Preparation of Calcined Zirconia-Carbon Composite from Metal Organic Frameworks and Its Application to Adsorption of Crystal Violet and Salicylic Acid
Abstract
:1. Introduction
2. Results and Discussions
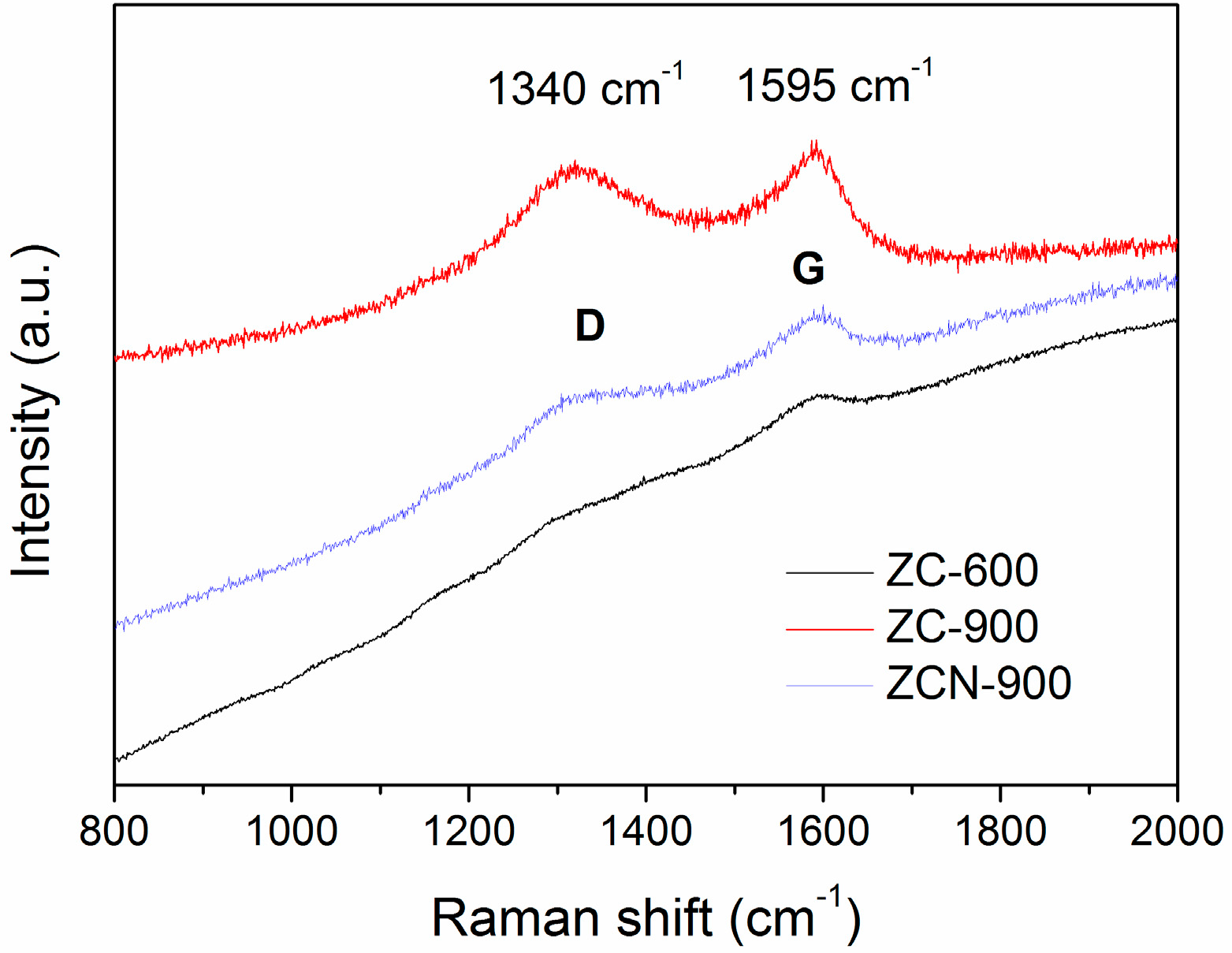
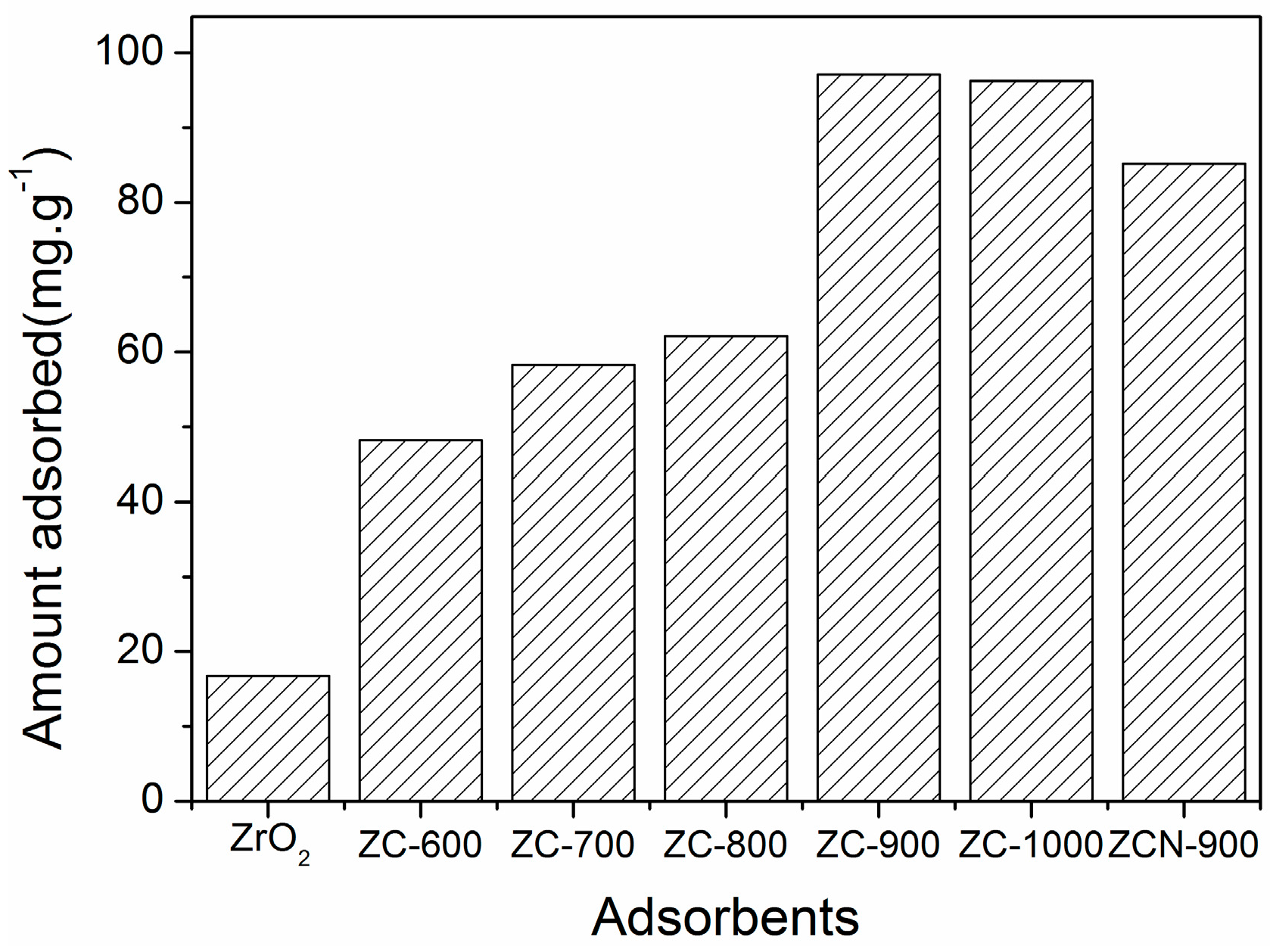
2.1. Characterization of the MOFs and ZC Composites
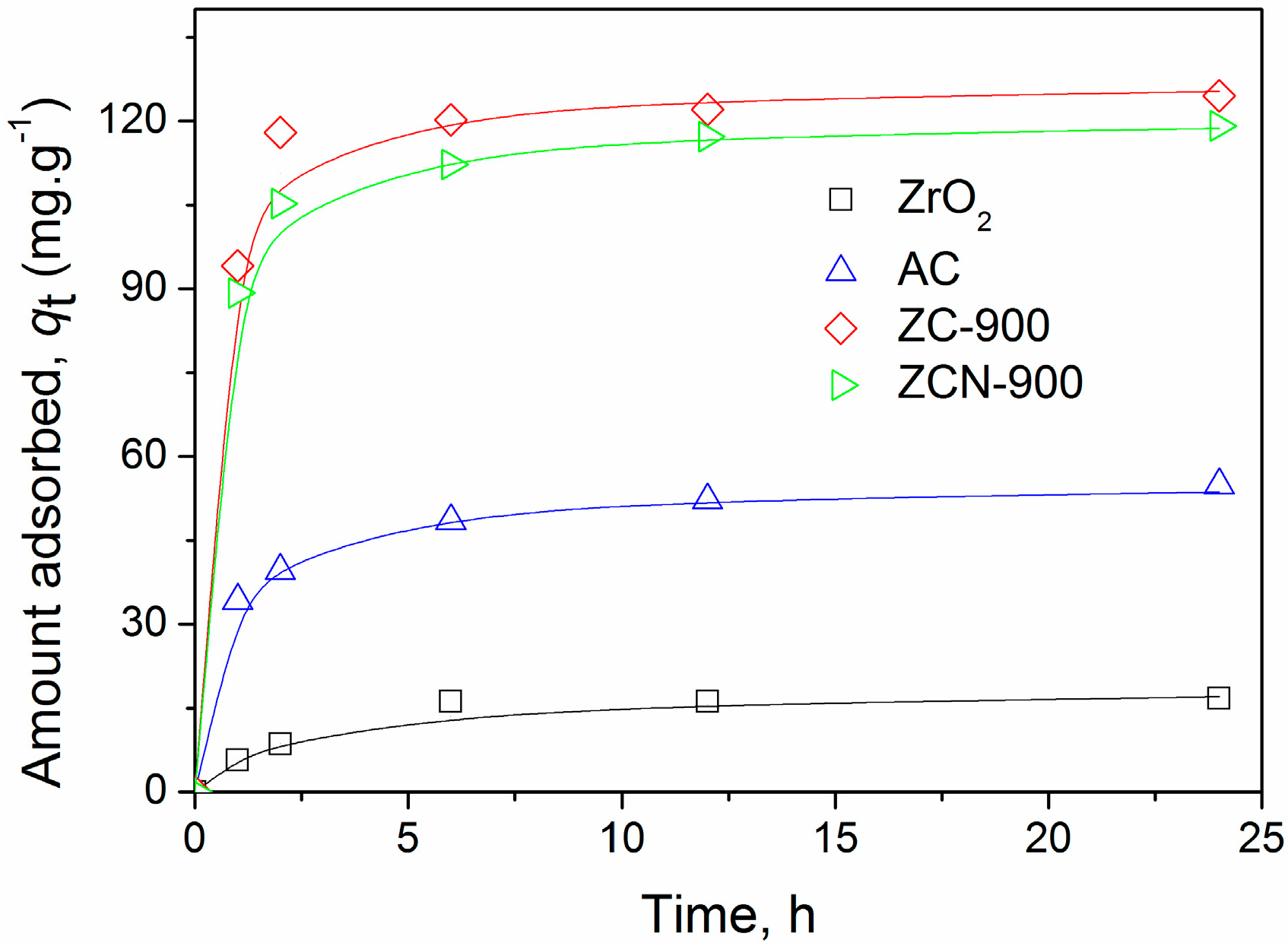
2.2. CV Adsorption on the Composites
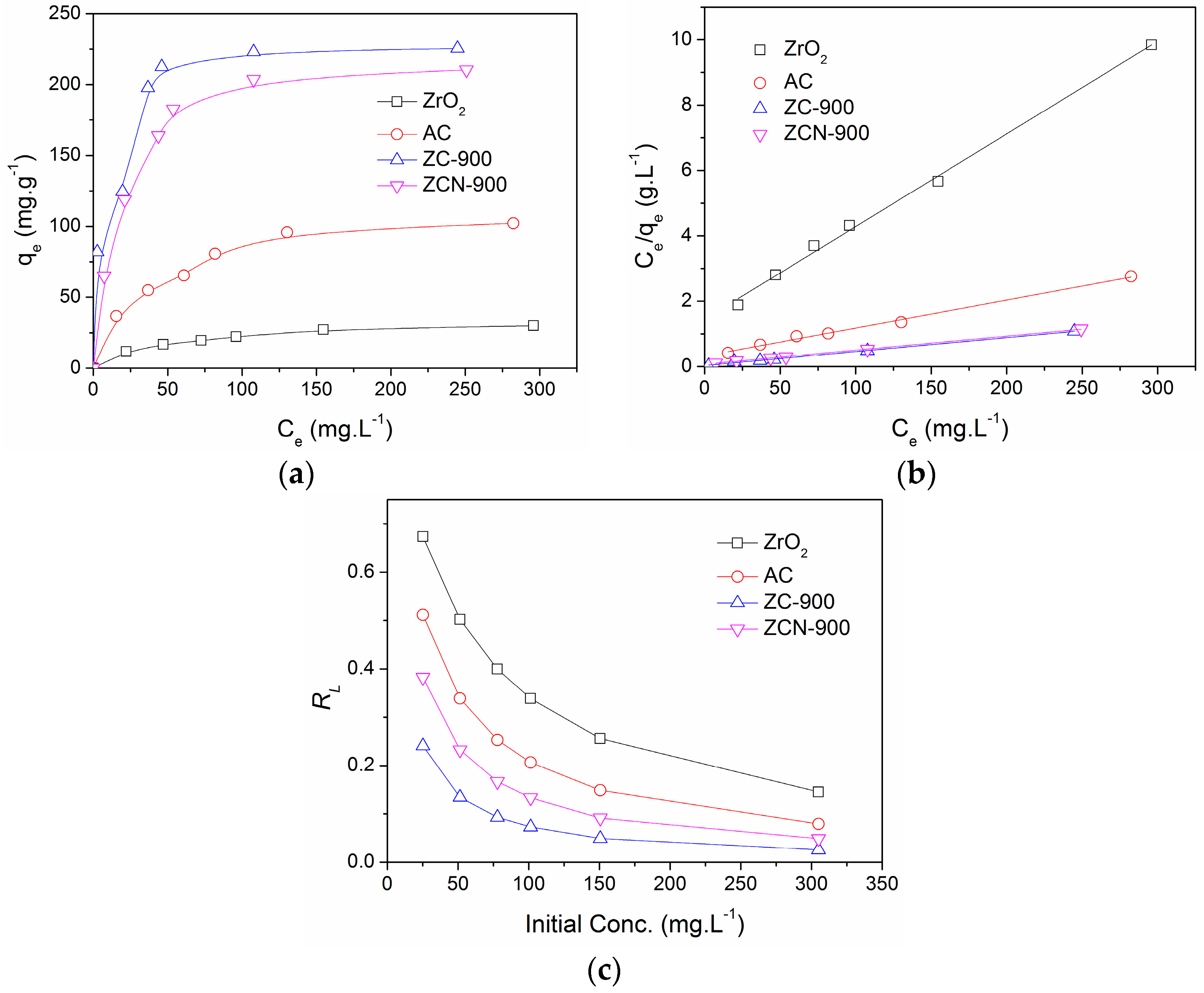
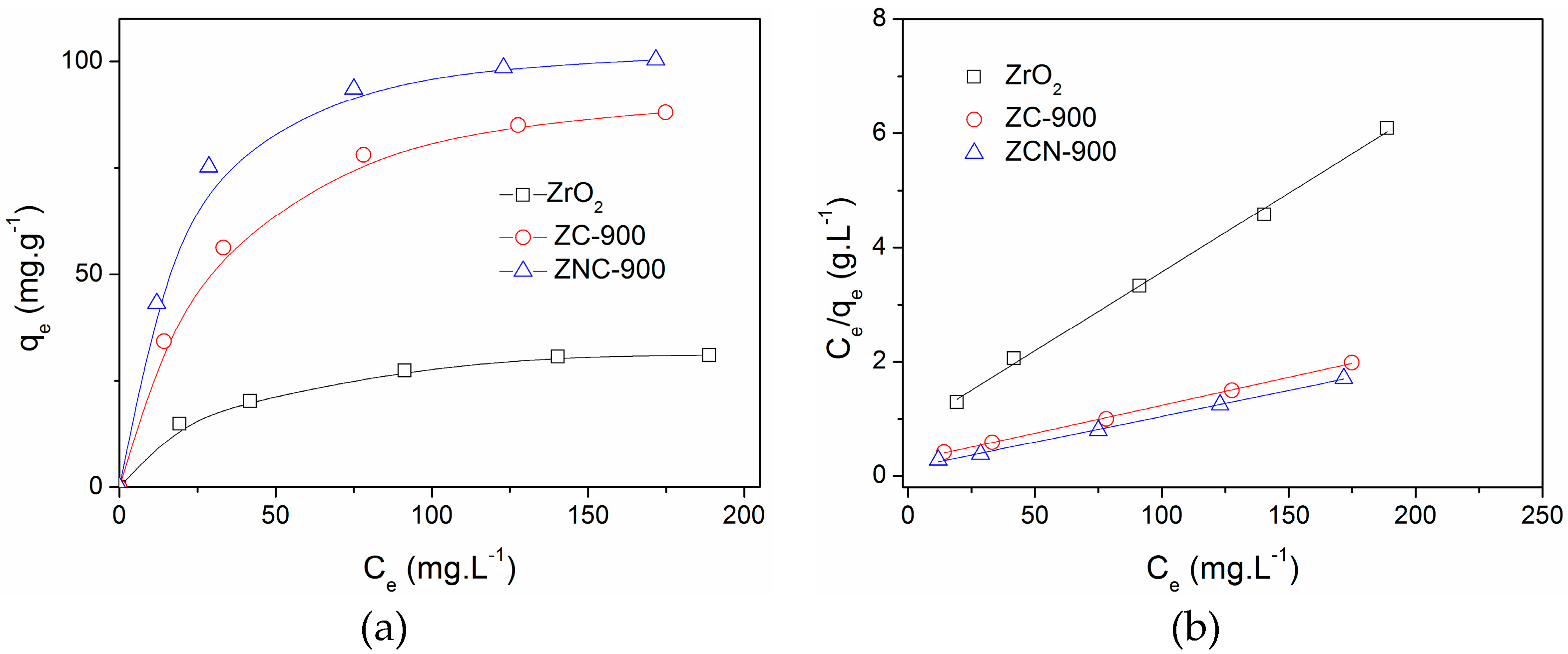
2.3. Adsorption Isotherms
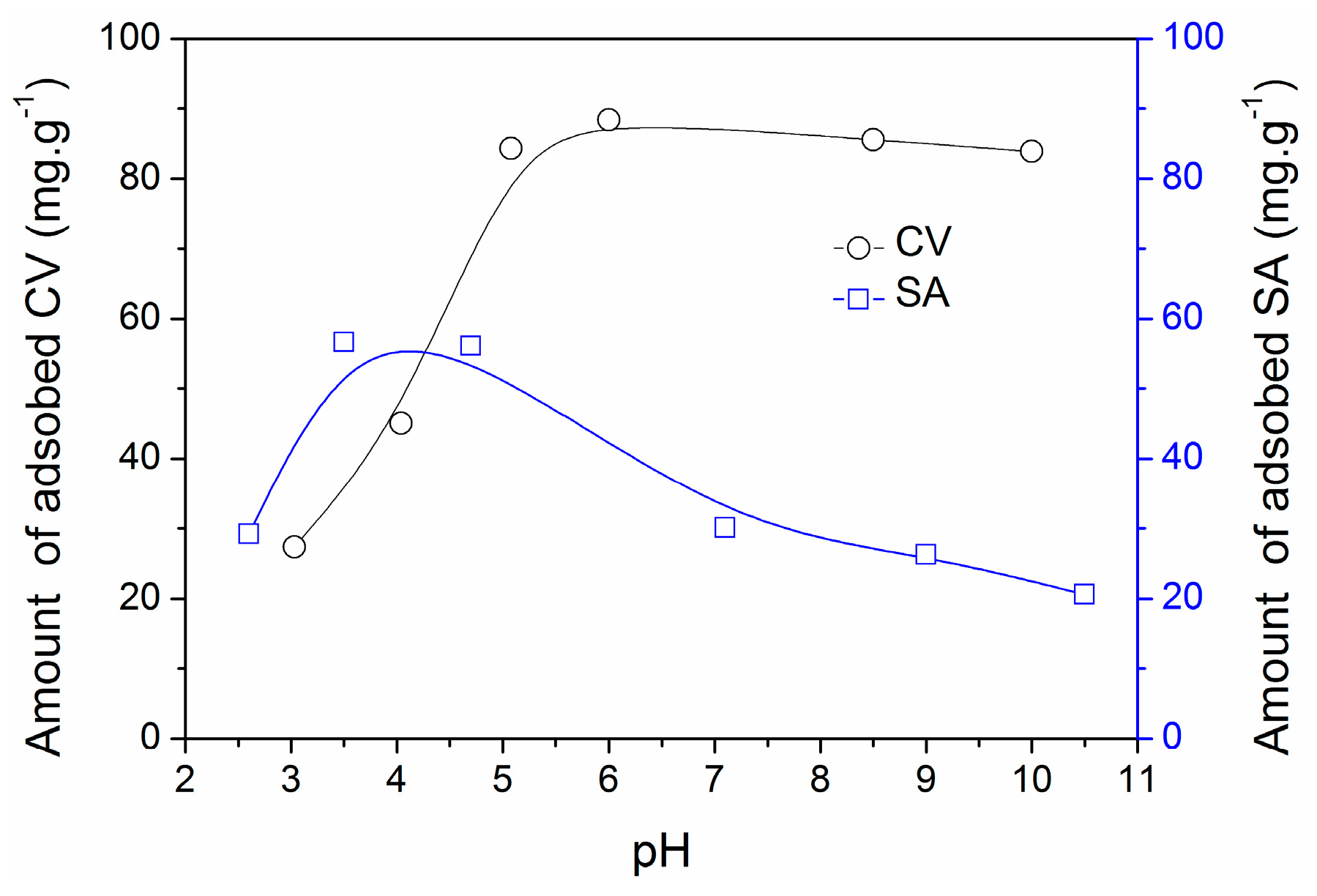
2.4. Adsorption Mechanisms
3. Materials and Methods
3.1. Materials
3.2. Synthesis of UiO-66 and NH2-UiO-66
3.3. Preparation of ZC Composites
3.4. Characterization of Adsorbents
3.5. Zero Point Charge pH Measurement
3.6. Adsorption Experiments
3.7. Calculation of Adsorption Kinetics
3.8. Calculation of Maximum Adsorption Capacity (Q0) and Separation Factor (RL)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shen, Y. Carbothermal synthesis of metal-functionalized nanostructures for energy and environmental applications. J. Mater. Chem. A 2015, 3, 13114–13188. [Google Scholar] [CrossRef]
- Yao, W.; Shen, C.; Lu, Y. Fe3O4@C@polyaniline trilaminar core-shell composite microspheres as separable adsorbent for organic dye. Compos. Sci. Technol. 2013, 87, 8–13. [Google Scholar] [CrossRef]
- Gusain, D.; Bux, F.; Sharma, Y.C. Abatement of chromium by adsorption on nanocrystalline zirconia using response surface methodology. J. Mol. Liq. 2014, 197, 131–141. [Google Scholar] [CrossRef]
- Deshpande, P.A.; Polisetti, S.; Madras, G. Rapid synthesis of ultrahigh adsorption capacity zirconia by a solution combustion technique. Langmuir 2011, 27, 3578–3587. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, C.; Huang, Z.; Wu, G.; Gong, J. A Ni@ZrO2 nanocomposite for ethanol steam reforming: Enhanced stability via strong metal-oxide interaction. Chem. Commun. 2013, 49, 4226–4228. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, L.; Lin, H.; Nong, Q.; Wu, Y.; Wu, T.; He, Y. Synthesis and characterization of a ZrO2/g-C3N4 composite with enhanced visible-light photoactivity for rhodamine degradation. RSC Adv. 2014, 4, 40029–40035. [Google Scholar] [CrossRef]
- Deshpande, P.A.; Polisetti, S.; Madras, G.; Jyothi, D.; Chandrasekaran, S. Dispersed ZrO2 nanoparticles in MCM-48 with high adsorption activity. AIChE 2012, 58, 2987–2996. [Google Scholar] [CrossRef]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Jhung, S.H.; Khan, N.A.; Hasan, Z. Analogous porous metal-organic frameworks: Synthesis, stability and application in adsorption. CrystEngComm 2012, 14, 7099–7109. [Google Scholar] [CrossRef]
- Chen, J.-J.; Chen, Y.-T.; Raja, D.S.; Kang, Y.-H.; Tseng, P.-C.; Lin, C.-H. Metal-organic frameworks to metal/metal oxide embedded carbon matrix: Synthesis, characterization and gas sorption properties. Materials 2015, 8, 5336–5347. [Google Scholar] [CrossRef]
- Hasan, Z.; Jhung, S.H. Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.-Y. Applications of metal-organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [PubMed]
- Chaikittisilp, W.; Ariga, K.; Yamauchi, Y. A new family of carbon materials: Synthesis of MOF derived nanoporous carbons and their promising applications. J. Mater. Chem. A 2013, 1, 14–19. [Google Scholar] [CrossRef]
- Suna, J.-K.; Xu, Q. Functional materials derived from open framework templates/precursors: Synthesis and applications. Energy Environ. Sci. 2014, 7, 2071–2100. [Google Scholar] [CrossRef]
- Yan, X.; Lu, N.; Fan, B.; Bao, J.; Pan, D.; Wang, M.; Li, R. Synthesis of mesoporous and tetragonal zirconia with inherited morphology from metal-organic frameworks. CrystEngComm 2015, 17, 6426–6433. [Google Scholar] [CrossRef]
- Yue, H.; Shi, Z.; Wang, Q.; Cao, Z.; Dong, H.; Qiao, Y.; Yin, Y.; Yang, S. MOF-derived cobalt-doped ZnO@C composites as a high performance anode material for lithium-ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 17067–17074. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.-W.; Jeon, B.-H.; Chon, C.-M.; Schwartz, F.W.; Jeong, Y.; Song, H. Magnetic chitosan composite for adsorption of cationic and anionic dyes in aqueous solution. J. Ind. Eng. Chem. 2015, 28, 60–66. [Google Scholar] [CrossRef]
- Kamal, T.; Ul-Islam, M.; Khan, S.B.; Asiria, A.M. Adsorption and photocatalyst assisted dye removal and bactericidal performance of ZnO/chitosan coating layer. Int. J Biol. Macromol. 2015, 81, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R. Studies on adsorption of crystal violet dye from aqueous solution onto coniferous pinus bark powder (CPBP). J. Hazard. Mater. 2009, 171, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Kyzasa, G.Z.; Fu, J.; Lazaridisa, N.K.; Bikiaris, D.N.; Matis, K.A. New approaches on the removal of pharmaceuticals from wastewaters with adsorbent materials. J. Mol. Liq. 2015, 209, 87–93. [Google Scholar] [CrossRef]
- Carmona, E.; Andreu, V.; Picó, Y. Occurrence of acidic pharmaceuticals and personal care products in Turia river basin: From waste to drinking water. Sci. Total Environ. 2014, 484, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Ghoreishi, S.M.; Kashani, F.Z.; Khoobi, A.; Enhessari, M. Fabrication of a nickel titanate nanoceramic modified electrode for electrochemical studies and detection of salicylic acid. J. Mol. Liq. 2015, 211, 970–980. [Google Scholar] [CrossRef]
- Fu, Z.; Li, H.; Yang, L.; Yuan, H.; Jiao, Z.; Chen, L.; Huang, J.; Liu, Y.-N. Magnetic polar post-cross-linked resin and its adsorption towards salicylic acid from aqueous solution. Chem. Eng. J. 2015, 273, 240–246. [Google Scholar] [CrossRef]
- Vilaseca, M.; López-Grimau, V.; Gutiérrez-Bouzán, C. Valorization of waste obtained from oil extraction in moringa oleifera seeds: Coagulation of reactive dyes in textile effluents. Materials 2014, 7, 6569–6584. [Google Scholar] [CrossRef]
- Niu, H.; Wang, Q.; Liang, H.; Chen, M.; Mao, C.; Song, J.; Zhang, S.; Gao, Y.; Chen, C. Visible-light active and magnetically recyclable nanocomposites for the degradation of organic dye. Materials 2014, 7, 4034–4044. [Google Scholar] [CrossRef]
- Esplugas, S.; Bila, D.M.; Krause, L.G.T.; Dezotti, M. Ozonation and advanced oxidation technologies to remove endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents. J. Hazard. Mater. 2007, 149, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Hasan, Z.; Khan, N.A.; Jhung, S.H. Adsorptive removal of diclofenac sodium from water with Zr based metal-organic frameworks. Chem. Eng. J. 2016, 284, 1406–1413. [Google Scholar] [CrossRef]
- Liu, S.-C.; Yue, Z.F.; Liu, Y. Mesoporous carbon-ZrO2 composites prepared using thermolysis of zirconium based metal-organic frameworks and their adsorption properties. J. Porous Mater. 2015, 22, 465–471. [Google Scholar] [CrossRef]
- Pendolino, F.; Capurso, G.; Maddalena, A.; Russo, S.L. The structural change of graphene oxide in a methanol dispersion. RSC Adv. 2014, 4, 32914–32917. [Google Scholar] [CrossRef]
- Liang, B.; Fang, L.; Hu, Y.; Yang, G.; Zhu, Q.; Ye, X. Fabrication and application of flexible graphene silk composite film electrodes decorated with spiky Pt, Nanospheres. Nanoscale 2014, 6, 4264–4274. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Sun, J.; Jiang, S.; Zeng, Y.; Li, Y.; Yan, S.; Geng, J.; Huang, Y. Grafting P3HT brushes on GO sheets: Distinctive properties of the GO/P3HT composites due to different grafting approaches. J. Mater. Chem. 2012, 22, 21583–21591. [Google Scholar] [CrossRef]
- Yang, G.; Han, H.; Li, T.; Du, C. Synthesis of nitrogen-doped porous graphitic carbons using nano-CaCO3 as template, graphitization catalyst, and activating agent. Carbon 2012, 50, 3753–3765. [Google Scholar] [CrossRef]
- Bradder, P.; Ling, S.K.; Wang, S.; Liu, S. Dye adsorption on layered graphite oxide. J. Chem. Eng. Data 2011, 56, 138–141. [Google Scholar] [CrossRef]
- Ho, Y.-S.; Chiang, T.-H.; Hsueh, Y.-M. Removal of basic dye from aqueous solution using tree fern as a biosorbent. Process Biochem. 2005, 40, 119–124. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, M.; Wang, X.; Huang, Q.; Min, Y.; Ma, T.; Niu, J. Removal of crystal violet by a novel cellulose-based adsorbent: Comparison with native cellulose. Ind. Eng. Chem. Res. 2014, 53, 5498–5506. [Google Scholar] [CrossRef]
- Singh, K.P.; Gupta, S.; Singh, A.K.; Sinha, S. Optimizing adsorption of crystal violet dye from water by magnetic nanocomposite using response surface modeling approach. J. Hazard. Mater. 2011, 186, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Qiu, F.; Rong, X.; Yan, J.; Zhao, H.; Yang, D. Adsorption behavior of crystal violet from aqueous solutions with chitosan-graphite oxide modified polyurethane as an adsorbent. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Lin, Y.; He, X.; Han, G.; Tian, Q.; Hu, W. Removal of crystal violet from aqueous solution using powdered mycelial biomass of Ceriporia lacerate. J. Env. Sci. 2011, 23, 2055–2062. [Google Scholar] [CrossRef]
- El-Sayed, G.O. Removal of methylene blue and crystal violet from aqueous solutions by palm kernel fiber. Desalination 2011, 272, 225–232. [Google Scholar] [CrossRef]
- Yang, K.; Xing, B. Adsorption of organic compounds by carbon nanomaterials in aqueous phase: Polanyi theory and its application. Chem. Rev. 2010, 110, 5989–6008. [Google Scholar] [CrossRef] [PubMed]
- Faria, P.C.C.; Órfão, J.J.M.; Pereira, M.F.R. Adsorption of anionic and cationic dyes on activated carbons with different surface chemistries. Water Res. 2004, 38, 2043–2052. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Utrilla, J.; Bautista-Toledo, I.; Ferro-García, M.A.; Moreno-Castilla, C. Activated carbon surface modifications by adsorption of bacteria and their effect on aqueous lead adsorption. J. Chem. Technol. Biotechnol. 2001, 76, 1209–1215. [Google Scholar] [CrossRef]
- Al-Degs, Y.S.; El-Barghouthi, M.I.; El-Sheikh, A.H.; Walker, G.M. Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigment. 2008, 77, 16–23. [Google Scholar] [CrossRef]
- Ho, Y.-S. Second-order kinetic model for the sorption of cadmium onto tree fern: A comparison of linear and non-linear methods. Water Res. 2006, 40, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Nouri, L.; Ghodbane, I.; Hamdaoui, O.; Chiha, M. Batch sorption dynamics and equilibrium for the removal of cadmium ions from aqueous phase using wheat bran. J. Hazard. Mater. 2007, 149, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.H.; Rahman, A.A. Removal of phenol from aqueous solutions by adsorption onto activated carbon prepared from biomass material. J. Hazard. Mater. 2008, 160, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-H.; Juang, R.-S. Adsorption of phenol and its derivatives from water using synthetic resins and low-cost natural adsorbents: A review. J. Environ. Manag. 2009, 90, 1336–1349. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.W.; Chakkravorti, R.K. Pore and solid diffusion models for fixed-bed adsorbers. AIChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Önal, Y.; Akmil-Basar, C.; Sarıcı-Özdemir, C. Elucidation of the naproxen sodium adsorption onto activated carbon prepared from waste apricot: Kinetic, equilibrium and thermodynamic characterization. J. Hazard. Mater. 2007, 148, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.P.; Mohan, D.; Sinha, S.; Tondon, G.S. Color removal from wastewater using low-cost activated carbon derived from agricultural waste material. Ind. Eng. Chem. Res. 2003, 42, 1965–1976. [Google Scholar] [CrossRef]



| Adsorbent | BET Surface Area (m2·g−1) | Total Pore Volume (cm3·g−1) | Mean Pore Size (nm) | Pseudo-Second-Order Kinetics Parameters a (g·mg−1·h−1) | Q0 b (mg·g−1) | b c (L·mg−1) | r2 (Langmuir Plot) | |
|---|---|---|---|---|---|---|---|---|
| k2 | r2 | |||||||
| AC | 870 | 0.44 | 3.6 | 0.016 | 0.999 | 116 | 0.019 | 0.995 |
| ZrO2 | 30 | 0.44 | - | 0.022 | 0.989 | 35 | 0.039 | 0.996 |
| ZC-600 | 56 | 0.11 | 1.5 | - | - | - | - | - |
| ZC-700 | 168 | 0.15 | 1.7 | - | - | - | - | - |
| ZC-800 | 184 | 0.16 | 1.9 | - | - | - | - | - |
| ZC-900 | 370 | 0.26 | 2.9 | 0.027 | 0.998 | 243 | 0.119 | 0.996 |
| ZC-1000 | 345 | 0.25 | 2.2 | - | - | - | - | - |
| ZCN-900 | 276 | 0.17 | 2.3 | 0.025 | 0.999 | 222 | 0.064 | 0.998 |
| Entry | Adsorbents | Maximum Adsorption Capacity (mg·g−1) | Reference |
|---|---|---|---|
| 1 | Cellulose-based adsorbent | 182 | [37] |
| 2 | Magnetic nanocomposite | 113 | [38] |
| 3 | Chitosan-graphite oxide modified polyurethane foam | 58 | [39] |
| 4 | Mycelial biomass | 239 | [40] |
| 5 | Palm kernel fier | 79 | [41] |
| 6 | Activated carbon (commercial) | 116 | This study |
| 7 | ZC-900 | 243 | This study |
| Adsorbents | Q0 a (mg·g−1) | b b (L·mg−1) | r2 (Langmuir Plot) |
|---|---|---|---|
| ZrO2 | 36 | 0.034 | 0.998 |
| ZC-900 | 102 | 0.371 | 0.996 |
| ZCN-900 | 109 | 0.047 | 0.998 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, Z.; Cho, D.-W.; Nam, I.-H.; Chon, C.-M.; Song, H. Preparation of Calcined Zirconia-Carbon Composite from Metal Organic Frameworks and Its Application to Adsorption of Crystal Violet and Salicylic Acid. Materials 2016, 9, 261. https://doi.org/10.3390/ma9040261
Hasan Z, Cho D-W, Nam I-H, Chon C-M, Song H. Preparation of Calcined Zirconia-Carbon Composite from Metal Organic Frameworks and Its Application to Adsorption of Crystal Violet and Salicylic Acid. Materials. 2016; 9(4):261. https://doi.org/10.3390/ma9040261
Chicago/Turabian StyleHasan, Zubair, Dong-Wan Cho, In-Hyun Nam, Chul-Min Chon, and Hocheol Song. 2016. "Preparation of Calcined Zirconia-Carbon Composite from Metal Organic Frameworks and Its Application to Adsorption of Crystal Violet and Salicylic Acid" Materials 9, no. 4: 261. https://doi.org/10.3390/ma9040261






