, 5337–5343. [Google Scholar] [CrossRef]
- Rouse, J.H.; Lillehei, P.T. Electrostatic assembly of polymer/single walled carbon nanotube multilayer films. Nano Lett. 2003, 3, 59–62. [Google Scholar] [CrossRef]
- Artyukhin, A.B.; Bakajin, O.; Stroev, P.; Noy, A. Layer-by-Layer Electrostatic Self-Assembly of Polyelectrolyte Nanoshells on Individual Carbon Nanotube Templates. Langmuir 2004, 20, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, T.; Lu, X. Facile fabrication of polystyrene/carbon nanotube composite nanospheres with core-shell structure via self-assembly. Polymer 2010, 51, 3715–3721. [Google Scholar] [CrossRef]
- He, Q.; Cui, Y.; Ai, S.; Tian, Y.; Li, J. Self-assembly of composite nanotubes and their applications. Curr. Opin. Colloid Interface Sci. 2009, 14, 115–125. [Google Scholar] [CrossRef]
- Mamedov, A.A.; Kotov, N.A.; Prato, M.; Guldi, D.M.; Wicksted, J.P.; Hirsch, A. Molecular design of strong single-wall carbon nanotube/polyelectrolyte multilayer composites. Nat. Mater. 2002, 1, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, P.; Kaushik, A.K.; Arruda, E.M.; Waas, A.M.; Shim, B.S.; Xu, J.D.; Nandivada, H.; Pumplin, B.G.; Lahann, J.; Ramamoorthy, A.; Kotov, N.A. Ultrastrong and Stiff Layered Polymer Nanocomposites. Science 2007, 318, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Halpin, J.C.; Kardos, J.L. The Halpin-Tsai equations: A review. Polym. Eng. Sci. 1976, 16, 344–352. [Google Scholar]
- Terrones, M.; Martín, O.; González, M.; Pozuelo, J.; Serrano, B.; Cabanelas, J.C.; Vega-Díaz, S.M.; Baselga, J. Interphases in Graphene Polymer-based Nanocomposites: Achievements and Challenges. Adv. Mater. 2011, 23, 5302–5310. [Google Scholar] [CrossRef] [PubMed]
- Cadek, M.; Coleman, J.N.; Ryan, K.P.; Nicolosi, V.; Bister, G.; Fonseca, A.; Nagy, J.B.; Szostak, K.; Béguin, F.; Blau, W.J. Reinforcement of Polymers with Carbon Nanotubes: The Role of Nanotube Surface Area. Nano Lett. 2004, 4, 353–356. [Google Scholar] [CrossRef]
- Haggenmueller, R.; Gommans, H.H.; Rinzler, A.G.; Fischer, J.E.; Winey, K.I. Aligned single-wall carbon nanotubes in composites by melt processing methods. Chem. Phys. Lett. 2000, 330, 219–225. [Google Scholar] [CrossRef]
- Haggenmueller, R.; Zhou, W.; Fisher, J.E.; Winey, K.I. Production and characterization of polymer nanocomposites with highly aligned single-walled carbon nanotubes. J. Nanosci. Nanotechnol. 2003, 3, 105–110. [Google Scholar] [CrossRef]
- Chen, X.Q.; Saito, T.; Yamada, H.; Matsushige, K. Aligning single-wall carbon nanotubes with an alternating-current electric field. Appl. Phys. Lett. 2001, 78, 3714–3716. [Google Scholar] [CrossRef]
- Kumar, M.S.; Lee, S.H.; Kim, T.Y.; Kim, T.H.; Song, S.M.; Yang, J.W.; Nahm, K.S.; Suh, E.K. DC electric field assisted alignment of carbon nanotubes on metal electrodes. Solid-State Electron. 2003, 47, 2075–2080. [Google Scholar] [CrossRef]
- Kumar, M.S.; Kim, T.H.; Lee, S.H.; Song, S.M.; Yang, J.W.; Nahm, K.S.; Suh, E.K. Influence of electric field type on the assembly of single walled carbon nanotubes. Chem. Phys.Lett. 2004, 383, 235–239. [Google Scholar] [CrossRef]
- Martin, C.A.; Sandler, J.K.W.; Windle, A.H.; Schwarz, M.K.; Bauhofer, W.K.; Shaffer, M.S.P. Electric field-induced aligned multi-wall carbon nanotube networks in epoxy composites. Polymer 2005, 46, 877–886. [Google Scholar] [CrossRef]
- Strobl, C.J.; Schaflein, C.; Beierlein, U.; Ebbecke, J.; Wixforth, A. Carbon nanotube alignment by surface acoustic waves. Appl. Phys. Lett. 2004, 85, 1427–1429. [Google Scholar] [CrossRef]
- Kamat, P.V.; Thomas, K.G.; Barazzouk, S.; Girishkumar, G.; Vinodgopal, K.; Meisel, D. Self-Assembled Linear Bundles of Single Wall Carbon Nanotubes and Their Alignment and Deposition as a Film in a dc Field. J. Am. Chem. Soc. 2004, 126, 10757–10762. [Google Scholar] [CrossRef] [PubMed]
- Camponeschi, E.; Vance, R.; Al-Haik, M.S.; Garmestani, H.; Tannebaum, R. Properties of carbon nanotube-polymer composites in a magnetic field. Carbon 2007, 45, 2037–2046. [Google Scholar] [CrossRef]
- Garmestani, H.; Al-Haik, M.S.; Dahmen, K.; Tannenbaum, R.; Li, D.; Sablin, S.S.; Hussaini, M.Y. Polymer-Mediated Alignment of Carbon Nanotubes under High Magnetic Fields. Adv. Mater. 2003, 15, 1918–1921. [Google Scholar] [CrossRef]
- Steinart, B.W.; Dean, D.R. Magnetic field alignment and electrical properties of solution cast PET-carbon nanotube composite films. Polymer 2009, 50, 898–904. [Google Scholar] [CrossRef]
- Yang, J.; Wang, C.; Wang, K.; Zhang, Q.; Chen, F.; Du, R.; Fu, Q. Direct Formation of Nanohybrid Shish-Kebab in the Injection Molded Bar of Polyethylene/Multiwalled Carbon Nanotubes Composite. Macromolecules 2009, 42, 7016–7023. [Google Scholar] [CrossRef]
- Bin, Y.; Kitanaka, M.; Zhu, D.; Matsuo, M. Development of highly oriented polyethylene filled with aligned carbon nanotubes by gelation/crystallization from solutions. Macromolecules 2003, 36, 6213–6219. [Google Scholar] [CrossRef]
- Chen, W.; Tao, X. Production and characterization of polymer nanocomposite with aligned single wall carbon nanotubes. Appl. Surf. Sci. 2006, 252, 3547–3552. [Google Scholar] [CrossRef]
- Wang, Q.; Dai, J.F.; Li, W.; Wei, Z.Q.; Jiang, J.L. The effects of CNT alignment on electrical conductivity and mechanical properties of SWNT/epoxy nanocomposites. Compos. Sci. Technol. 2008, 68, 1644–1648. [Google Scholar] [CrossRef]
- Rafiee, M.A.; Rafiee, J.; Srivastava, I.; Wang, Z.; Song, H.; Yu, Z.Z.; Koratkar, N. Fracture and fatigue in graphene nanocomposites. Small 2010, 6, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, A.; Luo, J.J.; Daniel, I.M. Processing of expanded graphite reinforced polymer nanocomposites. Compos. Sci. Technol. 2006, 66, 1182–1189. [Google Scholar] [CrossRef]
- Usuki, A.; Hasegawa, N.; Kato, M. Polymer-Clay Nanocomposites. Adv. Polym. Sci. 2005, 179, 135–195. [Google Scholar]
- Usuki, A.; Kojima, Y.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Synthesis of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1179–1184. [Google Scholar] [CrossRef]
- Fornes, T.D.; Yoon, P.J.; Keskkula, H.; Paul, D.R. Nylon 6 nanocomposites: The effect of matrix molecular weight. Polymer 2001, 42, 09929–09940. [Google Scholar] [CrossRef]
- Hu, K.; Gupta, M.K.; Kulkarni, D.D.; Tsukruk, V.V. Ultra-Robust Graphene Oxide-Silk Fibroin Nanocomposite Membranes. Adv. Mater. 2013, 25, 2301–2307. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, D.D.; Choi, I.; Singamaneni, S.S.; Tsukruk, V.V. Graphene Oxide-Polyelectrolyte Nanomembranes. ACS Nano 2010, 4, 4667–4676. [Google Scholar] [CrossRef] [PubMed]
- Kotov, N.A.; Magonov, S.; Tropsha, E. Layer-by-Layer Self-Assembly of Alumosilicate-Polyelectrolyte Composites: Mechanism of Deposition, Crack Resistance, and Perspectives for Novel Membrane Materials. Chem. Mater. 1998, 10, 886–895. [Google Scholar] [CrossRef]
- Tang, Z.; Magonov, S.; Ozturk, B. Nanostructured artificial nacre. Nat. Mater. 2003, 2, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.; Jacques, D.; Minot, M.; Rantell, T. Fabrication of carbon multiwall nanotube/polymer composites by shear mixing. Macromol. Mater. Eng. 2002, 287, 395–403. [Google Scholar] [CrossRef]
- Xiao, K.Q.; Zhang, L.C.; Zarudi, I. Mechanical and rheological properties of CNT-reinforced polyethylene composites. Compos. Sci. Technol. 2007, 67, 177–182. [Google Scholar] [CrossRef]
- Gorrasi, J.; Sarno, M.; Di Bartolomeo, A.; Sannino, D.; Ciambelli, P.; Vittoria, V. Incorporation of carbon nanotubes into polyethylene by high energy ball milling: Morphology and physical properties. J. Polym. Sci. B Polym. Phys. 2007, 45, 597–606. [Google Scholar] [CrossRef]
- Moore, E.M.; Ortiz, D.L.; Marla, V.T.; Shambaugh, R.L.; Grady, B.P. Enhancing the strength of polypropylene fibers with CNTs. J. Appl. Polym. Sci. 2004, 93, 2926–2933. [Google Scholar] [CrossRef]
- Manchado, M.A.L.; Valentini, L.; Biagiotti, J.; Kenny, J.M. Thermal and mechanical properties of SWCNT-polypropylene composites prepared by melt processing. Carbon 2005, 43, 1499–1505. [Google Scholar] [CrossRef]
- Dondero, W.E.; Gorg, R.E.A. Morphological and mechanical properties of CNT-polymer composites via melt compounding. J. Polym. Sci. B Polym. Phys. 2006, 44, 864–878. [Google Scholar] [CrossRef]
- Jose, M.V.; Dean, D.; Tyner, J.; Price, G.; Nyairo, E. Polypropylene/CNT nanocomposite fibers: Process-morphology-property relationships. J. Appl. Polym. Sci. 2007, 103, 3844–3850. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, Z.; Xu, C.; Liang, J.; Wei, B.; Wu, D.; Zhu, S. Study on poly(methyl methacrylate)/carbon nanotube composites. Mater. Sci. Eng. A 1999, 271, 395–400. [Google Scholar] [CrossRef]
- Velasco-Santos, C.; Martinez-Hernandez, A.L.; Fisher, F.T.; Ruoff, R.; Castano, V.M. Improvement of thermal and mechanical properties of carbon nanotube composites through chemical functionalization. Chem. Mater. 2003, 15, 4470–4475. [Google Scholar] [CrossRef]
- Gorga, R.E.; Cohen, R.E. Toughness enhancements in PMMA by addition of oriented MWCNTs. J. Polym. Sci. B Polym. Phys. 2004, 42, 2690–2702. [Google Scholar] [CrossRef]
- Blond, D.; Barron, V.; Ruether, M.; Ryan, K.P.; Nicolosi, V.; Blau, W.J.; Coleman, J.N. Enhancement of Modulus, Strength, and Toughness in Poly(methyl methacrylate)-Based Composites by the Incorporation of Poly(methyl methacrylate)-Functionalized Nanotubes. Adv. Funct. Mater. 2006, 16, 1608–1613. [Google Scholar] [CrossRef]
- Hou, Y.; Tang, J.; Zhang, H.; Qian, C.; Feng, Y.; Liu, J. Functionalised few-walled carbon nanotubes for mechanical reinforcement of polymeric composites. ACS Nano 2009, 3, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Bokobza, L.; Belin, C. Effect of strain in the properties of a styrene-butadiene rubber filled with MWCNTs. J. Appl. Polym. Sci. 2007, 105, 2054–2061. [Google Scholar] [CrossRef]
- Zhang, W.D.; Shen, L.; Phang, I.Y.; Liu, T. CNT reinforced nylon-6 composite prepared by simple melt compounding. Macromolecules 2004, 37, 256–259. [Google Scholar] [CrossRef]
- Liu, T.; Phang, I.Y.; Shen, L.; Chow, S.Y.; Zhang, Y.D. Morphology and mechanical properties of MWCNT reinforced nylon-6 composites. Macromolecules 2004, 37, 7214–7222. [Google Scholar] [CrossRef]
- Ga, J.; Zhao, B.; Itkis, M.E.; Bekyarova, E.; Hu, H.; Kranak, V.; Yu, A.; Haddon, R.C. Chemical Engineering of the Single-Walled Carbon Nanotube-Nylon 6 Interface. J. Am. Chem. Soc. 2006, 128, 7492–7496. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.J.; Zhang, D.; Li, Q.; Hou, H.; Graham, M.J.; Dai, L.; Harris, F.W.; Cheng, S.Z.D. Multiwalled Carbon Nanotubes with Chemically Grafted Polyetherimides. J. Am. Chem. Soc. 2005, 127, 9984–9985. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Tong, Y.; Zhang, W.D. Preparation and characterization of CNT-polyetherimide nanocomposite films. Compos. Sci. Technol. 2007, 67, 406–412. [Google Scholar] [CrossRef]
- Siochi, E.J.; Working, D.C.; Park, C.; Lillehei, P.T.; Rouse, J.H.; Topping, C.C.; Bhattacharyya, A.R.; Kumar, S. Melt processing of SWCNT-polyimide nanocomposite fibers. Compos. B 2004, 35, 439–446. [Google Scholar] [CrossRef]
- Xia, H.; Song, M. Preparation and characterization of polyurethane-carbon nanotube composites. Soft Matter 2005, 1, 386–394. [Google Scholar] [CrossRef]
- Jung, Y.C.; Sahoo, N.G.; Cho, J.W. Polymeric nanocomposites of polyurethane block copolymers and functionalized multi-walled carbon nanotubes as crosslinkers. Macromol. Rapid Commun. 2006, 27, 126–131. [Google Scholar] [CrossRef]
- Penumadu, D.; Dutta, A.; Pharr, G.M.; Files, B. Mechanical properties of blended SWCNT composites. J. Mater. Res. 2003, 18, 1849–1853. [Google Scholar] [CrossRef]
- Zheng, W.; Wong, S.C.; Sue, H.J. Transport behavior of PMMA/expanded graphite nanocomposites. Polymer 2002, 43, 6767–6773. [Google Scholar] [CrossRef]
- Ramanathan, T.; Stankovich, S.; Dikin, D.A.; Liu, H.; Shen, H.; Nguyen, S.T.; Brinson, L.C. Graphitic Nanofillers in PMMA Nanocomposites—An Investigation of Particle Size and Dispersion and Their Influence on Nanocomposite Properties. J. Polym. Sci. B Polym. Phys. 2007, 45, 2097–2112. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kim, M.S.; Jeong, H.M.; Shin, C.M. Graphite oxide/poly(methyl methacrylate) nanocomposites prepared by a novel method utilizing macroazoinitiator. Compos. Sci. Technol. 2009, 69, 186–191. [Google Scholar] [CrossRef]
- Zheng, W.; Lu, X.; Wong, S.C. Electrical and mechanical properties of expanded graphite-reinforced high-density polyethylene. J. Appl. Polym. Sci. 2004, 91, 2781–2788. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, L.; Wang, Y.; Ma, Y.; Guo, T.; Chen, Y. Molecular-Level Dispersion of Graphene into Poly(vinyl alcohol) and Effective Reinforcement of their Nanocomposites. Adv. Funct. Mater. 2009, 19, 2297–2302. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Q.; Chen, D. Enhanced mechanical properties of graphene-based poly(vinyl alcohol) composites. Macromolecules 2010, 43, 2357–2363. [Google Scholar] [CrossRef]
- Xu, Y.; Hong, W.; Bai, H.; Li, C.; Shi, G. Strong and ductile poly(vinyl alcohol)/graphene oxide composite films with a layered structure. Carbon 2009, 47, 3538–3543. [Google Scholar] [CrossRef]
- Lee, Y.R.; Raghu, A.V.; Jeong, H.M.; Kim, B.K. Properties of waterborne polyurethane/functionalized graphene sheet nanocomposites prepared by an in situ method. Macromol. Chem. Phys. 2009, 210, 1247–1254. [Google Scholar] [CrossRef]
- Feng, R.; Guan, G.; Zhou, W.; Li, C.; Zhang, D.; Xiao, Y. In situ synthesis of poly(ethylene terephthalate)/graphene composites using a catalyst supported on graphite oxide. J. Mater. Chem. 2011, 21, 3931–3939. [Google Scholar] [CrossRef]
- Garboczi, E.J.; Snyder, K.A.; Douglas, J.F.; Thorpe, M.F. Geometrical percolation threshold of overlapping ellipsoids. Phys. Rev. E 1996, 52, 819–828. [Google Scholar] [CrossRef]
- Steurer, P.; Wissert, R.; Thomann, R.; Mülhaupt, R. Functionalized Graphenes and Thermoplastic Nanocomposites Based upon Expanded Graphite Oxide. Macromol. Rapid Commun. 2009, 30, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.B.; Allaoui, A. Effect of the length and the aggregate size of MWNTs on the improvement efficiency of the mechanical and electrical properties of nanocomposites—Experimental investigation. Compos. A Appl. Sci. Manuf. 2003, 34, 689–694. [Google Scholar] [CrossRef]
- Celzard, A.; McRae, E.; Deleuze, C.; Dufort, M.; Furdin, G.; Marêché, J.F. Critical concentration in percolating systems containing a high-aspect-ratio filler. Phys. Rev. B 1996, 53, 6209–6214. [Google Scholar] [CrossRef]
- Qi, X.Y.; Yan, D.; Jiang, Z.; Cao, Y.K.; Yu, Z.Z.; Yavari, F.; Koratkar, N. Enhanced Electrical Conductivity in Polystyrene Nanocomposites at Ultra-Low Graphene Content. ACS Appl. Mater. Interfaces 2011, 3, 3130–3133. [Google Scholar] [CrossRef] [PubMed]
- Sandler, J.K.W.; Kirk, J.E.; Kinloch, I.A.; Shaffer, M.S.P.; Windle, A.H. Ultra-low electrical percolation threshold in carbon-nanotube-epoxy composites. Polymer 2003, 44, 5893–5899. [Google Scholar] [CrossRef]
- Li, J.; Sham, M.L.; Kim, J.K.; Marom, G. Morphology and properties of UV/ozone treated graphite nanoplatelet/epoxy nanocomposites. Compos. Sci. Technol. 2007, 67, 296–305. [Google Scholar] [CrossRef]
- Bauhofer, W.; Kovacs, J.Z. A review and analysis of electrical percolation in carbon nanotube polymer composites. Compos. Sci. Technol. 2009, 69, 1486–1498. [Google Scholar] [CrossRef]
- Xie, L.; Xu, F.; Qiu, F.; Lu, H.; Yang, Y. Single-walled carbon nanotubes functionalized with high bonding density of polymer layers and enhanced mechanical properties of composites. Macromolecules 2007, 40, 3296–3305. [Google Scholar] [CrossRef]
- Verdejo, R.; Barroso-Bujans, F.; Rodriguez-Perez, M.A.; Saja, J.A.D.; Lopez-Manchado, M.A. Functionalized graphene sheet filled silicone foam nanocomposites. J. Mater. Chem. 2008, 18, 2221–2226. [Google Scholar] [CrossRef]
- Veca, L.M.; Meziani, M.J.; Wang, W.; Wang, X.; Lu, F.; Zhang, P.; Lin, Y.; Fee, R.; Connell, J.W.; Sun, Y.P. Carbon nanosheets for polymeric nanocomposites with high thermal conductivity. Adv. Mater. 2009, 21, 2088–2092. [Google Scholar] [CrossRef]
- Wang, S.; Tambraparni, M.; Qiu, J.; Tipton, J.; Dean, D. Thermal Expansion of Graphene Composites. Macromolecules 2009, 42, 5251–5255. [Google Scholar] [CrossRef]
- Liu, N.; Luo, F.; Wu, H.; Liu, Y.; Zhang, C.; Chen, J. One-Step Ionic-Liquid-Assisted Electrochemical Synthesis of Ionic-Liquid-Functionalized Graphene Sheets Directly from Graphite. Adv. Funct. Mater. 2008, 18, 1518–1525. [Google Scholar] [CrossRef]
- Kayano, Y.; Keskkula, H.; Paul, D.R. Effect of polycarbonate molecular weight and processing conditions on mechanical behaviour of blends with a core-shell impact modifier. Polymer 1996, 37, 4505–4518. [Google Scholar] [CrossRef]
- Koerner, H.; Hampton, E.; Dean, D.; Turgut, Z.; Drummy, L.; Mirau, P.; Vaia, R. Generating Triaxial Reinforced Epoxy/Montmorillonite Nanocomposites with Uniaxial Magnetic Fields. Chem. Mater. 2005, 17, 1990–1996. [Google Scholar] [CrossRef]
- Koerner, H.; Jacobs, J.D.; Tomlin, D.W.; Busbee, J.D.; Vaia, R.A. Tuning Polymer Nanocomposite Morphology: AC Electric Field Manipulation of Epoxy-Montmorillonite (Clay) Suspensions. Adv. Mater. 2004, 16, 297–302. [Google Scholar] [CrossRef]
- Sasaki, T.; Shimizu, A.; Mourey, T.H.; Thurau, C.T.; Ediger, M.D. Glass transition of small polystyrene spheres in aqueous suspensions. J. Chem. Phys. 2003, 119, 8730–8735. [Google Scholar] [CrossRef]
- Ding, J.; Xue, G.; Dai, Q.; Cheng, R. Glass transition temperature of polystyrene microparticles. Polymer 1993, 34, 3325–3327. [Google Scholar] [CrossRef]
- Forrest, J.A.; Dalnoki-Veress, K.; Stevens, J.R.; Dutcher, J.R. Effect of Free Surfaces on the Glass Transition Temperature of Thin Polymer Films. Phys. Rev. Lett. 1996, 77, 2002–2005. [Google Scholar] [CrossRef] [PubMed]
- Rittigstein, P.; Priestley, R.D.; Broadbelt, L.J.; Torkelson, J.M. Model polymer nanocomposites provide an understanding of confinement effects in real nanocomposites. Nat. Mater. 2007, 6, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.H.; Aoyama, S.; Abdala, A.A.; Macosko, C.W. Does Graphene Change Tg of Nanocomposites? Macromolecules 2014, 47, 8311–8319. [Google Scholar] [CrossRef]
- Gaur, U.; Wunderlich, B. Study of Microphase Separation in Block Copolymers of Styrene and α-Methylstyrene in the Glass Transition Region Using Quantitative Thermal Analysis. Macromolecules 1980, 13, 1618–1625. [Google Scholar] [CrossRef]
- Roth, C.B.; Dutcher, J.R. Glass transition temperature of freely-standing films of atactic poly(methyl methacrylate). Eur. Phys. J. E 2003, 12, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Tate, R.S.; Fryer, D.S.; Pasqualini, S.; Montague, M.F.; de Pablo, J.J.; Nealey, P.F. Extraordinary elevation of the glass transition temperature of thin polymer films grafted to silicon oxide substrates. J. Chem. Phys. 2001, 115, 9982–9990. [Google Scholar] [CrossRef]
- Keddie, J.L.; Jones, R.A.L.; Cory, R.A. Interface and surface effects on the glass-transition temperature in thin polymer films. Faraday Discuss. 1994, 98, 219–230. [Google Scholar] [CrossRef]
- Rittigstein, P.; Torkelson, J.M. Polymer-nanoparticle interfacial interactions in polymer nanocomposites: Confinement effects on glass transition temperature and suppression of physical aging. J. Polym. Sci. B Polym. Phys. 2006, 44, 2935–2943. [Google Scholar] [CrossRef]
- Yuen, S.M.; Ma, C.M.; Lin, Y.Y.; Kuan, H.C. Preparation, morphology and properties of acid and amine modified multiwalled carbon nanotube/polyimide composite. Compos. Sci. Technol. 2007, 67, 2564–2573. [Google Scholar] [CrossRef]
- Grady, B.P. Effects of carbon nanotubes on polymer physics. J. Polym. Sci. B Polym. Phys. 2012, 50, 591–623. [Google Scholar] [CrossRef]
- Lee, K.M.; Han, C.D. Effect of hydrogen bonding on the rheology of polycarbonate/organoclay nanocomposites. Polymer 2003, 44, 4573–4588. [Google Scholar] [CrossRef]
- Dai, X.; Xu, J.; Guo, X.; Lu, Y.; Shen, D.; Zhao, N.; Luo, X.; Zhang, X. Study on Structure and Orientation Action of Polyurethane Nanocomposites. Macromolecules 2004, 37, 5615–5623. [Google Scholar] [CrossRef]
- Zhang, X.; Loo, L.S. Study of glass transition and reinforcement mechanism in polymer/layered silicate nanocomposites. Macromolecules 2009, 42, 5196–5207. [Google Scholar] [CrossRef]
- Krishnamoorti, R.; Vaia, R.A.; Giannelis, E.P. Structure and Dynamics of Polymer-Layered Silicate Nanocomposites. Macromolecules 1996, 8, 1728–1734. [Google Scholar] [CrossRef]
- Messersmith, P.B.; Giannelis, E.P. Synthesis and barrier properties of poly(e-caprolactone)-layered silicate nanocomposites. J. Polym. Sci. A Polym. Chem. 1995, 33, 1047–1057. [Google Scholar] [CrossRef]
- Yang, Y.H.; Bolling, L.; Priolo, M.A.; Grunlan, J.C. Super gas barrier and selectivity of graphene oxide-polymer multilayer thin films. Adv. Mater. 2013, 45, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Kuila, T.; Kim, N.H.; Kud, B.C.; Lee, J.H. In situ synthesis of the reduced graphene oxide-polyethyleneimine composite and its gas barrier properties. J. Mater. Chem. A 2013, 1, 3739–3746. [Google Scholar] [CrossRef]
- Tseng, I.H.; Liao, Y.F.; Chiang, J.C.; Tsai, M.H. Transparent polyimide/graphene oxide nanocomposite with improved moisture barrier property. Mater. Chem. Phys. 2012, 136, 247–253. [Google Scholar] [CrossRef]
- Morgan, A.B. Flame retarded polymer layered silicate nanocomposites:A review of commercial and open literature systems. Polym. Adv. Technol. 2006, 96, 206–217. [Google Scholar] [CrossRef]
- Schutz, M.R.; Kalo, H.; Lunkenbein, T.; Breu, J.; Wilkie, C.A. Intumescent-like behavior of polystyrene synthetic clay nanocomposites. Polymer 2012, 52, 3288–3294. [Google Scholar] [CrossRef]
- Bartholmai, M.; Schartel, B. Layered silicate polymer nanocomposites: New approach or illusion for fire retardancy? Investigations of the potentials and the tasks using a model system. Polym. Adv.Technol. 2004, 15, 355–364. [Google Scholar] [CrossRef]
- Costache, M.C.; Heidecker, M.J.; Manias, E.; Camino, G.; Frache, A.; Beyer, G.; Gupta, R.K.; Wilkie, C.A. The influence of carbon nanotubes, organically modified montmorillonites and layered double hydroxides on the thermal degradation and fire retardancy of polyethylene, ethylene-vinyl acetate copolymer and polystyrene. Polymer 2007, 48, 6352–6345. [Google Scholar] [CrossRef]
- May, P.; Khan, U.; O'Neill, A.; Coleman, J.N. Approaching the theoretical limit for reinforcing polymers with graphene. J. Mater. Chem. 2012, 22, 1278–1282. [Google Scholar] [CrossRef]
- Grimmer, C.S.; Dharan, C.K.H. High-cycle fatigue of hybrid carbon nanotube/glass fiber/polymer composites. J. Mater. Sci. 2008, 43, 4487–4492. [Google Scholar] [CrossRef]
- Kim, K.T.; Jo, W.H. Non-destructive functionalization of multi-walled carbon nanotubes with naphthalene-containing polymer for high performance Nylon66/multi-walled carbon nanotube composites. Carbon 2011, 49, 819–826. [Google Scholar] [CrossRef]
- Yuan, W.; Chan-Park, M.B. Covalent cum Noncovalent Functionalizations of Carbon Nanotubes for Effective Reinforcement of a Solution Cast Composite Film. ACS Appl. Mater. Interfaces 2012, 4, 2065–2073. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, S.; Park, Y.T.; Ougizawa, T.; Macosko, C.W. Melt crystallization of poly(ethylene terephthalate): Comparing addition of graphene vs. carbon nanotubes. Polymer 2014, 55, 2077–2085. [Google Scholar] [CrossRef]
- Calcagno, C.I.W.; Mariani, C.M.; Teixeira, S.R.; Mauler, R.S. The effect of organic modifier of the clay on morphology and crystallization properties of PET nanocomposites. Polymer 2007, 48, 966–974. [Google Scholar] [CrossRef]
- Chou, C.C.; McAtee, J.L. Thermal Decomposition of Organo-Ammonium Compounds Exchanged Onto Montmorillonite and Hectorite. Clay and Clay Miner. 1969, 17, 339–346. [Google Scholar] [CrossRef]
- Xie, W.; Gao, Z.; Pan, W.-P.; Hunter, D.; Singh, A.; Vaia, R. Thermal Degradation Chemistry of Alkyl Quaternary Ammonium Montmorillonite. Chem. Mater. 2001, 13, 2979–2990. [Google Scholar] [CrossRef]
- Nanostructured and Amorphous Materials Incorporated. Available online: http:www.nanoamor.com (accessed on 6 December 2015).
- Cheap Tubes. Available online: http://www.cheaptubes.com/ (accessed on 6 December 2015).
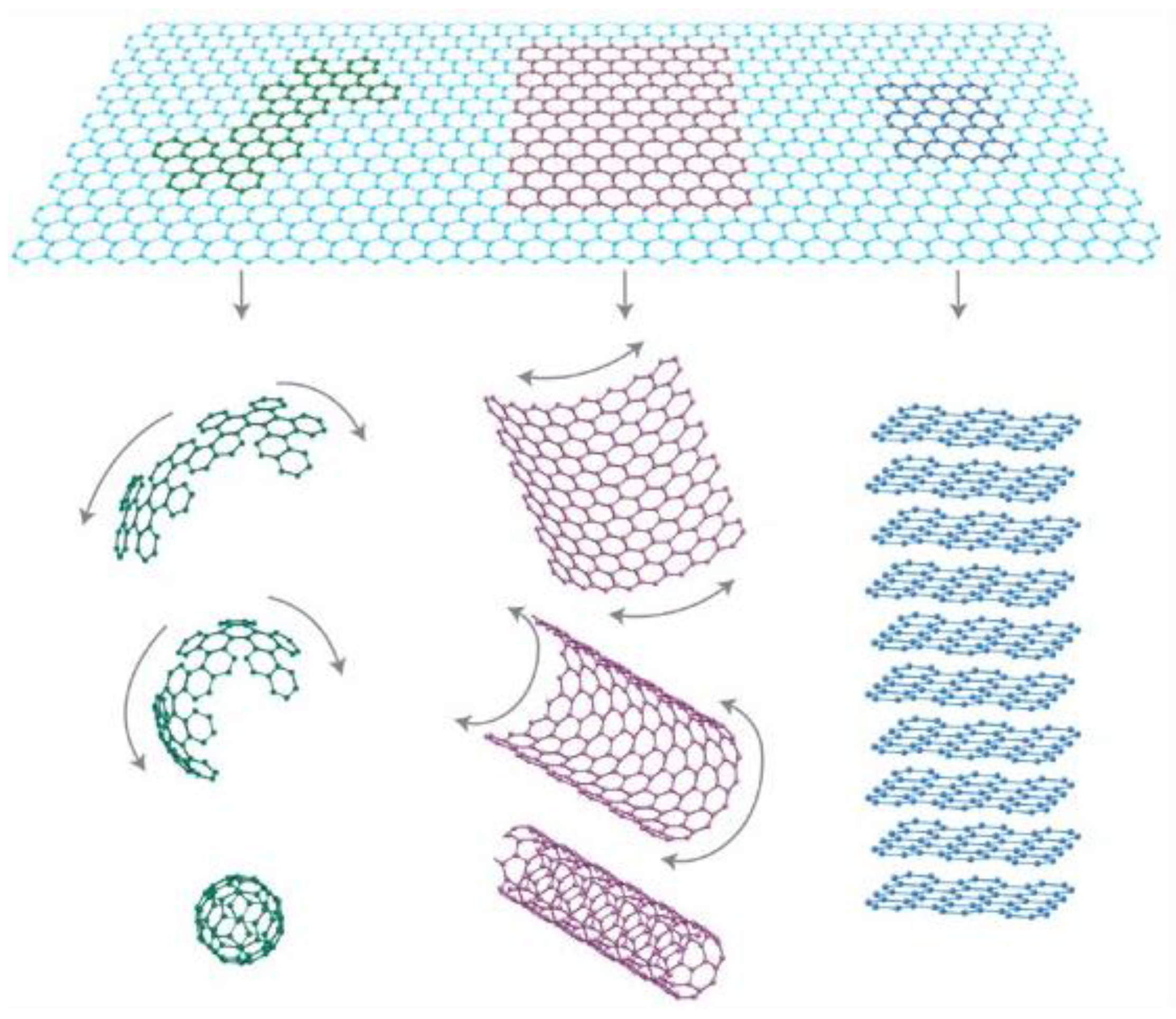


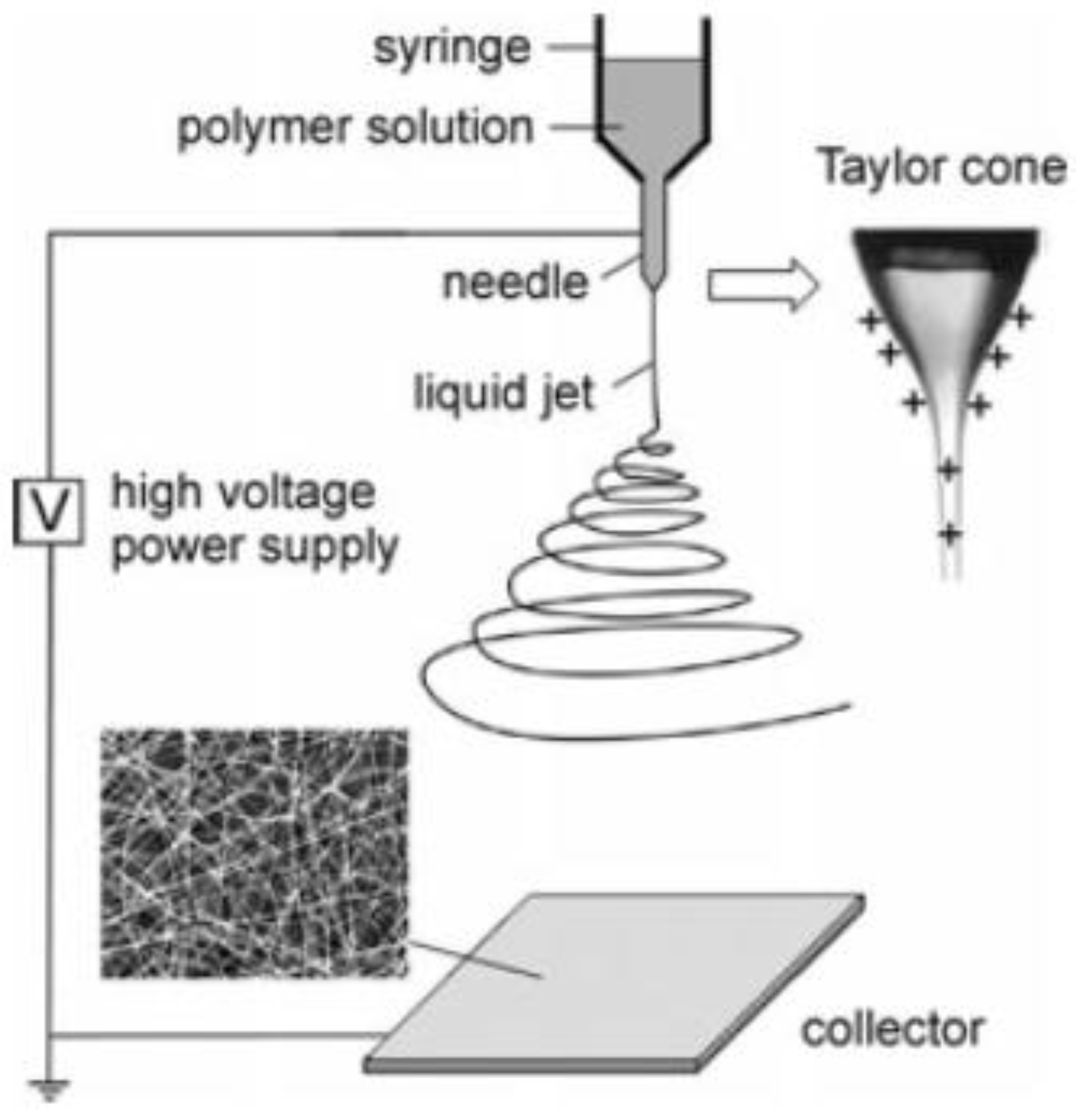

| Polymer | CNT Type | wt(%) | Processing Method | σ/σ0 | E/E0 | Reference |
|---|---|---|---|---|---|---|
| PS | MWCNT | 1.00 | Solution Casting | 1.25 | 1.42 | [31] |
| PS | MWCNT | 5.00 | Solution Casting | 1.5 | 2.2 | [149] |
| PS | MWCNT | 40.00 | Melt Mixing | 1.1 | 2.0 | [262] |
| LDPE | MWCNT | 10.00 | Melt Mixing | 1.56 | 1.89 | [263] |
| LDPE | MWCNT | 3.00 | Ball Milling | 3.50 | 1.3 | [264] |
| PP | SWCNT | 1.00 | Solution mixing-fiber spinning | 1.5 | 2.0 | [265] |
| PP | SWCNT | 0.75 | Shear Mixing | 1.15 | 1.4 | [266] |
| PP | MWCNT | 0.25 | Melt fiber spinning | 1.1 | 2.3 | [267] |
| PP | MWCNT | 1.00 | Melt fiber spinning | 5.0 | 3.7 | [268] |
| PMMA | Oxidized MWCNT | 5.00 | In situ bulk polymerization | 1.30 | – | [269] |
| PMMA | Oxidized MWCNT | 1.50 | In situ bulk polymerization | 1.75 | – | [270] |
| PMMA | MWCNT | 1.00 | Melt Extrusion | 1.0 | 1.0 | [271] |
| PMMA | SWCNT | 2.00 | Solution casting | 1.9 | – | [51] |
| PMMA | PMMA-g-MWCNT | 0.15 | Solution casting | 3.6 | 1.9 | [272] |
| PVA | Hydroxy-modified SWCNT | 0.80 | Solution casting | 1.47 | 1.79 | [49] |
| PVA | Oxidized MWCNT | 0.20 | Solution casting | 1.52 | 1.46 | [273] |
| PVA | Oxidized SWCNT | 0.20 | Solution casting | 1.42 | 1.29 | [273] |
| SBR | MWCNT | 10.00 | Solution casting | 4.0 | 5.0 | [274] |
| Nylon 6 | MWCNT | 1.00 | Melt Blending | 1.2 | 1.1 | [275] |
| Nylon 6 | MWCNT | 2.00 | Melt Blending | 1.62 | 2.14 | [276] |
| Nylon 6 | Oxidized SWCNT | 0.5 | In situ polymerization | 2.5 | 3.5 | [277] |
| PI | Oxidized MWCNT | 0.38 | Solution casting | 1.6 | 1.5 | [278] |
| PI | Oxidized MWCNT | 1.00 | Solution casting | 1.23 | 1.40 | [279] |
| PI | SWCNT | 1.00 | Melt Extrusion | 1.0 | 1.5 | [280] |
| PU | Oxidized MWCNT | 1.50 | In situ condensation | 1.3 | 2.4 | [169] |
| PU | Oxidized MWCNT | 2.00 | In situ condensation | 1.15 | 1.4 | [281] |
| PU | Oxidized MWCNT | 4.00 | In situ condensation | 1.4 | 2.3 | [282] |
| Epoxy | SWCNT | 5.00 | Solution casting | 1.07 | 1.0 | [283] |
| Epoxy | MWCNT | 0.5 | Solution casting | 1.62 | 1.54 | [154] |
| Polymer Matrix | Graphene Type | wt(%) | Processing Method | σ/σ0 | E/E0 | Reference |
|---|---|---|---|---|---|---|
| Epoxy | Expanded | 1 | Sonication | 0.8 | 1.08 | [254] |
| Expanded | 1 | Shear | 0.93 | 1.11 | ||
| Expanded | 1 | Sonication and Shear | 0.94 | 1.15 | ||
| PMMA | Expanded | 21 | Solution casting | – | 1.21 | [284] |
| GNP | 5 | Solution casting | – | 2.33 | [285] | |
| GO | – | In situ Polymerization | – | 1.54 | [286] | |
| TRG | 1.2 | Solution mixing | 1.80 | – | [285] | |
| HDPE | Expanded | 3 | Melt processing | 1.04 | 2.0 | [287] |
| PVA | GO | 0.7 | Solution casting | 1.76 | – | [288] |
| Graphene | 4.5 | Solution casting | 2.5 | – | [289] | |
| GO | 6.3 | Solution casting | 1.7 | 2.23 | [290] | |
| TPU | Graphene | 13.3 | Solution casting | – | 3.0 | [291] |
| TRG | 4.0 | Melt | – | 3.5 | [123] | |
| 4.0 | Solution casting | – | 7.8 | |||
| 3.78 | In situ polymerization | – | 3.1 | |||
| PC | TGO | 2.5 | Melt Processing | – | 1.21 | [121] |
| PC | Graphite | 15 | Melt Processing | – | 2.48 |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattacharya, M. Polymer Nanocomposites—A Comparison between Carbon Nanotubes, Graphene, and Clay as Nanofillers. Materials 2016, 9, 262. https://doi.org/10.3390/ma9040262
Bhattacharya M. Polymer Nanocomposites—A Comparison between Carbon Nanotubes, Graphene, and Clay as Nanofillers. Materials. 2016; 9(4):262. https://doi.org/10.3390/ma9040262
Chicago/Turabian StyleBhattacharya, Mrinal. 2016. "Polymer Nanocomposites—A Comparison between Carbon Nanotubes, Graphene, and Clay as Nanofillers" Materials 9, no. 4: 262. https://doi.org/10.3390/ma9040262
APA StyleBhattacharya, M. (2016). Polymer Nanocomposites—A Comparison between Carbon Nanotubes, Graphene, and Clay as Nanofillers. Materials, 9(4), 262. https://doi.org/10.3390/ma9040262





