Computational Study of the Effect of Cortical Porosity on Ultrasound Wave Propagation in Healthy and Osteoporotic Long Bones
Abstract
:1. Introduction
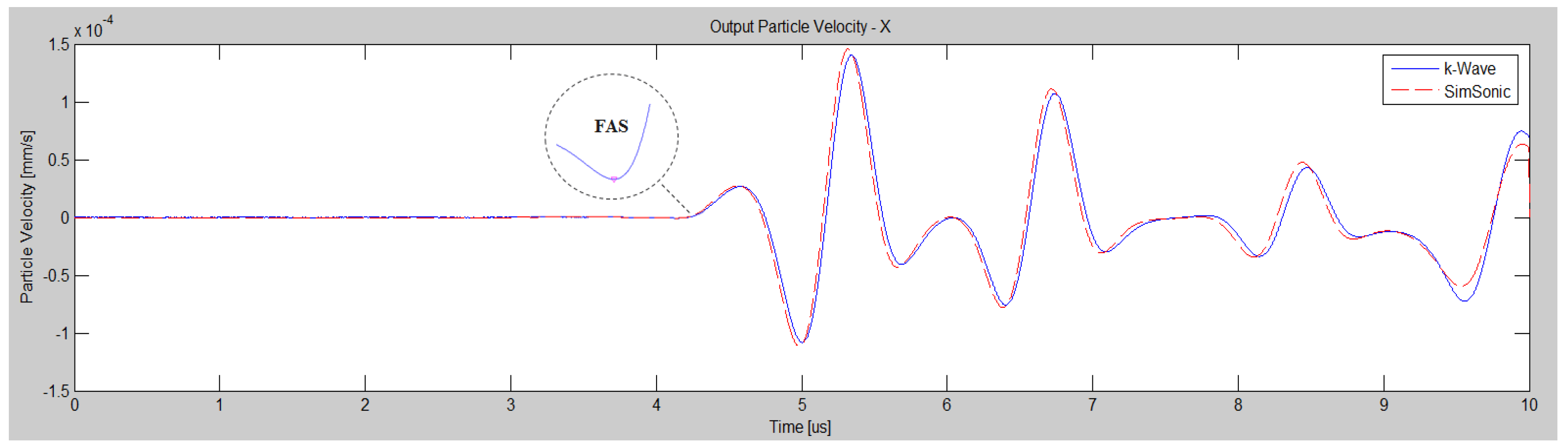
2. Validation of the Numerical Method: A Benchmark Problem
3. Numerical Evaluation of Cortical Porosity Using Ultrasonic Techniques
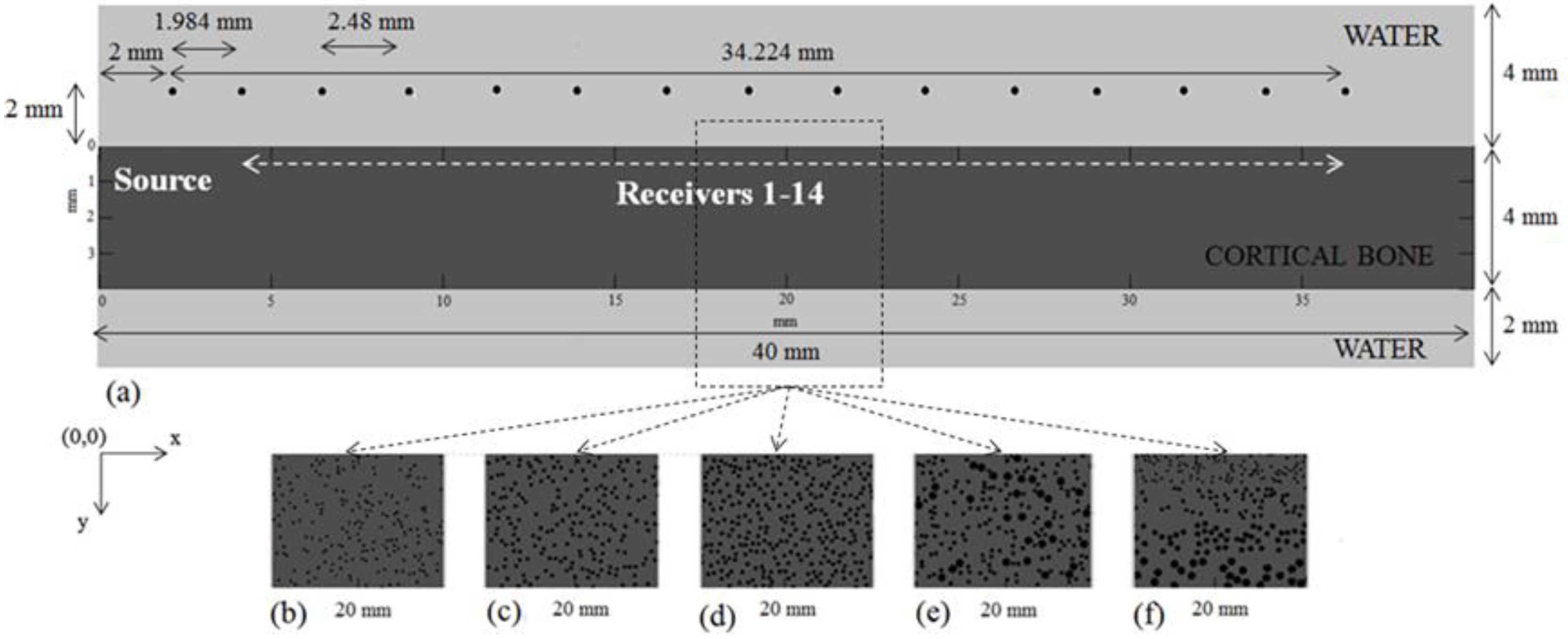
3.1. Model Geometry
3.2. Material Properties
3.3. Ultrasound Configuration
3.4. Determination of the Ultrasonic Wave Propagation Path and Velocity
3.5. Boundary Conditions
3.6. Numerical Simulation and Signal Analysis in the Time Domain
Convergence Study
3.7. Statistical Analysis
4. Results
4.1. First Set of Simulations (Series I_Po0-16)
4.1.1. Implanted Transducers
4.1.2. Transducers at a Distance of Two Millimeters from the Cortical Cortex
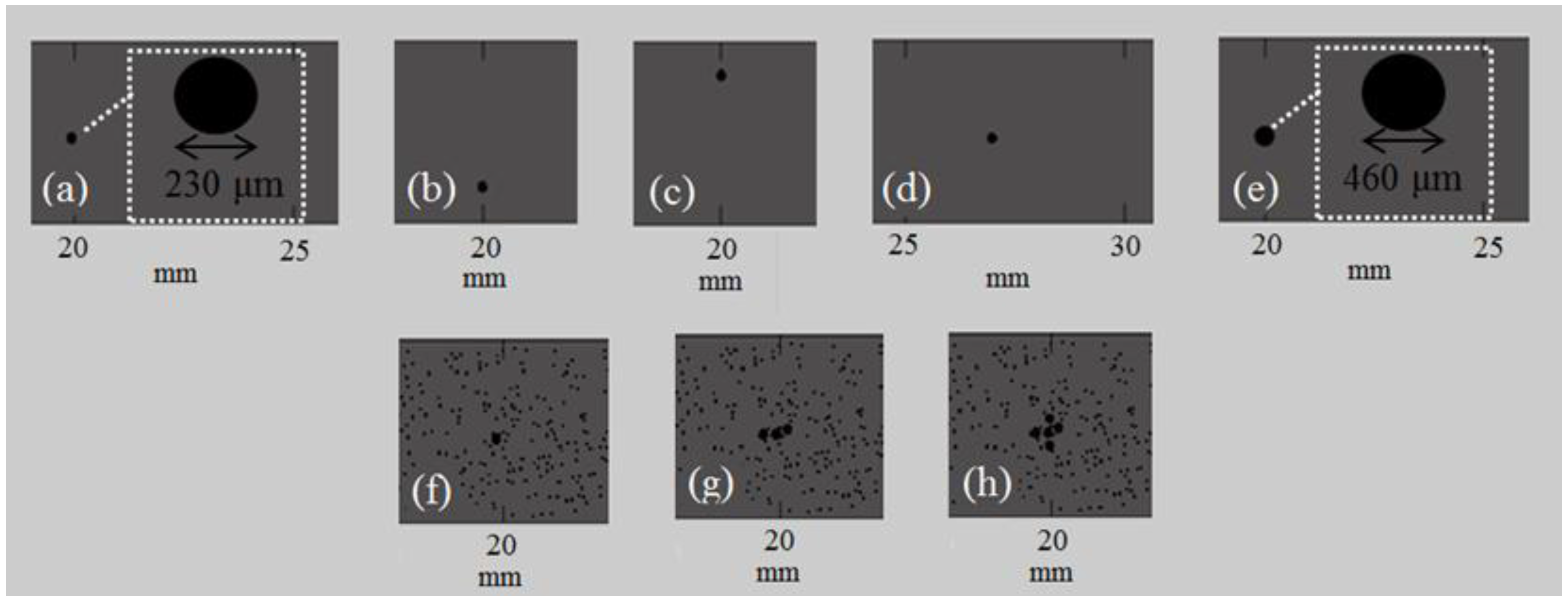
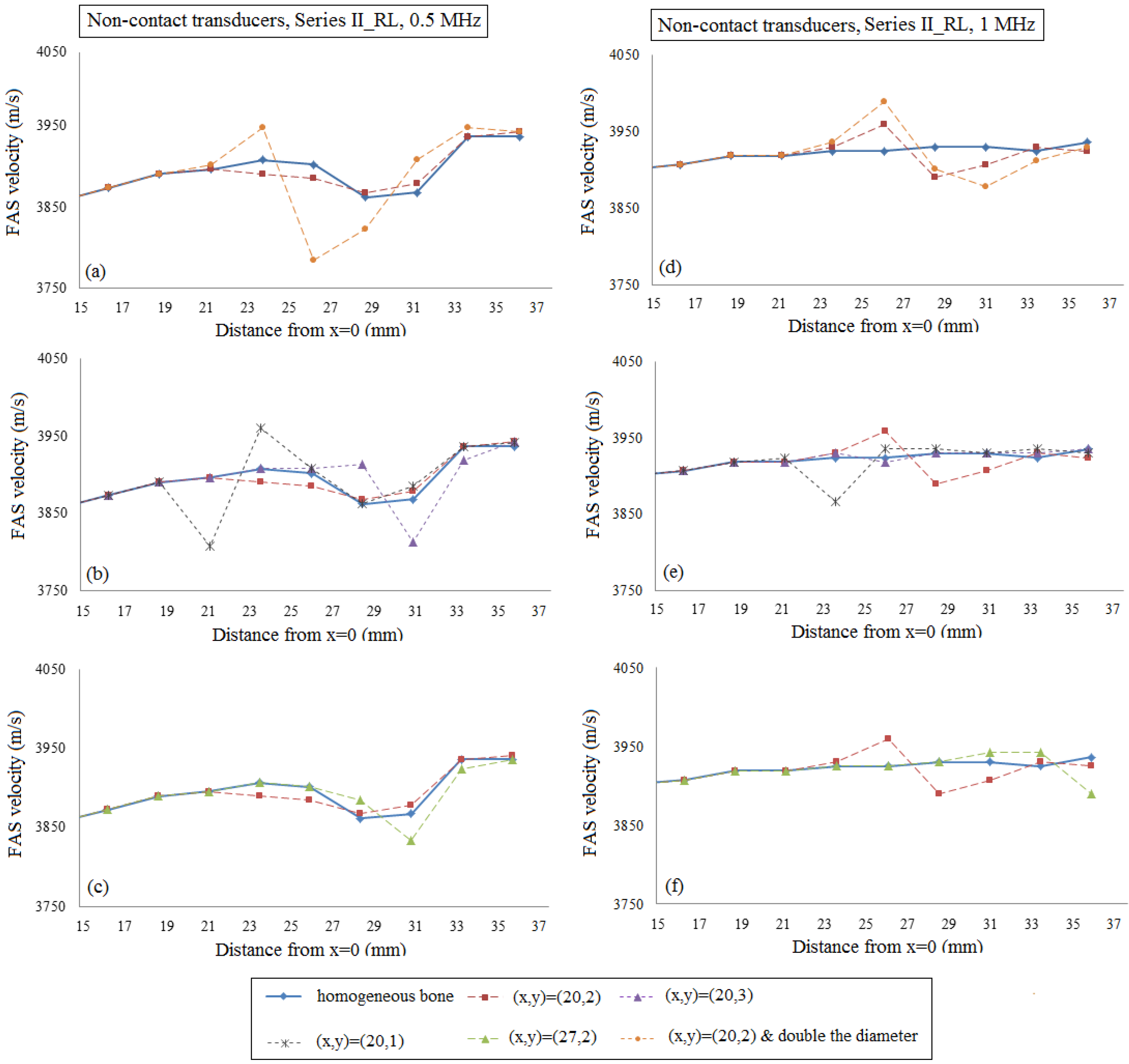
4.2. Second Set of Simulations (Series II_RL)
4.2.1. Implanted Transducers
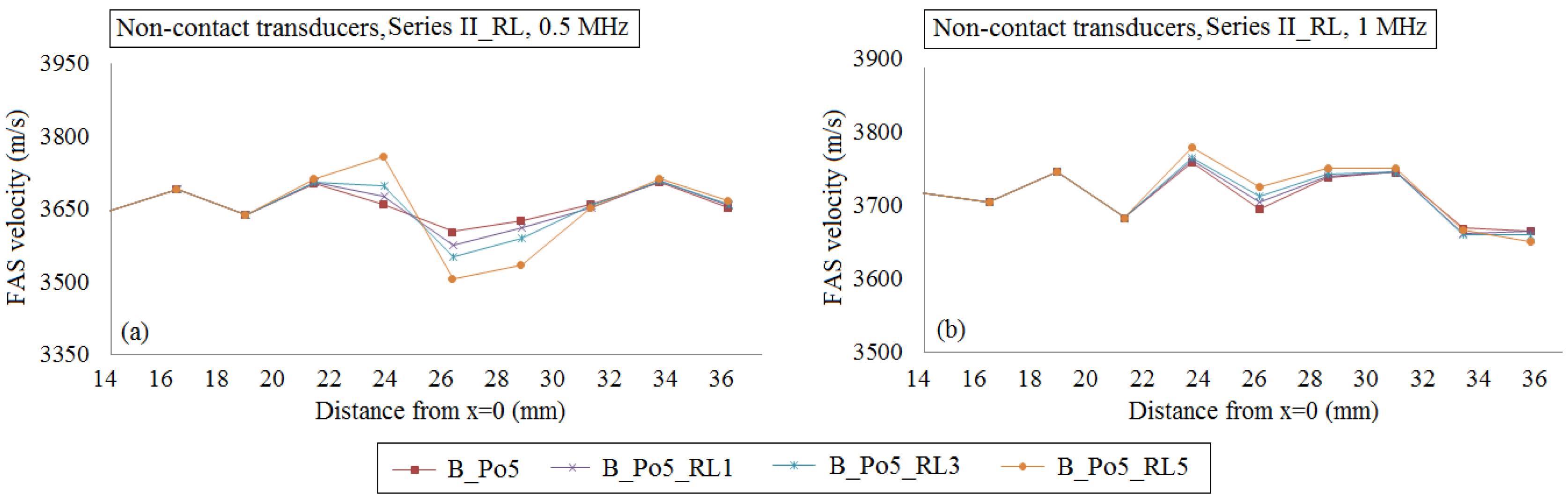
4.2.2. Transducers at a Distance of Two Millimeters from the Cortical Cortex
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Laugier, P.; Haïat, G. Bone Quantitative Ultrasound; Springer: New York, NY, USA, 2010. [Google Scholar]
- Rohde, K.; Rohrbach, D.; Gluer, C.C.; Laugier, P.; Grimal, Q.; Raum, K.; Barkmann, R. Influence of Porosity, Pore Size, and Cortical Thickness on the Propagation of Ultrasonic Waves Guided Through the Femoral Neck Cortex: A Simulation Study. IEEE Trans. Ultras. Ferroel. Freq. Control 2014, 61, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Grondin, J.; Grimal, Q.; Engelke, K.; Laugier, P. Potential of first arriving signal to assess cortical bone geometry at the hip with QUS: A model based study. Ultrasound Med. Biol. 2010, 36, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, P.; Talmant, M.; Bousson, V.; Nicholson, P.H.F.; Cheng, S.; Timonen, J.; Laugier, P. Ultrasonically determined thickness of long cortical bones: two-dimensional simulations of in vitro experiments. J. Acoust. Soc. Am. 2007, 122, 1818–1826. [Google Scholar] [CrossRef] [PubMed]
- Malo, M.K.H.; Rohrbach, D.; Isaksson, H.; Töyräs, J.; Jurvelin, J.S.; Tamminena, I.S.; Kröger, H.; Raum, K. Longitudinal elastic properties and porosity of cortical bone tissue vary with age in human proximal femur. Bone 2013, 53, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liu, D.; Ta, D.; Hu, B.; Wang, W. Quantification of guided mode propagation in fractured long bones. Ultrasonics 2014, 54, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Papacharalampopoulos, A.; Vavva, M.; Protopappas, V.; Polyzos, D.; Fotiadis, D.I. A numerical study on the propagation of Rayleigh and guided waves in cortical bone according to Mindlin’s Form II gradient elastic theory. J. Acoust. Soc. Am. 2011, 130, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- Machado, C.B.; Pereira, W.C.D.A.; Granke, M.; Talmant, M.; Padilla, F.; Laugier, P. Experimental and simulation results on the effect of cortical bone mineralization in ultrasound axial transmission measurements: A model for fracture healing ultrasound monitoring. Bone 2011, 48, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Rohrbach, D.; Lakshmanan, S.; Peyrin, F.; Langer, M.; Gerisch, A.; Grimal, Q.; Laugier, P.; Raum, K. Spatial distribution of tissue level properties in human a femoral cortical bone. J. Biomech. 2012, 45, 2264–2270. [Google Scholar] [CrossRef] [PubMed]
- Potsika, V.T.; Vavva, M.G.; Protopappas, V.C. Computational Modeling of Ultrasound Wave Propagation in Bone. In Computational Medicine in Data Mining and Modeling; Springer: New York, NY, USA, 2014; pp. 349–376. [Google Scholar]
- Moreau, L.; Minonzio, J.G.; Talmant, M.; Laugier, P. Measuring the wavenumber of guided modes in waveguides with linearly varying thickness. J. Acoust. Soc. Am. 2014, 135, 2614–2624. [Google Scholar] [CrossRef] [PubMed]
- Protopappas, V.C.; Fotiadis, D.I.; Malizos, K.N. Guided ultrasound wave propagation in intact and healing long bones. Ultras. Med. Biol. 2006, 32, 693–708. [Google Scholar] [CrossRef] [PubMed]
- Malo, M.K.H.; Töyräs, J.; Karjalainena, J.P.; Isakssonc, H.; Riekkinena, O.; Jurvelin, J.S. Ultrasound backscatter measurements of intact human proximal femurs—Relationships of ultrasound parameters with tissue structure and mineral density. Bone 2014, 64, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Bourgnon, Α.; Sitzer, Α.; Chabraborty, A.; Rohde, K.; Varga, P.; Wendlandt, R.; Raum, K. Impact of microscale properties measured by 50-MHz acoustic microscopy on mesoscale elastic and ultimate mechanical cortical bone properties. In Proceedings of IEEE International Ultrasonics Symposium, Chicago, IL, USA, 3–6 September 2014; pp. 636–638.
- Nicholson, P.; Moilanen, P.; Kärkkäinen, T.; Timonen, J.; Cheng, S. Guided ultrasonic waves in long bones: modelling, experiment and application. Physiol. Meas. 2002, 23, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Bossy, E.; Talmant, M.; Laugier, P. Effect of bone cortical thickness on velocity measurements using ultrasonic axial transmission: a 2d simulation study. J. Acoust. Soc. Am. 2002, 112, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, P.; Talmant, M.; Nicholson, P.H.F.; Cheng, S.; Timonen, J.; Laugier, P. Ultrasonically determined thickness of long cortical bones: Three-dimensional simulations of in vitro experiments. J. Acoust. Soc. Amer. 2007, 122, 2439–2445. [Google Scholar] [CrossRef] [PubMed]
- Grimal, Q.; Rohrbach, D.; Grondin, J.; Barkmann, R.; Gluer, C.C.; Raum, K.; Laugier, P. Modeling of femoral neck cortical bone for the numerical simulation of ultrasound propagation. Ultrasound Med. Biol. 2013, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Granke, M.; Grimal, Q.; Saïed, A.; Nauleau, P.; Peyrin, F.; Laugier, P. Change in porosity is the major determinant of the variation of cortical bone elasticity at the millimeter scale in aged women. Bone 2011, 49, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.D. Age changes in bone mineralization, cortical thickness, and haversian canal area. Calcif. Tissue Int. 1980, 31, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Treeby, B.E.; Jaros, J.; Rendell, A.P.; Cox, B.T. Modeling nonlinear ultrasound propagation in heterogeneous media with power law absorption using a k-space pseudospectral method. J. Acoust. Soc. Am. 2012, 131, 4324–4336. [Google Scholar] [CrossRef] [PubMed]
- Bossy, E.; Grimal, Q. Numerical methods for ultrasonic bone characterization. In Bone Quantitative Ultrasound; Laugier, P., Haiat, G., Eds.; Springer: New York, NY, USA, 2010; pp. 181–228. [Google Scholar]
- Bossy, E.; Laugier, P.; Peyrin, F.; Padilla, F. Attenuation in trabecular bone: A comparison between numerical simulation and experimental results in human femur. J. Acoust. Soc. Am. 2007, 122, 2469–2475. [Google Scholar] [CrossRef] [PubMed]
- Muller, M; Moilanen, P.; Bossy, E.; Nicholson, P.; Kilappa, V.; Timonen, J.; Talmant, M.; Cheng, S.; Laugier, P. Comparison of three ultrasonic axial transmission methods for bone assessment. Ultrasound Med. Biol. 2005, 31, 633–642. [Google Scholar]
- Dodd, S.P.; Cunningham, J.L.; Miles, A.W.; Gheduzzi, S.; Humphrey, V.F. Ultrasound transmission loss across transverse and oblique bone fractures: An in vitro study. Ultrasound Med. Biol. 2008, 34, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Potsika, V.; Spiridon, I.; Protopappas, V.; et al. Computational study of the influence of callus porosity on ultrasound propagation in healing bones. In Proceedings of 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 3–6 September 2014; pp. 636–638.
- Potsika, V.T.; Grivas, Κ.Ν.; Protopappas, V.C.; Vavva, M.G.; Raum, K.; Rohrbach, D.; Polyzos, D.; Fotiadis, D.I. Application of an effective medium theory for modeling ultrasound wave propagation in healing long bones. Ultrasonics 2014, 54, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, P.; Nicholson, P.H.F.; Kilappa, V.; Cheng, S.; Timonen, J. Assessment of the cortical bone thickness using ultrasonic guided waves: modelling and in vitro study. Ultrasound Med. Biol. 2007, 33, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Raum, K.; Grimal, Q.; Varga, P.; Barkmann, R.; Glüer, C.C.; Laugier, P. Ultrasound to Assess Bone Quality. Curr. Osteoporos. Rep. 2014, 12, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Raum, K.; Leguerney, I.; Chandelier, F.; Talmant, M.; Saïed, A.; Peyrin, F.; Laugier, P. Site-matched assessment of structural and tissue properties of cortical bone using scanning acoustic microscopy and synchrotron radiation μCT. Phys. Med. Biol. 2006, 51, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Potsika, V.T.; Gortsas, T.; Grivas, Κ.Ν.; Protopappas, V.C.; Vavva, M.G.; Raum, K.D.; Polyzos, D.; Fotiadis, D.I. Computational modeling of guided ultrasound wave propagation in healthy and osteoporotic bones. In ΕLΕΜΒΙΟ 6th National Conference, Patras, Greece, 2014.
- Roschger, P.; Misof, B.; Paschalis, E.; Fratzl, P.; Klaushofer, K. Changes in the Degree of Mineralization with Osteoporosis and its Treatment. Curr. Osteoporos. Rep. 2014, 12, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Cortet, B.; Boutry, N.; Dubois, P.; Legroux-Gérot, I.; Cotton, A.; Marchandise, X. Does quantitative ultrasound of bone reflect more bone mineral density than bone microarchitecture. Calcif. Tissue Int. 2004, 74, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Sasso, M; Haïat, G.; Yamato, Y.; Naili, S.; Matsukawa, M. Frequency Dependence of Ultrasonic Attenuation in Bovine Cortical Bone: An In Vitro Study. Ultrasound Med. Biol. 2007, 33, 1933–1942. [Google Scholar]







| Propagation Medium | C11 (GPa) | C12 (GPa) | C66 (GPa) | Ρ (g/cm3) |
|---|---|---|---|---|
| Water | 2.25 | 2.25 | 0 | 1.00 |
| Bone | 28.71 | 10.67 | 9.02 | 1.85 |
| Description | Porosity (%) | No. of Pores | Pores’ Radius (μm) | No. of RL/RLs | Radius of RL/RLs (μm) | |
|---|---|---|---|---|---|---|
| B_Hom | Homogeneous bone | 0 | 0 | – | – | – |
| B_Po5 | Porous bone, normal pores | 5 | 1592 | 40 | – | – |
| B_Po5_RL1 | Porous bone, normal pores and 1 RL | 5 | 1592 | 40 | 1 | 115 |
| B_Po5_RL3 | Porous bone, normal pores and 3 RLs | 5 | 1592 | 40 | 3 | 115 |
| B_Po5_RL5 | Porous bone, normal pores and 5 RLs | 5 | 1592 | 40 | 5 | 115 |
| B_Po10 | Porous bone, normal pores | 10 | 1592 | 60 | – | – |
| B_Po16 | Porous bone, normal pores | 16 | 2263 | 60 | – | – |
| B_Po16_RL | Porous bone, normal pores and RLs | 16 | 1400 | 60 | 235 | 115 |
| B_Po16_Gradual | Gradual distribution of the pores, normal pores and RLs | 16 | 1775 | 40, 60, 80 | 192 | 115 |
| Implanted Transducers | Transducers at a Distance of 2 mm from the Cortical Surface | |||||
|---|---|---|---|---|---|---|
| y = ax + b | R2 | RMSE | y = ax + b | R2 | RMSE | |
| B_Hom | y = 2.27x + 3844 | 0.54 | 16.56 | y = 3.00x + 3815 | 0.52 | 22.79 |
| B_Po5 | y = 0.17x + 3661 | 0.01 | 37.94 | y = 2.81x + 3577 | 0.17 | 48.84 |
| B_Po10 | y = 0.72x + 3475 | 0.01 | 78.51 | y = 0.28x + 3489 | 0.01 | 66.92 |
| B_Po16 | y = −4.37x + 3417 | 0.09 | 110.08 | y = −3.64x + 3385 | 0.53 | 24.58 |
| B_Po16_RL | y = −4.09x + 3434 | 0.06 | 123.45 | y = −3.23x + 3410 | 0.06 | 92.93 |
| B_Po16_Gradual | y = 1.38x + 3409 | 0.02 | 75.88 | y = 1.57x + 3397 | 0.03 | 59.72 |
| Implanted Transducers | Transducers at a Distance of 2 mm from the Cortical Surface | |||||
|---|---|---|---|---|---|---|
| y = ax + b | R2 | RMSE | y = ax + b | R2 | RMSE | |
| B_Hom | y = 1.08x + 3898 | 0.80 | 4.26 | y = 1.65x + 3879 | 0.80 | 6.37 |
| B_Po5 | y = −1.41x + 3748 | 0.28 | 17.82 | y = −1.81x + 3759 | 0.19 | 29.16 |
| B_Po10 | y = −1.54x + 3639 | 0.02 | 93.23 | y = −1.78x + 3628 | 0.06 | 57.31 |
| B_Po16 | y = −1.36x + 3549 | 0.01 | 100.58 | y = −0.06x + 3510 | 0.01 | 30.84 |
| B_Po16_RL | y = −5.38x +3 702 | 0.13 | 111.10 | y = −3.21x + 3647 | 0.28 | 36.73 |
| B_Po16_Gradual | y = 3.96x + 3440 | 0.15 | 72.96 | y = 4.49x + 3406 | 0.16 | 73.61 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
T. Potsika, V.; N. Grivas, K.; Gortsas, T.; Iori, G.; C. Protopappas, V.; Raum, K.; Polyzos, D.; I. Fotiadis, D. Computational Study of the Effect of Cortical Porosity on Ultrasound Wave Propagation in Healthy and Osteoporotic Long Bones. Materials 2016, 9, 205. https://doi.org/10.3390/ma9030205
T. Potsika V, N. Grivas K, Gortsas T, Iori G, C. Protopappas V, Raum K, Polyzos D, I. Fotiadis D. Computational Study of the Effect of Cortical Porosity on Ultrasound Wave Propagation in Healthy and Osteoporotic Long Bones. Materials. 2016; 9(3):205. https://doi.org/10.3390/ma9030205
Chicago/Turabian StyleT. Potsika, Vassiliki, Konstantinos N. Grivas, Theodoros Gortsas, Gianluca Iori, Vasilios C. Protopappas, Kay Raum, Demosthenes Polyzos, and Dimitrios I. Fotiadis. 2016. "Computational Study of the Effect of Cortical Porosity on Ultrasound Wave Propagation in Healthy and Osteoporotic Long Bones" Materials 9, no. 3: 205. https://doi.org/10.3390/ma9030205
APA StyleT. Potsika, V., N. Grivas, K., Gortsas, T., Iori, G., C. Protopappas, V., Raum, K., Polyzos, D., & I. Fotiadis, D. (2016). Computational Study of the Effect of Cortical Porosity on Ultrasound Wave Propagation in Healthy and Osteoporotic Long Bones. Materials, 9(3), 205. https://doi.org/10.3390/ma9030205








