Synthesis of Shape-Tailored WO3 Micro-/Nanocrystals and the Photocatalytic Activity of WO3/TiO2 Composites
Abstract
:1. Introduction
2. Results
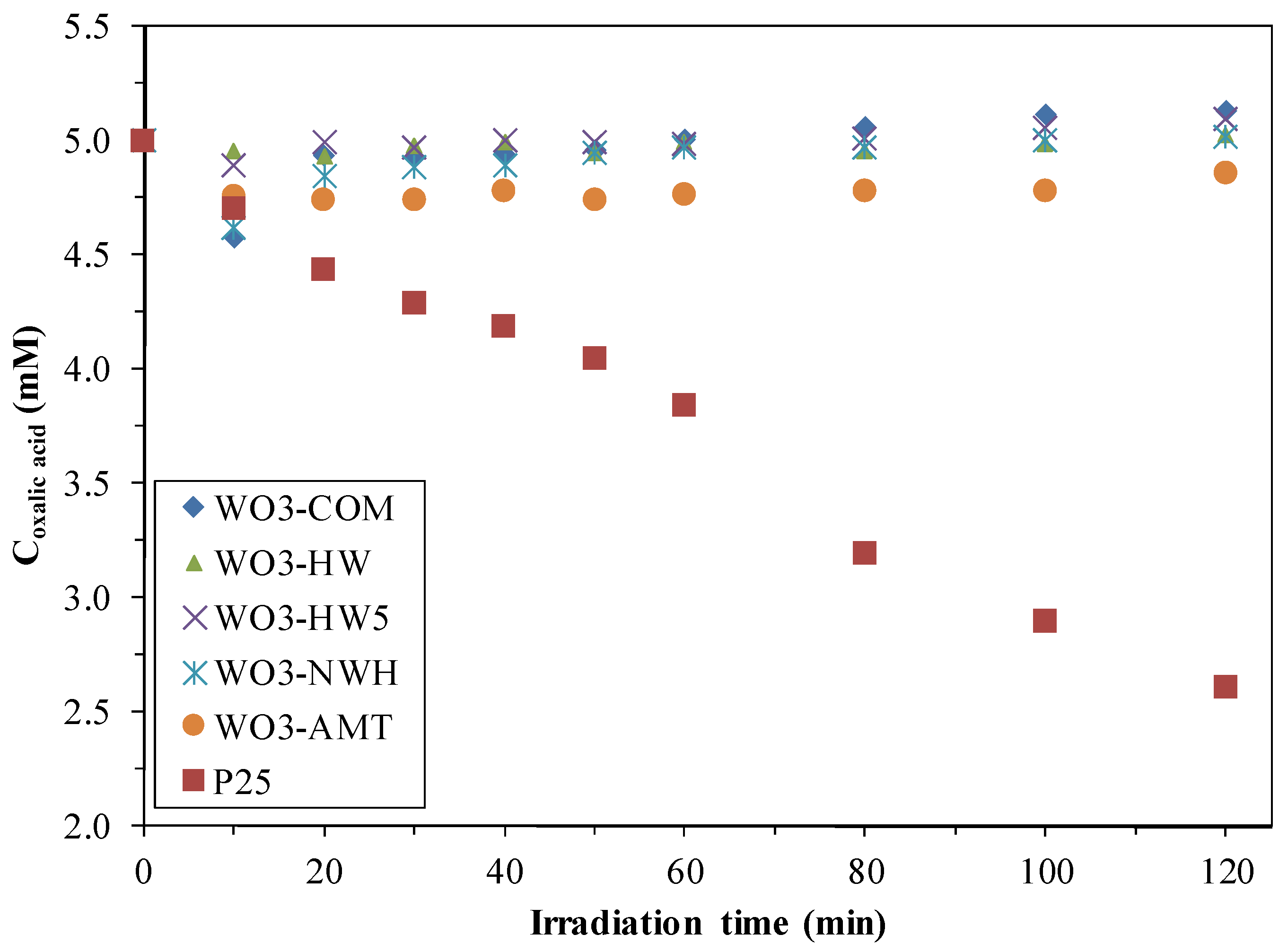
2.1. Photocatalytic Activity
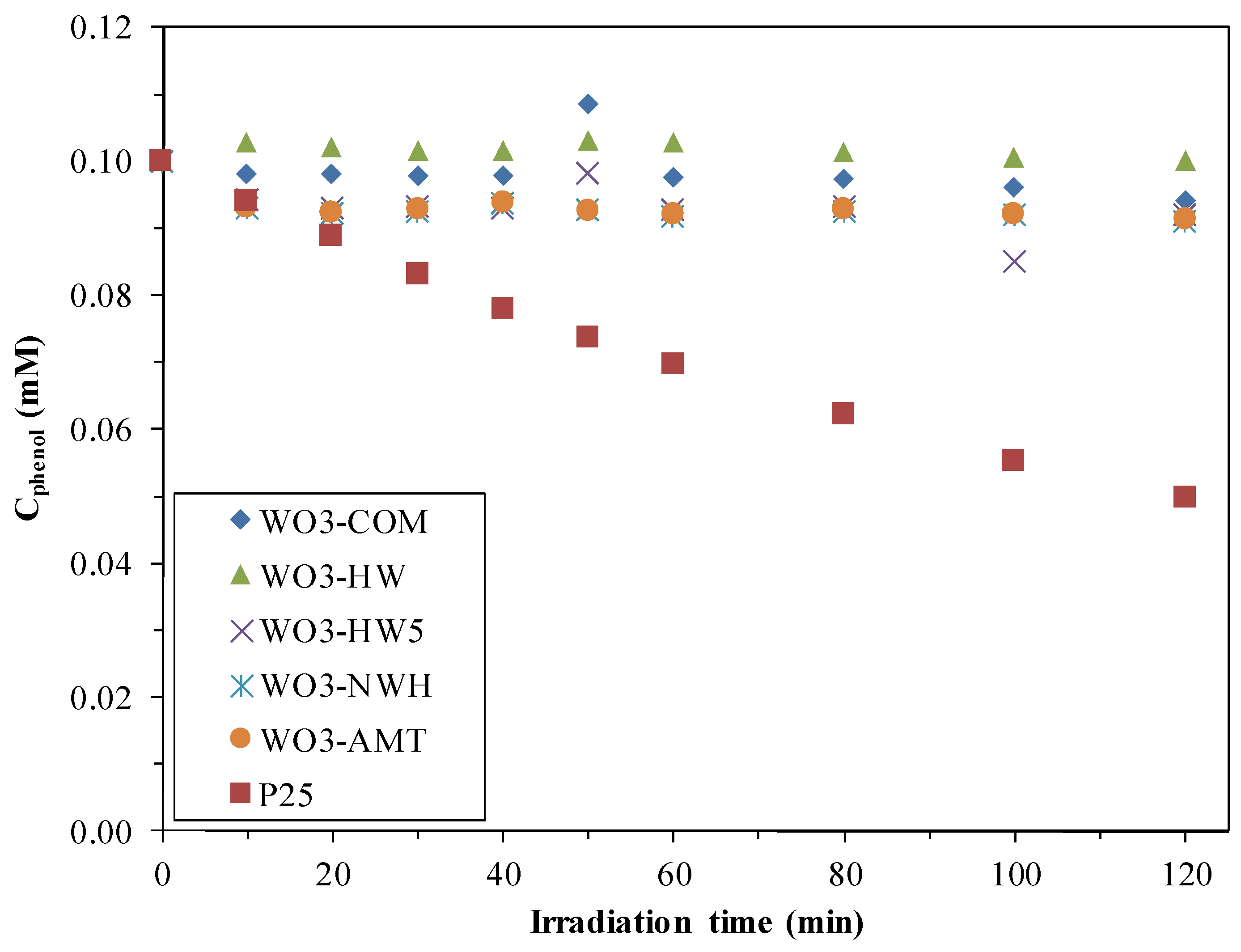
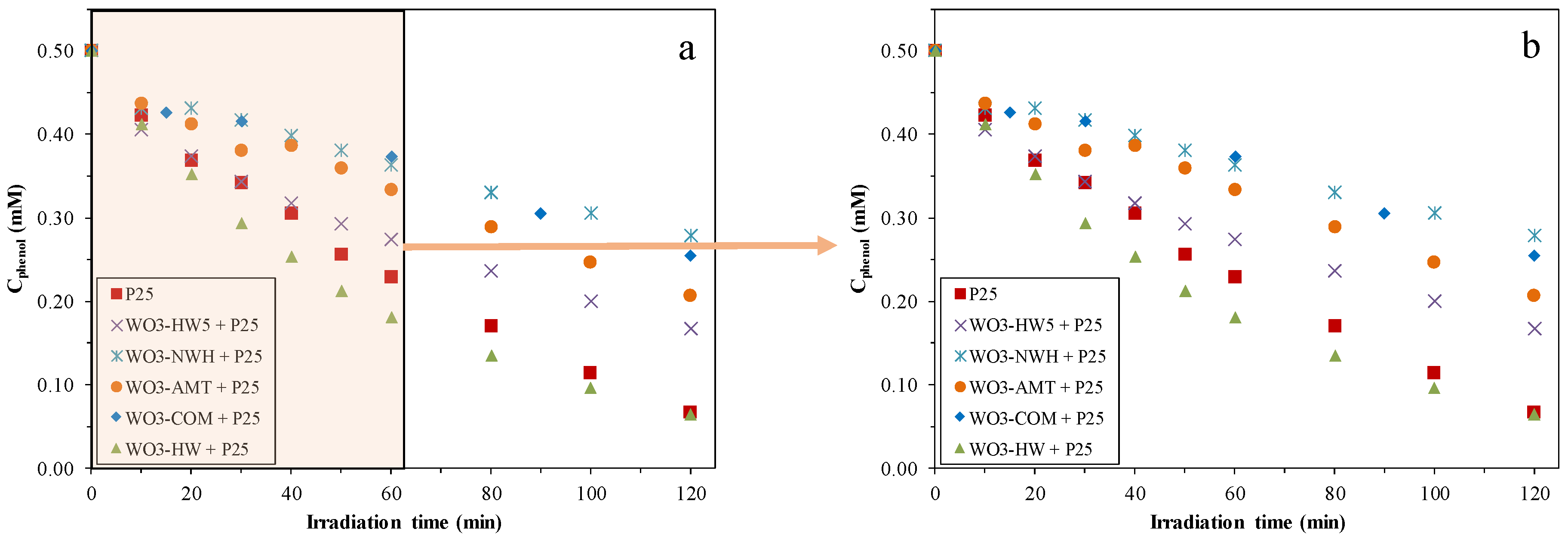
2.2. Phenol Conversion Rates
2.3. Reaction Rates of the Phenol Degradation
2.4. Reaction of the Methyl-Orange Degradation
3. Discussions of the Photocatalytic Activity Results in the Frame of the Structural and Morphological Features
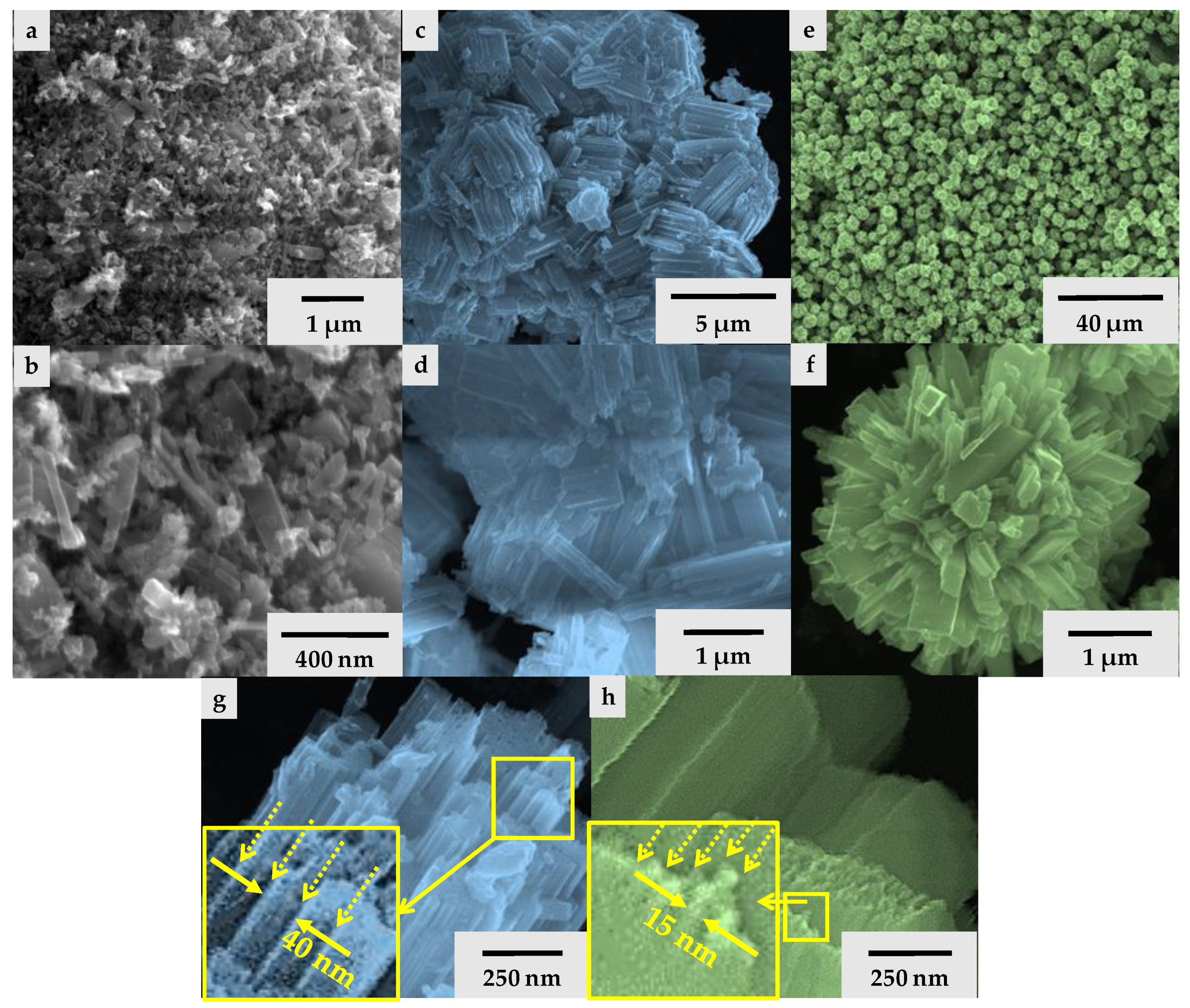
3.1. Morphological Aspects of the Obtained WO3 Microcrystals
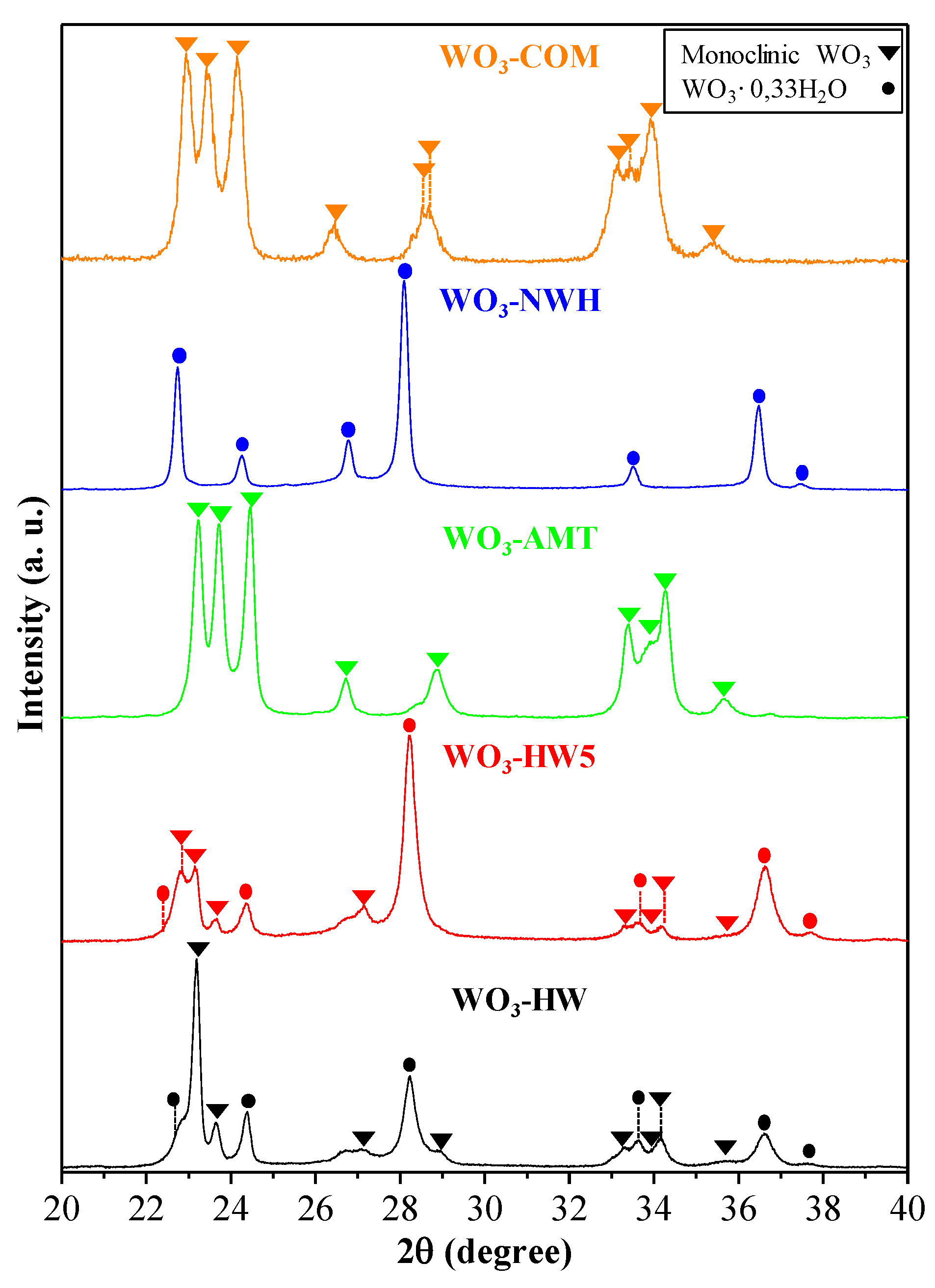
3.2. Crystalline Structure of the Shape-Tailored WO3
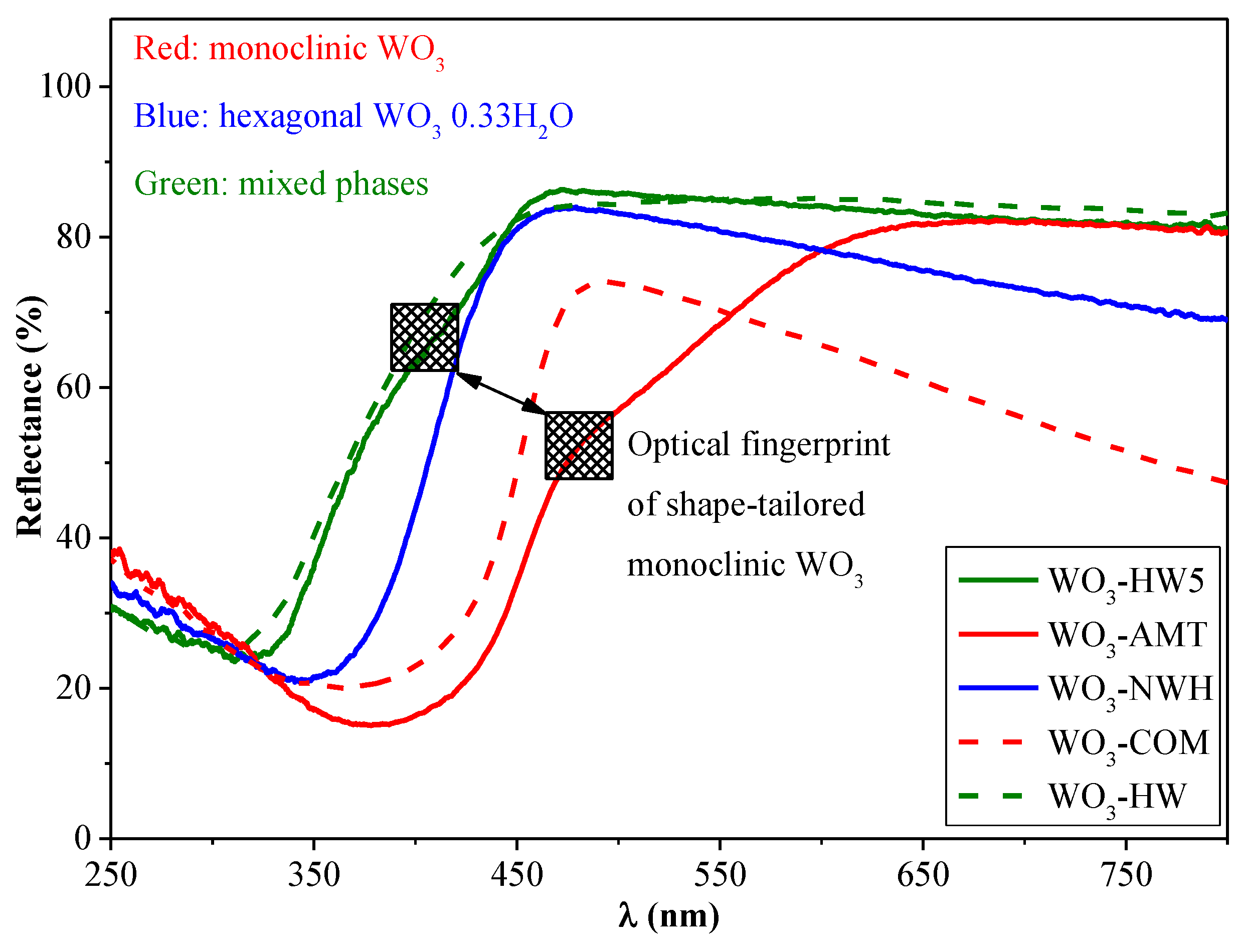
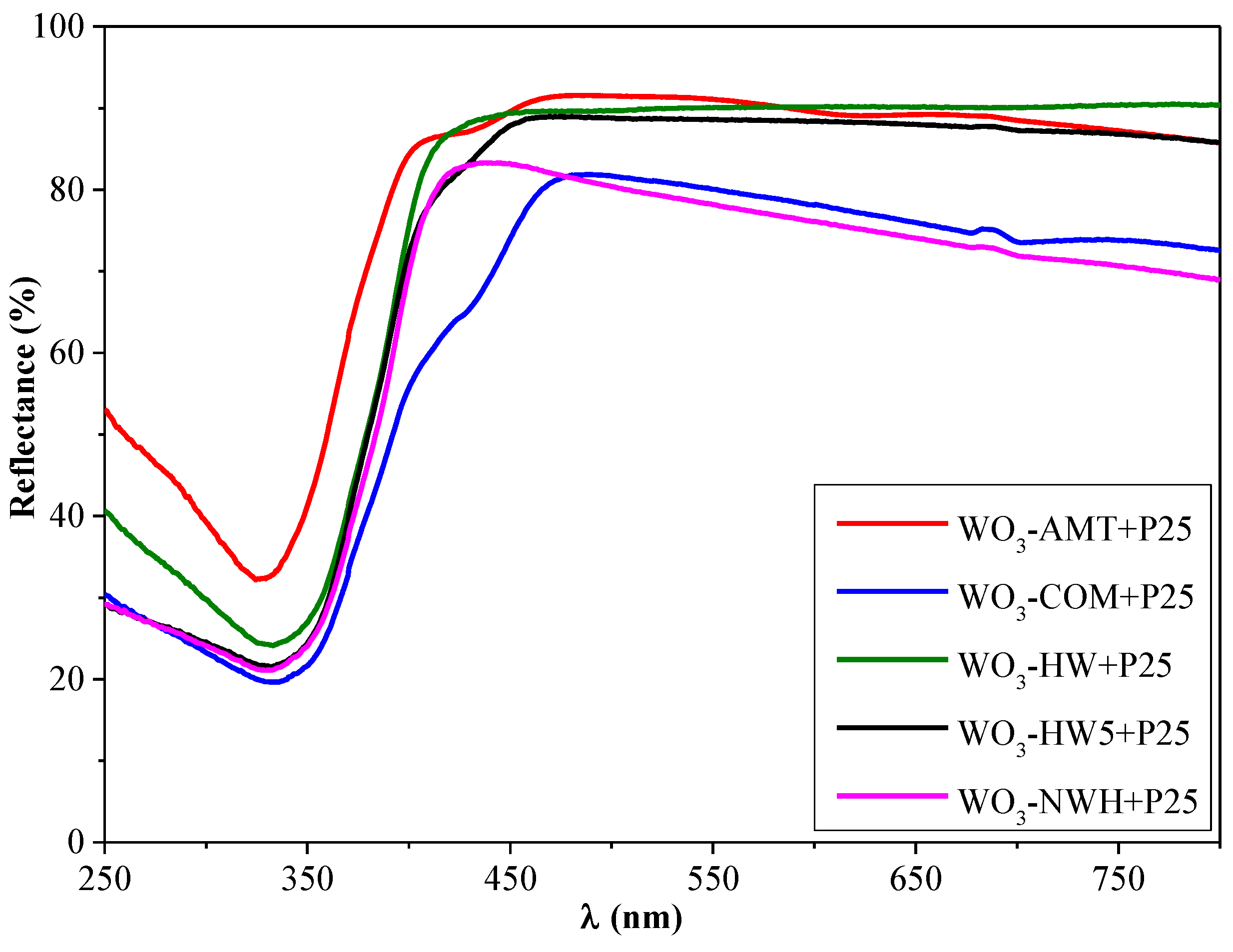
3.3. Optical Properties of the Individual WO3 and Composites
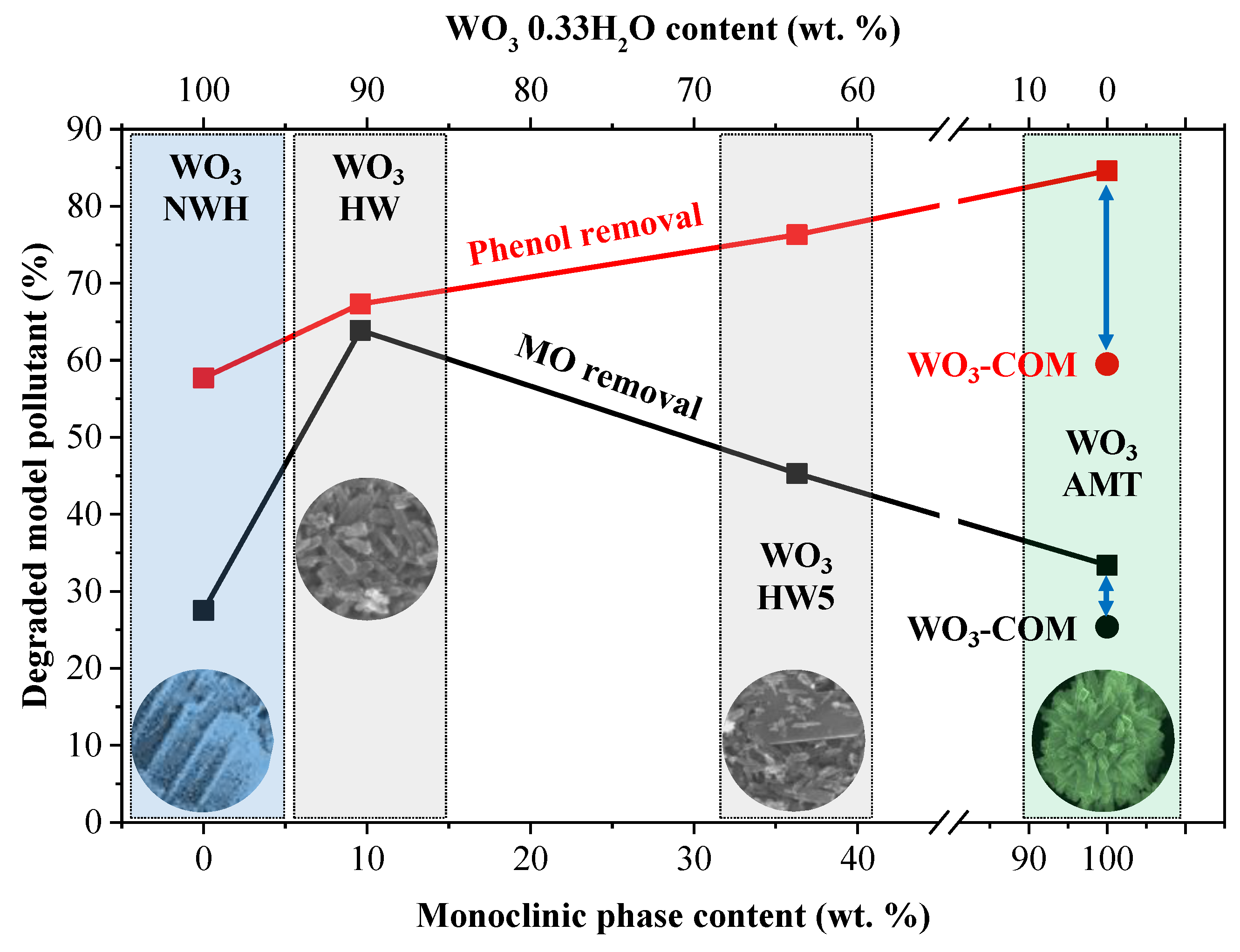
3.4. The Structure-Morphology-Photocatalytic Activity Relationship
4. Materials and Methods
4.1. Chemicals
4.2. Synthesis of the WO3 Semiconductors
4.2.1. Synthesis of WO3 Nanoplates-Intermediate Peroxo-Complex Approach
4.2.2. Synthesis of WO3-High Ionic Strength Approach
4.2.3. Synthesis of WO3 Nanostars-Low Mobility Anion Approach
4.3. Synthesis of the WO3/TiO2 Nanocomposites
4.4. Methods and Instrumentation
Characterization Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Biswas, S.K.; Baeg, J.O. A facile one-step synthesis of single crystalline hierarchical WO3 with enhanced activity for photoelectrochemical solar water oxidation. Int. J. Hydrog. Energy 2013, 8, 3177–3188. [Google Scholar] [CrossRef]
- Hoel, A.; Reyes, L.F.; Heszler, P.; Lantto, V.; Granqvist, C.G. Nanomaterials for environmental applications: Novel WO3-based gas sensors made by advanced gas deposition. Curr. Appl. Phys. 2004, 4, 547–553. [Google Scholar] [CrossRef]
- Tripathi, A.; Tripathi, V.; Pandey, N.K.; Tiwari, K. Resistive Type Moisture Sensor based on WO3. Nanomat. Sens. Transduc. J. 2012, 143, 152–161. [Google Scholar]
- Dickerson, J.H.; Boccaccini, A.R. Electrophoretic Deposition of Nanomaterials; Springer: New York, NY, USA, 2011. [Google Scholar]
- Lassner, E.; Schubert, W.D. Tungsten: Properties, Chemistry, Technology of the Element, Alloys, and Chemical Compounds; Kluwer Academic: New York, NY, USA, 1999. [Google Scholar]
- Karácsonyi, É.; Baia, L.; Dombi, A.; Danciu, V.; Mogyorósi, K.; Pop, L.C.; Kovács, G.; Coșoveanu, V.; Vulpoi, A.; Simon, S.; et al. The photocatalytic activity of TiO2/WO3/noble metal (Au or Pt) nanoarchitectures obtained by selective photodeposition. Catal. Today 2013, 208, 19–27. [Google Scholar] [CrossRef]
- Riboni, F.; Bettini, L.G.; Bahnemann, D.W.; Selli, E. WO3–TiO2 vs. TiO2 photocatalysts: Effect of the W precursor and amount on the photocatalytic activity of mixed oxides. Catal. Today 2013, 209, 28–34. [Google Scholar] [CrossRef]
- Lee, W.J.; Fang, Y.K.; Ho, J.J.; Hsieh, W.T.; Ting, S.F.; Huang, D.; Fang, H.C. Effects of surface porosity on tungsten trioxide (WO3) films’ electrochromic performance. J. Electron. Mater. 2000, 29, 29–183. [Google Scholar] [CrossRef]
- Shi, F.; Liu, J.X.; Huang, X.; Yu, L.; Liu, S.H.; Feng, X.; Wang, X.K.; Shao, G.L.; Hu, S.C.; Yang, B.; et al. Hydrothermal synthesis of mesoporous WO3–TiO2 powders with enhanced photocatalytic activity. Adv. Powder Technol. 2015, 26, 1435–1441. [Google Scholar] [CrossRef]
- Tennakone, K.; Ileperuma, O.A.; Bandara, J.M.S.; Kiridena, W.C.B. TiO2 and WO3 semiconductor particles in contact: Photochemical reduction of WO3 to the non-stoichiometric blue form. Semicond. Sci. Technol. 1992, 7, 423–424. [Google Scholar] [CrossRef]
- Ke, D.; Liu, H.; Peng, T.; Liu, X.; Dai, K. Preparation and photocatalytic activity of WO3/TiO2 nanocomposite particles. Mater. Lett. 2008, 62, 447–450. [Google Scholar] [CrossRef]
- Kim, J.; Bondarchuk, O.; Kay, B.D.; White, J.M.; Dohnálek, Z. Preparation and characterization of monodispersed WO3 nanoclusters on TiO2 (110). Catal. Today 2007, 120, 186–195. [Google Scholar] [CrossRef]
- Al-Kandari, H.; Al-Kharafi, F.; Al-Awadi, N.; El-Dusouqui, O.M.; Katrib, A. Surface electronic structure–catalytic activity relationship of partially reduced WO3 bulk or deposited on TiO2. J. Electron. Spectrosc. 2006, 151, 128–134. [Google Scholar] [CrossRef]
- Williams, D.E.; Aliwell, S.R.; Pratt, K.F.E.; Caruana, D.J.; Jones, R.L.; Cox, R.A.; Hansford, G.M. Modeling the response of a tungsten oxide semiconductor as a gas sensor for the measurement of ozone. Meas. Sci. Technol. 2002, 13, 923–931. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Yang, J.; Guo, W.; Zhu, L.; Zheng, W. Temperature and acidity effects on WO3 nanostructures and gas-sensing properties of WO3 nanoplates. Mater. Res. Bull. 2014, 57, 260–267. [Google Scholar] [CrossRef]
- Baia, L.; Orbán, E.; Fodor, S.; Hampel, B.; Kedves, E.Z.; Saszet, K.; Székely, I.; Karácsonyi, É.; Réti, B.; Berki, P.; et al. Preparation of TiO2/WO3 composite photocatalysts by the adjustment of the semiconductors’ surface charge. Mater. Sci. Semicond. Proc. 2016, 42, 66–71. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Liu, L.; Li, W.; Cao, P. Controlled morphologies and growth direction of WO3 nanostructures hydrothermally synthesized with citric acid. Mater. Lett. 2014, 130, 248–251. [Google Scholar] [CrossRef]
- Sungpanich, J.; Thongtem, T.; Thongtem, S. Photocatalysis of WO3 nanoplates synthesized by conventional hydrothermal and microwave-hydrothermal methods and of commercial WO3 nanorods. J. Nanomater. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Adhikari, S.; Sarkar, D. High Efficient Electrochromic WO3 Nanofibers. Electrochim. Acta 2014, 138, 115–123. [Google Scholar] [CrossRef]
- Zhou, L.; Zou, J.; Yu, M.; Lu, P.; Wei, J.; Qian, Y.; Wang, Y.; Yu, C. Green Synthesis of Hexagonal-Shaped WO3·0.33H2O Nanodiscs, Composed of Nanosheets. Cryst. Growth Des. 2008, 8, 3993–3998. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Yang, T.; Guo, X.; Wu, S. Synthesis of Pt nanoparticles functionalized WO3 nanorods and their gas sensing properties. Sens. Actuators B 2011, 156, 918–923. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A 2010, 216, 179–182. [Google Scholar] [CrossRef]
- Veréb, G.; Ambrus, Z.; Pap, Z.; Kmetykó, Á.; Dombi, A.; Danciu, V.; Cheesman, A.; Mogyorósi, K. Comparative study on UV and visible light sensitive bare and doped titanium dioxide photocatalysts for the decomposition of environmental pollutants in water. Appl. Catal. A 2012, 417–418, 26–36. [Google Scholar] [CrossRef]
- Kovács, G.; Fodor, S.; Vulpoi, A.; Schrantz, K.; Dombi, A.; Hernádi, K.; Danciu, V.; Pap, Z.; Baia, L. Polyhedral Pt vs. spherical Pt nanoparticles on commercial titanias: Is shape tailoring a guarantee of achieving high activity? J. Catal. 2015, 325, 156–167. [Google Scholar] [CrossRef]
- Ryu, J.; Choi, W. Substrate-specific photocatalytic activities of TiO2 and multiactivity test for water treatment application. Environ. Sci. Technol. 2008, 42, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Liu, H.; Sun, J.; Tian, Y.; Chen, S.; Song, J.; Luo, R.; Li, D.; Chen, A.; Liu, C. Improvement of TiO2 photocatalytic properties under visible light by WO3/TiO2 and MoO3/TiO2 composites. Appl. Surf. Sci. 2015, 338, 61–68. [Google Scholar] [CrossRef]
- Liu, S.; Huang, J.; Cao, L.; Li, J.; Ouyang, H.; Tao, X.; Liu, C. One-pot synthesis of TiO2–WO3 composite nanocrystallites with improved photocatalytic properties under natural sunlight irradiation. Mater. Sci. Semicond. Proc. 2014, 25, 106–111. [Google Scholar] [CrossRef]
- Aslam, M.; Iqbal, M.; Ismail, I.; Chandrasekaran, S.; Hameed, A. Morphology controlled bulk synthesis of disc-shaped WO3 powder and evaluation of its photocatalytic activity for the degradation of phenols. J. Hazard. Mater. 2014, 276, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Vamvasakis, I.; Georgaki, I.; Vernardou, D.; Kenanakis, G.; Katsarakis, N. Synthesis of WO3 catalytic powders: Evaluation of photocatalytic activity under NUV/visible light irradiation and alkaline reaction pH. J. Sol-Gel Sci. Technol. 2015, 76, 120–128. [Google Scholar] [CrossRef]
- Luo, X.; Deng, F.; Min, L.; Luo, S.; Guo, B.; Zeng, G.; Au, C. Facile one-step synthesis of inorganic-framework molecularly imprinted TiO2/WO3 nanocomposite and its molecular recognitive photocatalytic degradation of target contaminant. Envir. Sci. Technol. 2013, 47, 737–744. [Google Scholar]
- DePuccio, D.P.; Botella, P.; O’Rourke, B.; Landry, C.C. Degradation of Methylene Blue Using Porous WO3, SiO2−WO3, and Their Au-Loaded Analogs: Adsorption and Photocatalytic Studies. ACS Appl. Mater. Interf. 2015, 7, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Deng, C.; Xu, J.; Zheng, Q.; Chen, G.; Ge, X. Facile synthesis of hierarchical WO3 nanocakes displaying the excellent visible light photocatalytic performance. Mater. Lett. 2015, 161, 17–19. [Google Scholar] [CrossRef]
- Vajda, K.; Saszet, K.; Kedves, Z.; Kása, Z.; Danciu, V.; Baia, L.; Magyari, K.; Hernádi, K.; Kovács, G.; Pap, Z. Shape-controlled agglomeration of TiO2 nanoparticles New insights on polycrystallinity vs. single crystals in photocatalysis. Ceram. Int. 2016, 42, 3077–3087. [Google Scholar] [CrossRef]
- Kovács, G.; Baia, L.; Vulpoi, A.; Radu, T.; Karácsonyi, É.; Dombi, A.; Hernádi, K.; Danciu, V.; Simon, S.; Pap, Z. TiO2/WO3/Au nanoarchitectures’ photocatalytic activity, “from degradation intermediates to catalysts’ structural peculiarities”, Part I: Aeroxide P25 based composites. Appl. Catal. B. Environ. 2014, 147, 508–517. [Google Scholar] [CrossRef]
- Baia, L.; Vulpoi, A.; Radu, T.; Karácsonyi, É.; Dombi, A.; Hernádi, K.; Danciu, V.; Simon, S.; Norén, K.; Canton, S.E.; et al. TiO2/WO3/Au nanoarchitectures’ photocatalytic activity “from degradation intermediates to catalysts’ structural peculiarities” Part II: Aerogel based composites—Fine details by spectroscopic means. Appl. Catal. B 2014, 148–149, 589–600. [Google Scholar] [CrossRef]
- Jenkins, R.; Snyder, R.L. Introduction to X-Ray Powder Diffractometry; John Wiley & Sons: New York, NY, USA, 1996; pp. 1417–1418. [Google Scholar]
- Pap, Z.; Danciu, V.; Cegléd, Z.; Kukovecz, Á.; Oszkó, A.; Dombi, A.; Mogyorósi, K. The influence of rapid heat treatment in still air on the photocatalytic activity of titania photocatalysts for phenol and monuron degradation. Appl. Catal. B. Environ. 2011, 101, 461–470. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. Ein Beitrag zur Optik der Farbanstriche. Zeits. Phys. 1931, 12, 593–601. [Google Scholar]

| Sample Name | Structure WO3 | Band-gap (eV) | ηphenol (%) | r0, phenol (mM·min−1) | ηMO (%) | r0, MO (mM·min−1) | |
|---|---|---|---|---|---|---|---|
| *MC | #HY | ||||||
| P25 | – | – | 3.11 | 86.8 | 8.90 × 10−3 | 82.8 | 2.26 |
| WO3-HW5 | 36.3 | 63.6 | 2.69 | 0 | – | 0 | – |
| WO3-HW | 9.3 | 90.6 | 2.75 | 0 | – | 0 | – |
| WO3-NWH | 0 | 100 | 2.69 | 0 | – | 0 | – |
| WO3-AMT | 100 | 0 | 2.25 | 0 | – | 0 | – |
| WO3-COM | 100 | 0 | 2.61 | 0 | – | 0 | – |
| P25 + WO3-HW5 | – | – | 3.04 | 66.7 | 8.86 × 10−3 | 76.3 | 1.06 |
| P25 + WO3-HW | – | – | 3.00 | 87.2 | 6.53 × 10−3 | 67.3 | 1.01 |
| P25 + WO3-NWH | – | – | 2.97 | 44.4 | 5.31 × 10−3 | 57.7 | 0.35 |
| P25 + WO3-AMT | – | – | 3.10 | 58.7 | 6.69 × 10−3 | 84.6 | 1.66 |
| P25 + WO3-COM | – | – | 2.94 | 49.1 | 11.18 × 10−3 | 59.5 | 5.02 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Székely, I.; Kovács, G.; Baia, L.; Danciu, V.; Pap, Z. Synthesis of Shape-Tailored WO3 Micro-/Nanocrystals and the Photocatalytic Activity of WO3/TiO2 Composites. Materials 2016, 9, 258. https://doi.org/10.3390/ma9040258
Székely I, Kovács G, Baia L, Danciu V, Pap Z. Synthesis of Shape-Tailored WO3 Micro-/Nanocrystals and the Photocatalytic Activity of WO3/TiO2 Composites. Materials. 2016; 9(4):258. https://doi.org/10.3390/ma9040258
Chicago/Turabian StyleSzékely, István, Gábor Kovács, Lucian Baia, Virginia Danciu, and Zsolt Pap. 2016. "Synthesis of Shape-Tailored WO3 Micro-/Nanocrystals and the Photocatalytic Activity of WO3/TiO2 Composites" Materials 9, no. 4: 258. https://doi.org/10.3390/ma9040258







