Fabrication and Compressive Properties of Low to Medium Porosity Closed-Cell Porous Aluminum Using PMMA Space Holder Technique
Abstract
:1. Introduction
2. Material and Methods
2.1. Raw Materials
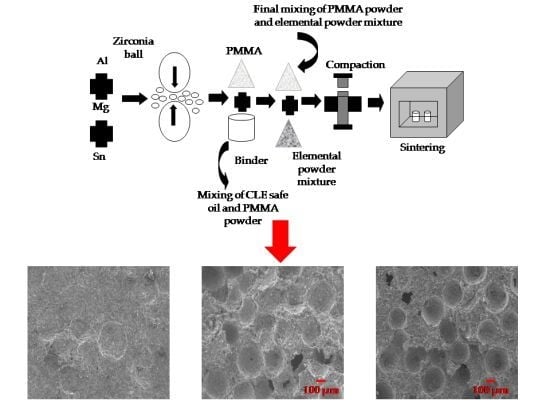
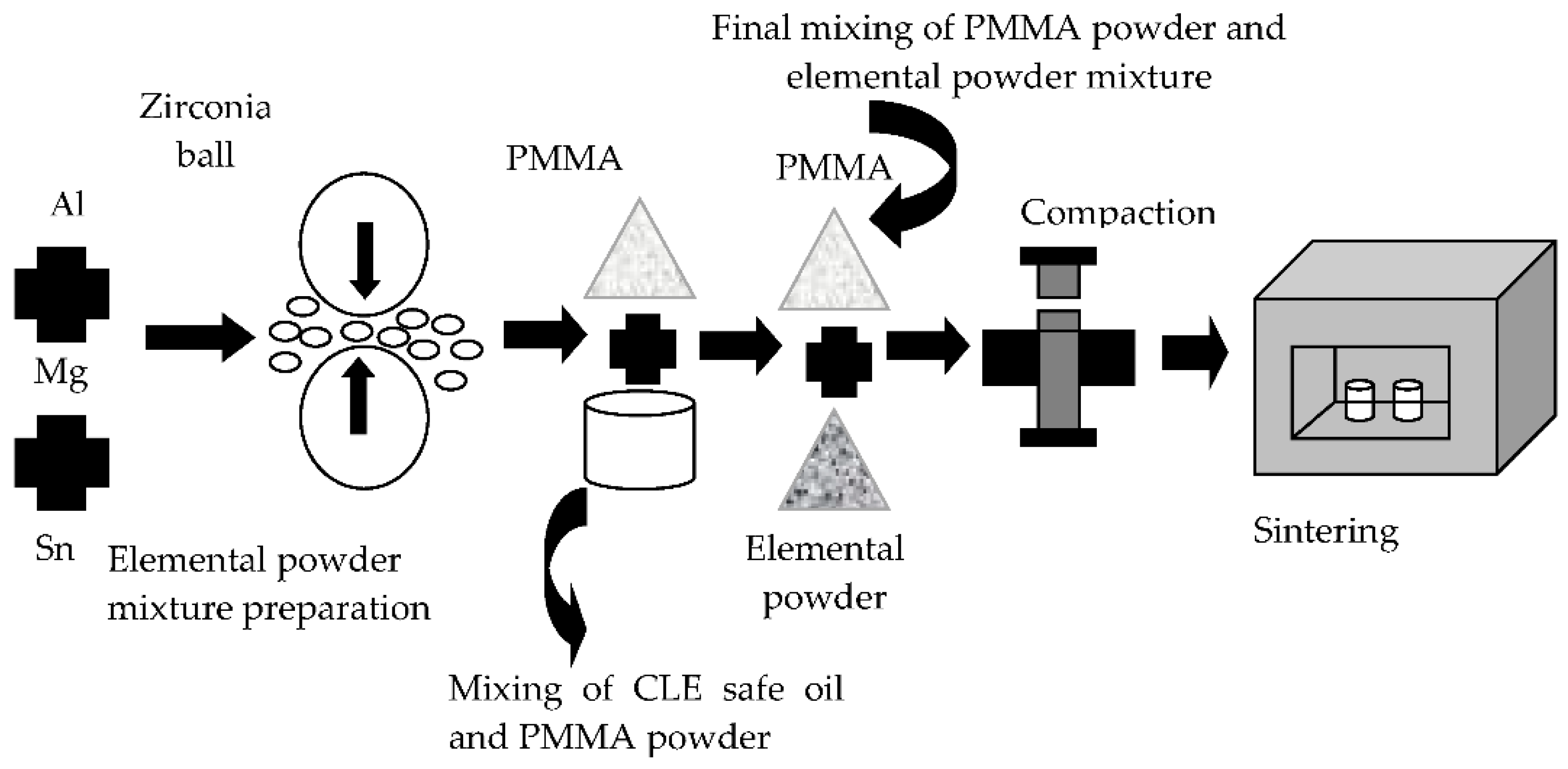
2.2. Preparation of Porous Al
2.3. Evaluation of Density and Porosity of Porous Al
2.4. Microstructural Characterization
2.5. Thermogravimetric (TGA) Analysis
2.6. X-ray Diffraction and Carbon Content Analysis
2.7. Compressive Behavior and Energy Absorption Capacity
3. Results and Discussion
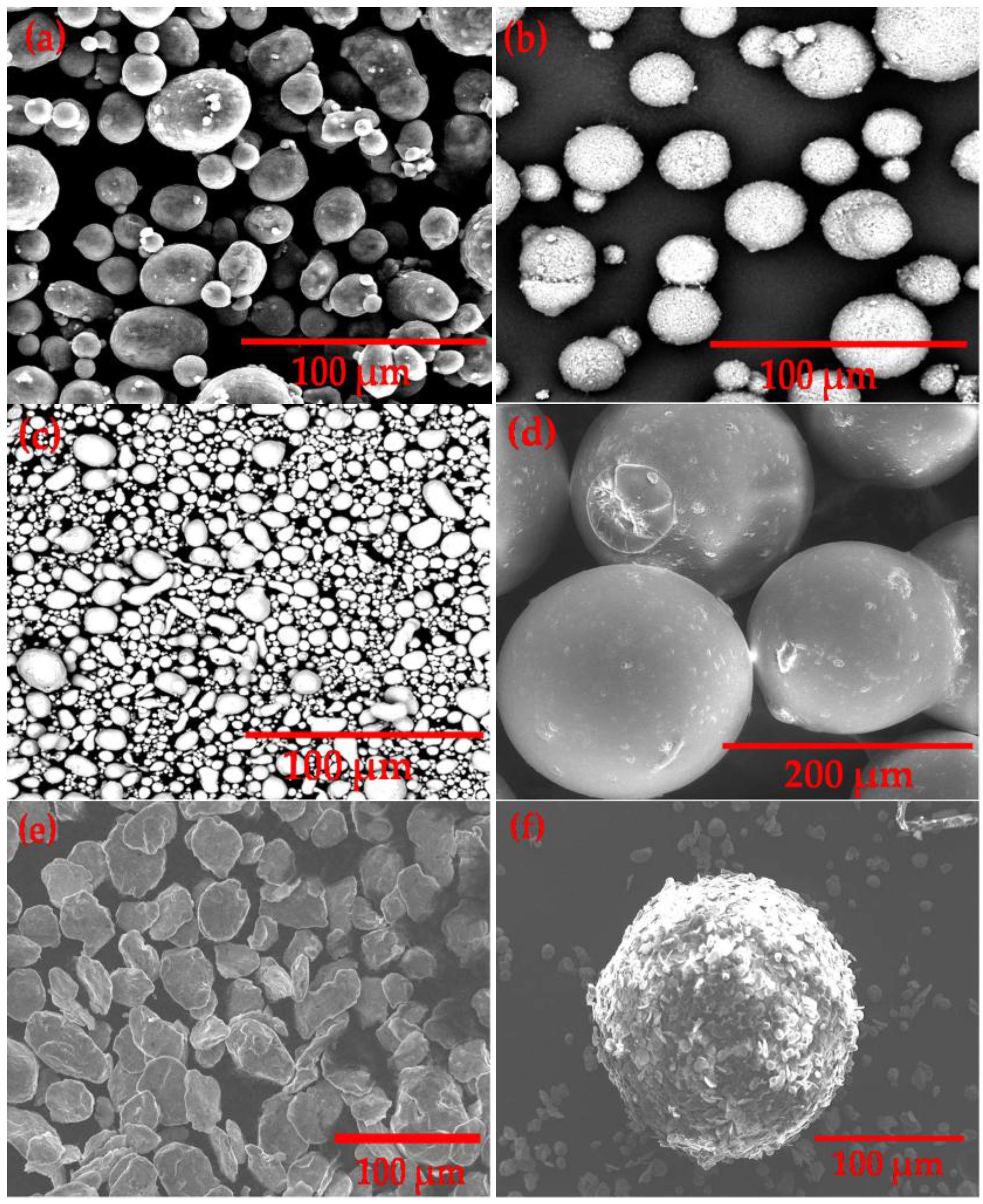
3.1. Morphology Characterization of Starting Powders
3.2. Thermogravimetric (TGA) Analysis of PMMA
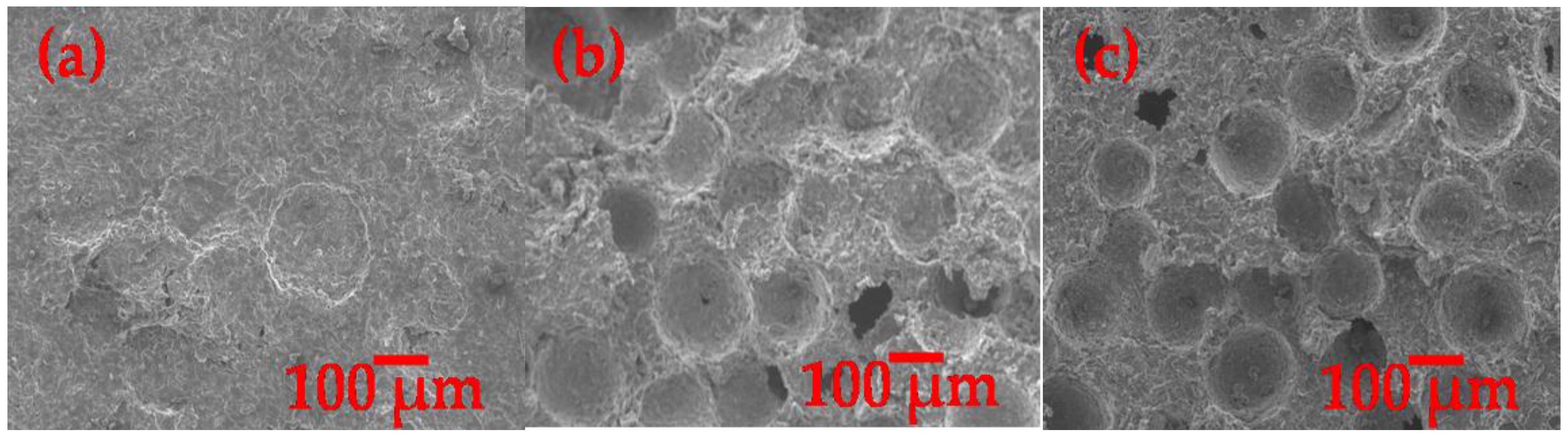
3.3. Microstructure Characterization of Porous Al
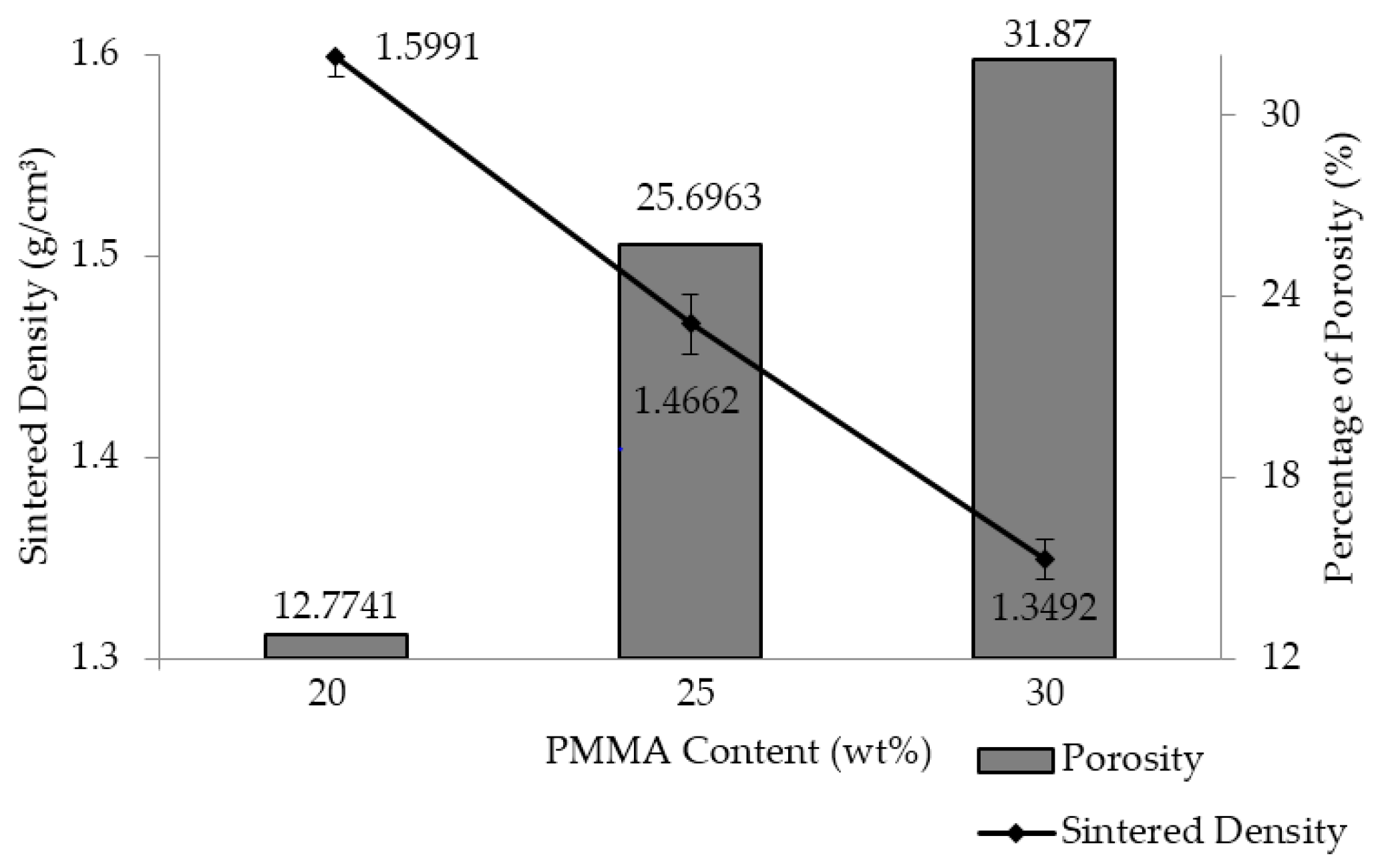
3.4. Sintered Density and Porosity of the As-Produced Porous Al
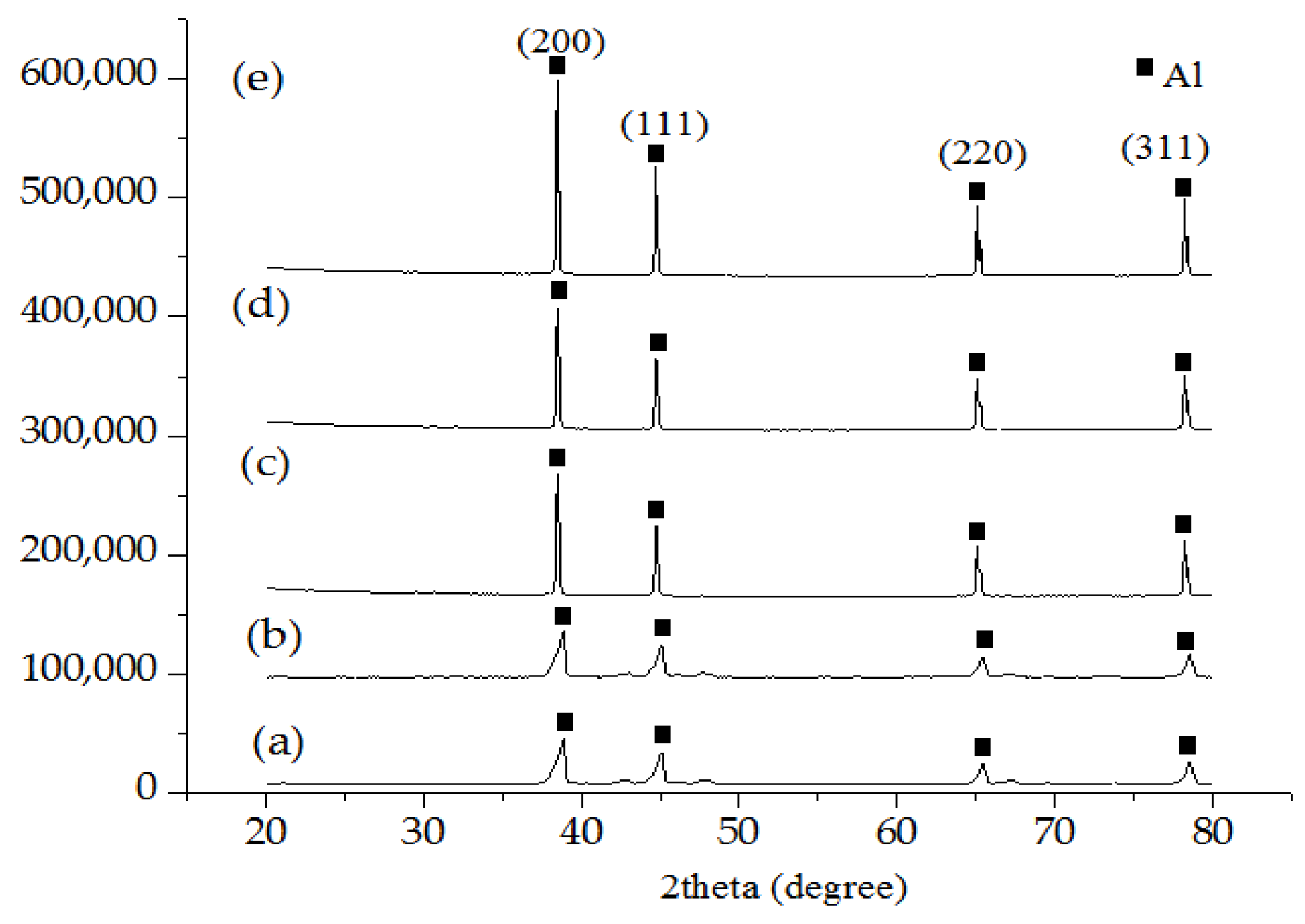
3.5. X-ray Diffraction Analysis
3.6. Carbon Content Analysis for Elemental Powder Mixture, Final Powder Mixture and Porous Al Specimen
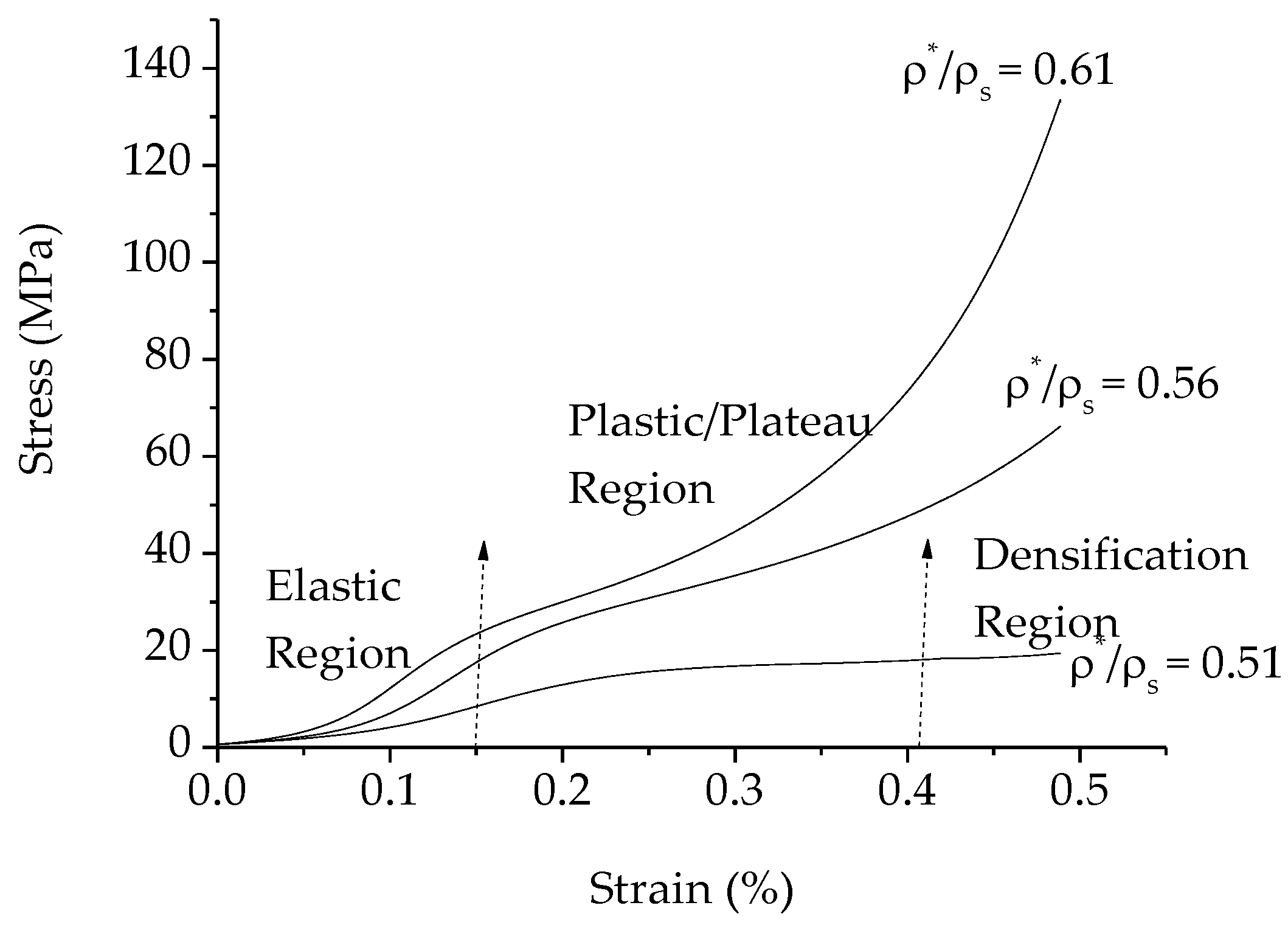
3.7. Compressive Behavior and Energy Absorption Capacity of the Resultant Porous Al
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kevorkijan, V. Low Cost Aluminium Foams Made By CaCO3 Particulates. Assoc. Metall. Eng. Serbia AMES 2010, 13, 205–219. [Google Scholar]
- Koizumi, T.; Kido, K.; Kita, K.; Mikado, K.; Gnyloskurenko, S.; Nakamura, T. Method of Preventing Shrinkage of Aluminum Foam Using Carbonates. Metals 2011, 2, 1–9. [Google Scholar] [CrossRef]
- Yu, S.; Liu, J.; Luo, Y.; Liu, Y. Compressive behavior and damping property of ZA22/SiCp composite foams. Mater. Sci. Eng. A 2007, 457, 325–328. [Google Scholar] [CrossRef]
- Heydari Astaraie, A.; Shahverdi, H.R.; Elahi, S.H. Compressive behavior of Zn–22Al closed-cell foams under uniaxial quasi-static loading. Trans. Nonferrous Met. Soc. China 2015, 25, 162–169. [Google Scholar] [CrossRef]
- Dewidar, M. Influence of processing parameters and sintering atmosphere on the mechanical properties and microstructure of porous 316L stainless steel for possible hard-tissue applications. Int. J. Mech. Mech. Eng. 2012, 12, 10–24. [Google Scholar]
- Smith, B.H.; Szyniszewski, S.; Hajjar, J.F.; Schafer, B.W.; Arwade, S.R. Steel foam for structures: A review of applications, manufacturing and material properties. J. Constr. Steel Res. 2012, 71, 1–10. [Google Scholar] [CrossRef]
- Ashby, M.F.; Evans, A.; Fleck, N.; Wadley, H.N.G. Metal Foams: A Design Guide; Butterworth-Heinemann: Boston, MA, USA, 2000. [Google Scholar]
- Kavei, G. Mechanical properties of aluminum foam fabricated by aluminum powders with Na or carbamide replica. AASCIT J. Mater. 2015, 1, 22–30. [Google Scholar]
- Jiang, B.; Zhao, N.Q.; Shi, C.S.; Du, X.W.; Li, J.J.; Man, H.C. A novel method for making open cell aluminum foams by powder sintering process. Mater. Lett. 2005, 59, 3333–3336. [Google Scholar] [CrossRef]
- Chen, S.; Bourham, M.; Rabiei, A. Applications of open-cell and closed-cell metal foams for radiation shielding. Procedia Mater. Sci. 2014, 4, 293–298. [Google Scholar] [CrossRef]
- Manonukul, A.; Muenya, N.; Léaux, F.; Amaranan, S. Effects of replacing metal powder with powder space holder on metal foam produced by metal injection moulding. J. Mater. Proc. Technol. 2010, 210, 529–535. [Google Scholar] [CrossRef]
- Gülsoy, H.Ö.; German, R.M. Production of micro-porous austenitic stainless steel by powder injection molding. Scr. Mater. 2008, 58, 295–298. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, G.-P.; Lu, T.-J.; He, S.-Y. Strain rate behavior of closed-cell Al-Si-Ti foams: Experiment and numerical modeling. Mech. Adv. Mater. Struct. 2015, 22, 556–563. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, E. On the compressive behavior of sintered porous coppers with low-to-medium porosities-Part II: Preparation and microstructure. Int. J. Mech. Sci. 2008, 50, 550–558. [Google Scholar] [CrossRef]
- Li, B.; Li, Z.; Lu, X. Effect of the macro-pore structure on the anodic polarization behavior of porous titanium. Anti Corros. Methods Mater. 2012, 59, 57–62. [Google Scholar] [CrossRef]
- Liu, J.; Yu, S.; Zhu, X.; Wei, M.; Luo, Y.; Liu, Y. The compressive properties of closed-cell Zn-22Al foams. Mater. Lett. 2008, 62, 683–685. [Google Scholar] [CrossRef]
- Golabgir, M.H.; Ebrahimi-Kahrizsangi, R.; Torabi, O.; Saatchi, A. Fabrication of open cell Fe-10%Al foam by space-holder technique. Arch. Metall. Mater. 2014, 59, 41–45. [Google Scholar] [CrossRef]
- Paulin, I.; Sustarsic, B.; Kevorkijan, V.; Skapin, S.D.; Jenko, M. Synthesis of aluminium foams by the powder metallurgy process: Compacting of precursors. Mater. Tehnol. 2011, 45, 13–19. [Google Scholar]
- Matijasevic, B.; Banhart, J. Improvement of aluminium foam technology by tailoring of blowing agent. Scr. Mater. 2006, 54, 503–508. [Google Scholar] [CrossRef]
- Li, B.Q.; Wang, C.Y.; Lu, X. Effect of pore structure on the compressive property of porous Ti produced by powder metallurgy technique. Mater. Des. 2013, 50, 613–619. [Google Scholar] [CrossRef]
- Jeon, K.C.; Kim, Y.D.; Suk, M.-J.; Oh, S.-T. Fabrication of porous Ti by thermal decomposition and sintering of PMMA/TiH2 powder compact. Arch. Metall. Mater. 2015, 60, 26–28. [Google Scholar] [CrossRef]
- Bi, Y.; Zheng, Y.; Li, Y. Microstructure and mechanical properties of sintered porous magnesium using polymethyl methacrylate as the space holder. Mater. Lett. 2015, 161, 583–586. [Google Scholar] [CrossRef]
- Bafti, H.; Habibolahzadeh, A. Production of aluminum foam by spherical carbamide space holder technique-processing parameters. Mater. Des. 2010, 31, 4122–4129. [Google Scholar] [CrossRef]
- Hassani, A.; Habibolahzadeh, A.; Bafti, H. Production of graded aluminum foams via powder space holder technique. Mater. Des. 2012, 40, 510–515. [Google Scholar] [CrossRef]
- Baumeister, J.; Banhart, J.; Weber, M. Aluminium foams for transport industry. Mater. Des. 1997, 18, 217–220. [Google Scholar] [CrossRef]
- Gokce, A.; Fındık, F. Mechanical and physical properties of sintered aluminum powders. J. Achiev. Mater. Manuf. Eng. 2008, 30, 157–164. [Google Scholar]
- Sukiman, N.L.; Gupta, R.K.; Buchheit, R.G.; Birbilis, N. Influence of microalloying additions on Al-Mg alloy. Part 1: Corrosion and electrochemical response. Corros. Eng. Sci. Technol. 2014, 49, 254–262. [Google Scholar] [CrossRef]
- Katsuyoshi, K.; Tachai, L.; Thotsaphon, T.; Atsushi, K. Local appearance of Sn liquid phase at surface of aluminum alloy powder during heating. Trans. JWRI 2007, 36, 29–33. [Google Scholar]
- Suryanarayana, C. Mechanical alloying and milling. Progress Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Kim, S.W.; Jung, H.-D.; Kang, M.-H.; Kim, H.-E.; Koh, Y.-H.; Estrin, Y. Fabrication of porous titanium scaffold with controlled porous structure and net-shape using magnesium as spacer. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2808–2815. [Google Scholar] [CrossRef] [PubMed]
- Arifvianto, B.; Zhou, J. Fabrication of metallic biomedical scaffolds with the space holder method: A review. Materials 2014, 7, 3588–3622. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, L.; Jing, D.; Ding, J. A comparative study of porous scaffolds with cubic and spherical macropores. Polymer 2005, 46, 4979–4985. [Google Scholar] [CrossRef]
- Bekoz, N.; Oktay, E. Effects of carbamide shape and content on processing and properties of steel foams. J. Mater. Proc. Technol. 2012, 212, 2109–2116. [Google Scholar] [CrossRef]
- Jha, N.; Mondal, D.P.; Dutta Majumdar, J.; Badkul, A.; Jha, A.K.; Khare, A.K. Highly porous open cell Ti-foam using NaCl as temporary space holder through powder metallurgy route. Mater. Des. 2013, 47, 810–819. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, F.; Fung, T. Optimisation of compaction and liquid-state sintering in sintering and dissolution process for manufacturing A1 foams. Mater. Sci. Eng. A 2004, 364, 117–125. [Google Scholar] [CrossRef]
- Mustapha, F.; Mustapha, M.; Noorsal, K.; Mamat, O.; Hussain, P.; Ahmad, F.; Muhamad, N.; Haris, S.M. Preliminary study on the fabrication of aluminium foam through pressure assisted sintering dissolution process. J. Mater. Proc. Technol. 2010, 210, 1598–1612. [Google Scholar] [CrossRef]
- Rabiei, A.; Vendra, L.; Reese, N.; Young, N.; Neville, B.P. Processing and characterization of a new composite metal foam. Mater. Trans. 2006, 47, 2148–2153. [Google Scholar] [CrossRef]
- Nesic, S.; Krupp, U.; Michels, W. Monotonic and cyclic loading behavior of closed-cell aluminum foams and sandwich structures. Procedia Mater. Sci. 2014, 4, 269–273. [Google Scholar] [CrossRef]
- Amsterdam, E.; de Hosson, J.T.M.; Onck, P.R. Failure mechanisms of closed-cell aluminum foam under monotonic and cyclic loading. Acta Mater. 2006, 54, 4465–4472. [Google Scholar] [CrossRef]
- Hamada, T.; Kanahashi, H.; Miyoshi, T.; Kanetake, N. Effects of the strain rate and alloying on the compression characteristics of closed cell aluminum foams. Mater. Trans. 2009, 50, 1418–1425. [Google Scholar] [CrossRef]

| Specimen | Carbon Content (wt %) |
|---|---|
| Elemental powder mixture(Al-Mg-Sn) | 0.22 ± 0.52 |
| Final powder mixture with 20 wt % PMMA | 11.29 ± 0.52 |
| Final powder mixture with 25 wt % PMMA | 12.17 ± 0.43 |
| Final powder mixture with 30 wt % PMMA | 13.03 ± 0.61 |
| Sintered porous Al with 20 wt % PMMA | 3.87 ± 0.54 |
| Sintered porous Al with 25 wt % PMMA | 0.41 ± 0.15 |
| Sintered porous Al with 30 wt % PMMA | 0.43 ± 0.16 |
| PMMA Content (wt %) | Plateau Stress (MPa) | Relative Density (ρ* /ρs) | Energy Absorption Capability (MJ/m3) |
|---|---|---|---|
| 20 | 29.41 ± 0.42 | 0.61 ± 0.32 | 1.61 ± 0.60 |
| 25 | 24.76 ± 0.55 | 0.56 ± 0.28 | 3.65 ± 0.57 |
| 30 | 17.17 ± 0.49 | 0.51 ± 0.41 | 1.41 ± 0.44 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamal, N.A.; Tan, A.W.; Yusof, F.; Katsuyoshi, K.; Hisashi, I.; Singh, S.; Anuar, H. Fabrication and Compressive Properties of Low to Medium Porosity Closed-Cell Porous Aluminum Using PMMA Space Holder Technique. Materials 2016, 9, 254. https://doi.org/10.3390/ma9040254
Jamal NA, Tan AW, Yusof F, Katsuyoshi K, Hisashi I, Singh S, Anuar H. Fabrication and Compressive Properties of Low to Medium Porosity Closed-Cell Porous Aluminum Using PMMA Space Holder Technique. Materials. 2016; 9(4):254. https://doi.org/10.3390/ma9040254
Chicago/Turabian StyleJamal, Nur Ayuni, Ai Wen Tan, Farazila Yusof, Kondoh Katsuyoshi, Imai Hisashi, S. Singh, and Hazleen Anuar. 2016. "Fabrication and Compressive Properties of Low to Medium Porosity Closed-Cell Porous Aluminum Using PMMA Space Holder Technique" Materials 9, no. 4: 254. https://doi.org/10.3390/ma9040254