Study of Polydiacetylene-Poly (Ethylene Oxide) Electrospun Fibers Used as Biosensors
Abstract
:1. Introduction
2. Results and Discussion
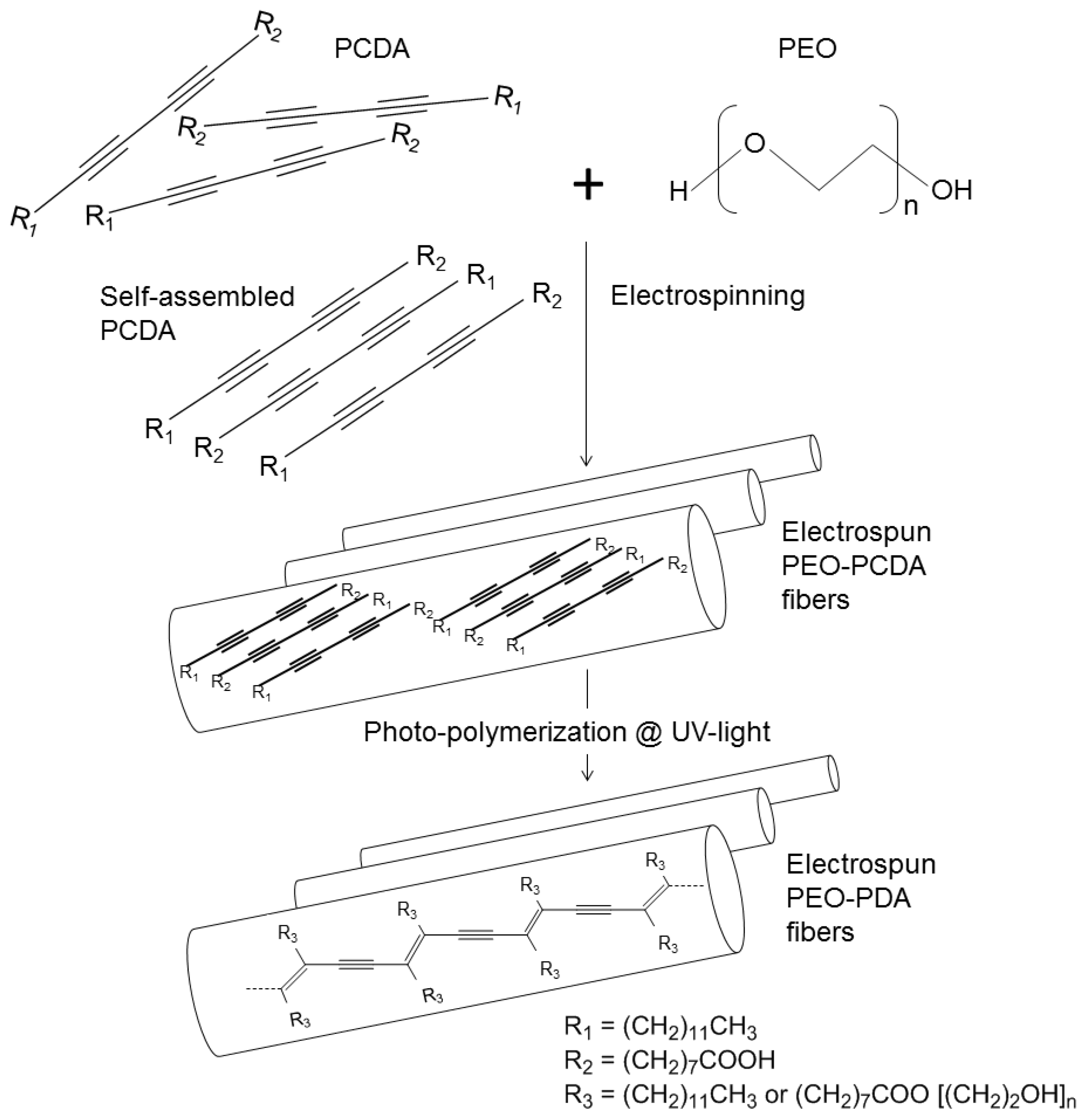
2.1. Preparation of PEO-PDA Fibers
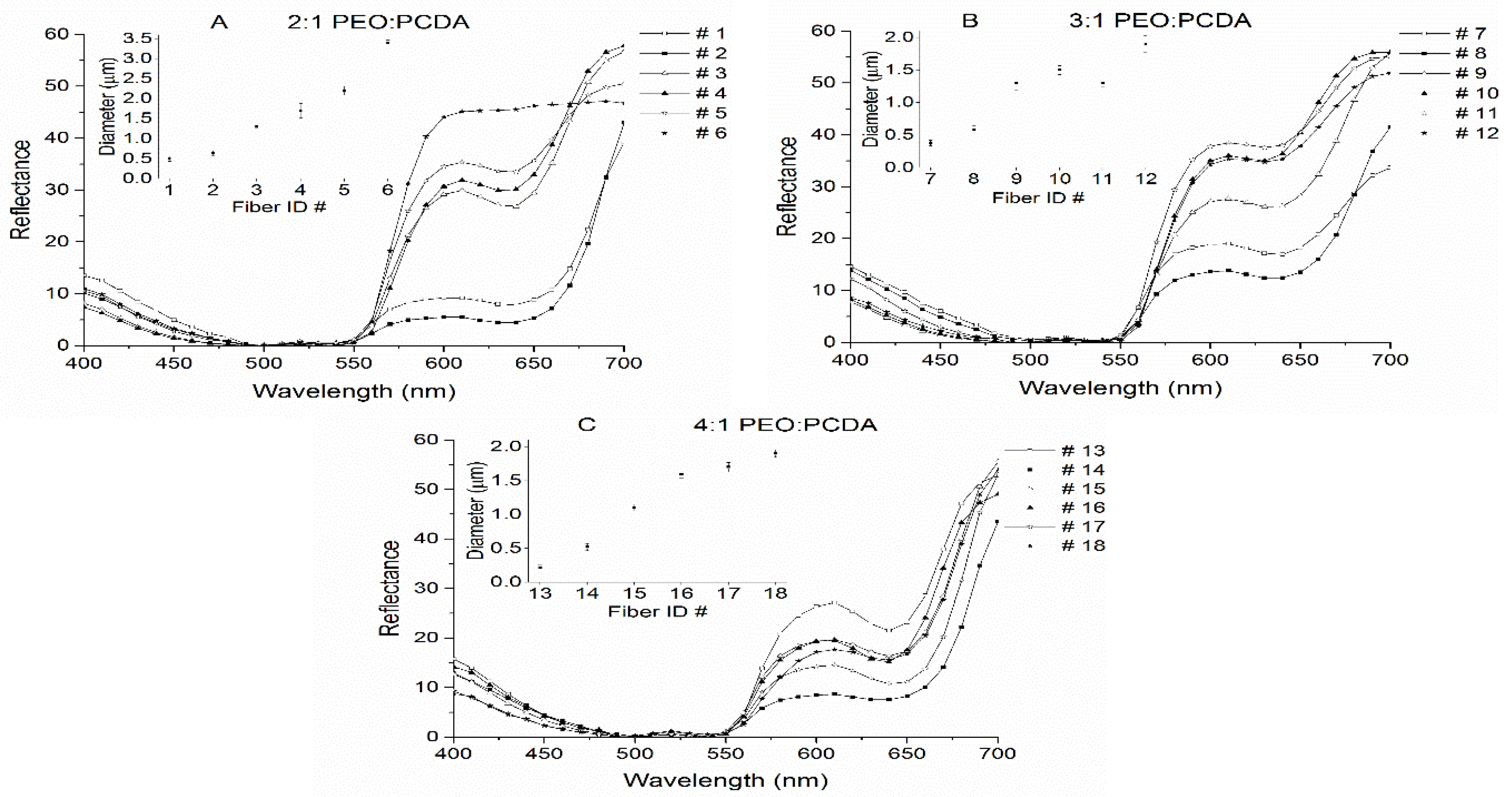
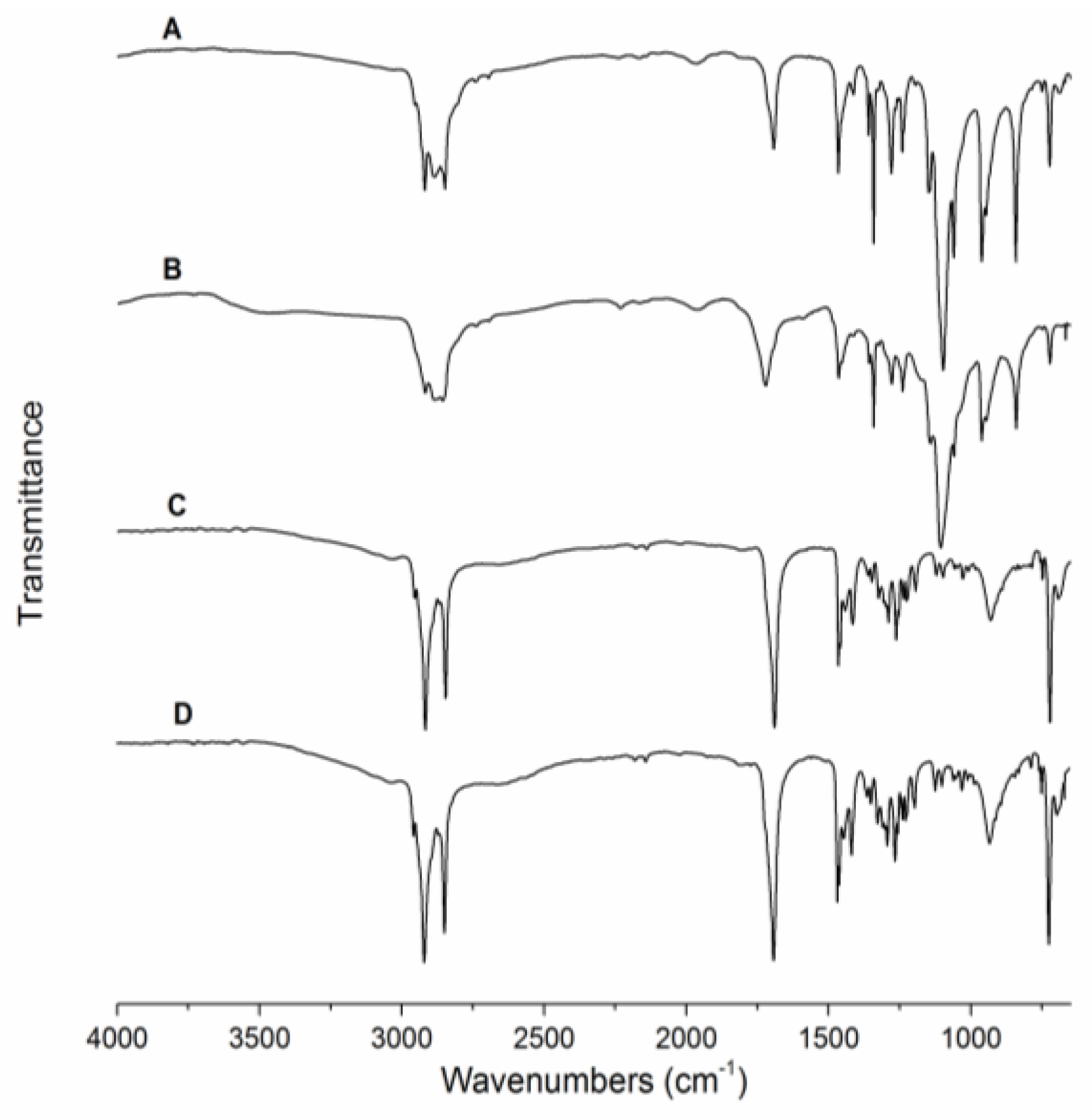
2.2. Characterization of Fiber Morphology
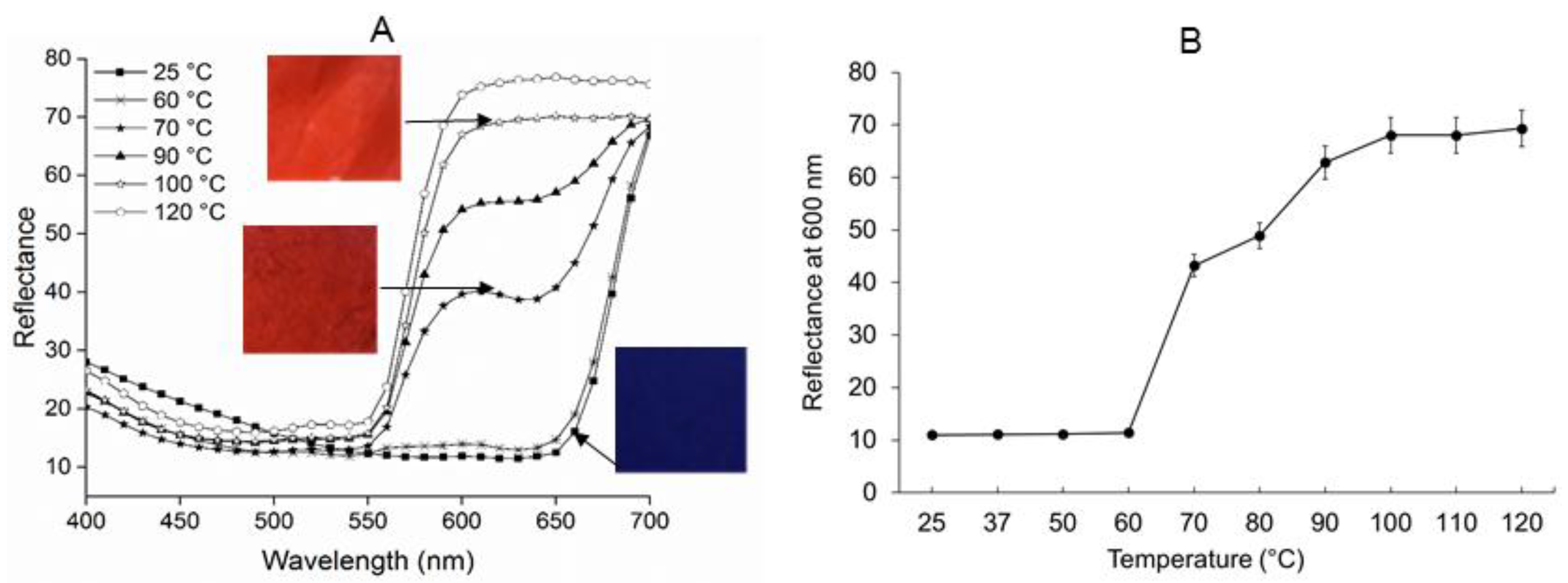
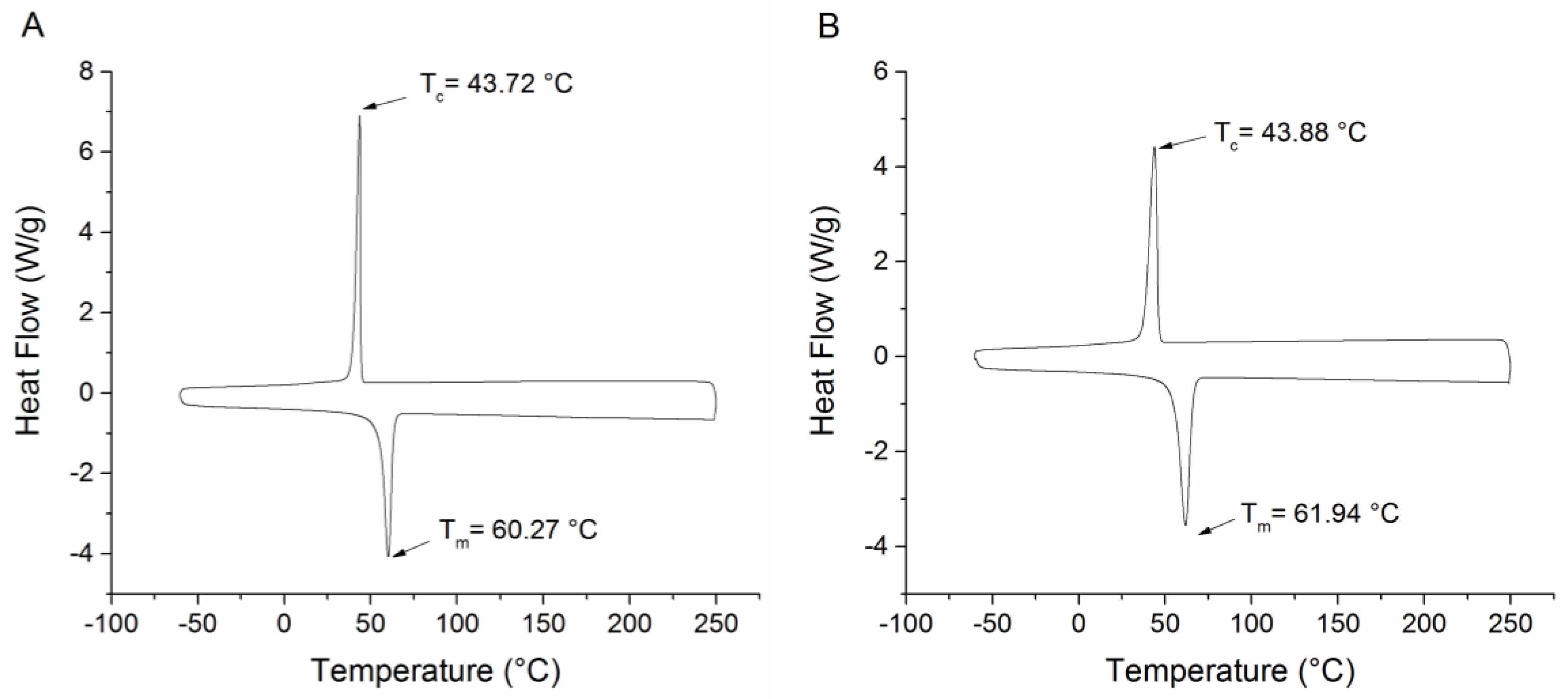
2.3. Temperature Sensitive Properties of PEO-PDA Fibers
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of PDA
3.2.2. Electrospinning of PEO-PDA Fibers
3.2.3. Fiber Characterization
3.2.4. Colorimetric Transition Analysis of the PEO-PDA Fibers Due to Temperature Change
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PCDA | 10, 12-Pentacosadiynoic acid |
| PDA | Polydiacetylene |
| PEO | Poly(ethylene oxide) |
| UV | Ultra-violet |
| SEM | Scanning electron microscope |
| DSC | Differential scanning calorimetry |
| FTIR | Fourier transform infrared spectroscopy |
| NMR | Nuclear magnetic resonance |
| VOC | Volatile organic compound |
| PPM | Parts per million |
| PMMA | Poly(methyl methacrylate) |
| TEOS | Tetraethyl orthosilicate |
| PS | Polystyrene |
References
- D’souza, S. Microbial biosensors. Biosens. Bioelectron. 2001, 16, 337–353. [Google Scholar] [CrossRef]
- Radke, S.M.; Alocilja, E.C. A high density microelectrode array biosensor for detection of E. Colio 157: H7. Biosens. Bioelectron. 2005, 20, 1662–1667. [Google Scholar] [CrossRef] [PubMed]
- Thusu, R. Strong growth predicted for biosensors market. Sensors 2010. [Google Scholar]
- Dargaville, T.R.; Farrugia, B.L.; Broadbent, J.A.; Pace, S.; Upton, Z.; Voelcker, N.H. Sensors and imaging for wound healing: A review. Biosens. Bioelectron. 2013, 41, 30–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velusamy, V.; Arshak, K.; Korostynska, O.; Oliwa, K.; Adley, C. An overview of foodborne pathogen detection: In the perspective of biosensors. Biotech. Adv. 2010, 28, 232–254. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Biswas, A.; Choi, K.; Pal, U. Methods for rapid detection of foodborne pathogens: An overview. Am. J. Food Technol. 2011, 6, 87–102. [Google Scholar] [CrossRef]
- Jelinek, R.; Ritenberg, M. Polydiacetylenes–recent molecular advances and applications. RSC Adv. 2013, 3, 21192–21201. [Google Scholar] [CrossRef]
- Sun, X.; Chen, T.; Huang, S.; Li, L.; Peng, H. Chromatic polydiacetylene with novel sensitivity. Chem. Soc. Rev. 2010, 39, 4244–4257. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Beck, C.; Ying, Y.; Federici, J.; Iqbal, Z. Thermochromism in polydiacetylene–zno nanocomposites. J. Phys. Chem. C 2013, 117, 19593–19600. [Google Scholar] [CrossRef]
- Pindzola, B.A.; Nguyen, A.T.; Reppy, M.A. Antibody-functionalized polydiacetylene coatings on nanoporous membranes for microorganism detection. Chem. Commun. 2006, 8, 906–908. [Google Scholar] [CrossRef] [PubMed]
- Kolusheva, S.; Kafri, R.; Katz, M.; Jelinek, R. Rapid colorimetric detection of antibody-epitope recognition at a biomimetic membrane interface. J. Am. Chem. Soc. 2001, 123, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Charych, D.H.; Nagy, J.O.; Spevak, W.; Bednarski, M.D. Direct colorimetric detection of a receptor-ligand interaction by a polymerized bilayer assembly. Science 1993, 261, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Lee, J.-S.; Choi, H.; Sohn, D.; Ahn, D.J. Rational design and in-situ ftir analyses of colorimetrically reversibe polydiacetylene supramolecules. Macromolecules 2005, 38, 9366–9376. [Google Scholar] [CrossRef]
- Lee, J.; Yarimaga, O.; Lee, C.H.; Choi, Y.K.; Kim, J.M. Network polydiacetylene films: Preparation, patterning, and sensor applications. Adv. Funct. Mater. 2011, 21, 1032–1039. [Google Scholar] [CrossRef]
- Mino, N.; Tamura, H.; Ogawa, K. Analysis of color transitions and changes on langmuir-blodgett films of a polydiacetylene derivative. Langmuir 1991, 7, 2336–2341. [Google Scholar] [CrossRef]
- Chance, R.; Baughman, R.; Müller, H.; Eckhardt, C.J. Thermochromism in a polydiacetylene crystal. J. Chem. Phys. 1977, 67, 3616–3618. [Google Scholar] [CrossRef]
- Chae, S.K.; Park, H.; Yoon, J.; Lee, C.H.; Ahn, D.J.; Kim, J.M. Polydiacetylene supramolecules in electrospun microfibers: Fabrication, micropatterning, and sensor applications. Adv. Mater. 2007, 19, 521. [Google Scholar] [CrossRef]
- Yoon, J.; Kim, J.M. Fabrication of conjugated polymer supramolecules in electrospun micro/nanofibers. Macromol. Chem. Phys. 2008, 209, 2194–2203. [Google Scholar] [CrossRef]
- Wu, J.; Lu, X.; Shan, F.; Guan, J.; Lu, Q. Polydiacetylene-embedded supramolecular electrospun fibres for a colourimetric sensor of organic amine vapour. RSC Adv. 2013, 3, 22841–22844. [Google Scholar] [CrossRef]
- Jeon, H.; Lee, J.; Kim, M.H.; Yoon, J. Polydiacetylene-based electrospun fibers for detection of HCl gas. Macromol. Rapid Commun. 2012, 33, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Steyaert, I.; Rahier, H.; De Clerck, K. Nanofibre-based sensors for visual and optical monitoring. In Electrospinning for high performance sensors; Springer: New York, NY, USA, 2015; pp. 157–177. [Google Scholar]
- Yoon, J.; Chae, S.K.; Kim, J.M. Colorimetric sensors for volatile organic compounds (vocs) based on conjugated polymer-embedded electrospun fibers. J. Am. Chem. Soc. 2007, 129, 3038–3039. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, S.L.; Bates, W.D.; Frisch, H.L.; Wnek, G.E. Role of chain entanglements on fiber formation during electrospinning of polymer solutions: Good solvent, non-specific polymer–polymer interaction limit. Polymer 2005, 46, 3372–3384. [Google Scholar] [CrossRef]
- Edwards-Jones, V. (Ed.) Essential Microbiology for Wound Care, 1st ed.; Oxford University Press: Oxford, UK, 2016.
- McKee, M.G.; Wilkes, G.L.; Colby, R.H.; Long, T.E. Correlations of solution rheology with electrospun fiber formation of linear and branched polyesters. Macromolecules 2004, 37, 1760–1767. [Google Scholar] [CrossRef]
- Nie, H.; He, A.; Wu, W.; Zheng, J.; Xu, S.; Li, J.; Han, C.C. Effect of poly (ethylene oxide) with different molecular weights on the electrospinnability of sodium alginate. Polymer 2009, 50, 4926–4934. [Google Scholar] [CrossRef]
- Sharma, S.; Khawaja, M.; Ram, M.K.; Goswami, D.Y.; Stefanakos, E. Characterization of 10, 12-pentacosadiynoic acid langmuir–blodgett monolayers and their use in metal–insulator–metal tunnel devices. Beilstein J. Nanotechnol. 2014, 5, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Sukwattanasinitt, M.; Lee, D.C.; Kim, M.; Wang, X.; Li, L.; Yang, K.; Kumar, J.; Tripathy, S.K.; Sandman, D.J. New processable, functionalizable polydiacetylenes. Macromolecules 1999, 32, 7361–7369. [Google Scholar] [CrossRef]
- Scoville, S.; Shirley, W. Investigations of chromatic transformations of polydiacetylene with aromatic compounds. J. Appl. Polym. Sci. 2011, 120, 2809–2820. [Google Scholar] [CrossRef]
- Ahn, D.J.; Chae, E.H.; Lee, G.S.; Shim, H.Y.; Chang, T.E.; Ahn, K.D.; Kim, J.M. Colorimetric reversibility of polydiacetylene supramolecules having enhanced hydrogen-bonding under thermal and ph stimuli. J. Am. Chem. Soc. 2003, 125, 8976–8977. [Google Scholar] [CrossRef] [PubMed]
- Luongo, J. Infrared study of oxygenated groups formed in polyethylene during oxidation. J. Polym. Sci. 1960, 42, 139–150. [Google Scholar] [CrossRef]
- Chance, R.; Patel, G.; Witt, J. Thermal effects on the optical properties of single crystals and solution-cast films of urethane substituted polydiacetylenes. J. Chem. Phys. 1979, 71, 206–211. [Google Scholar] [CrossRef]
- Champaiboon, T.; Tumcharern, G.; Potisatityuenyong, A.; Wacharasindhu, S.; Sukwattanasinitt, M. A polydiacetylene multilayer film for naked eye detection of aromatic compounds. Sensor Actuat. B-Chem. 2009, 139, 532–537. [Google Scholar] [CrossRef]
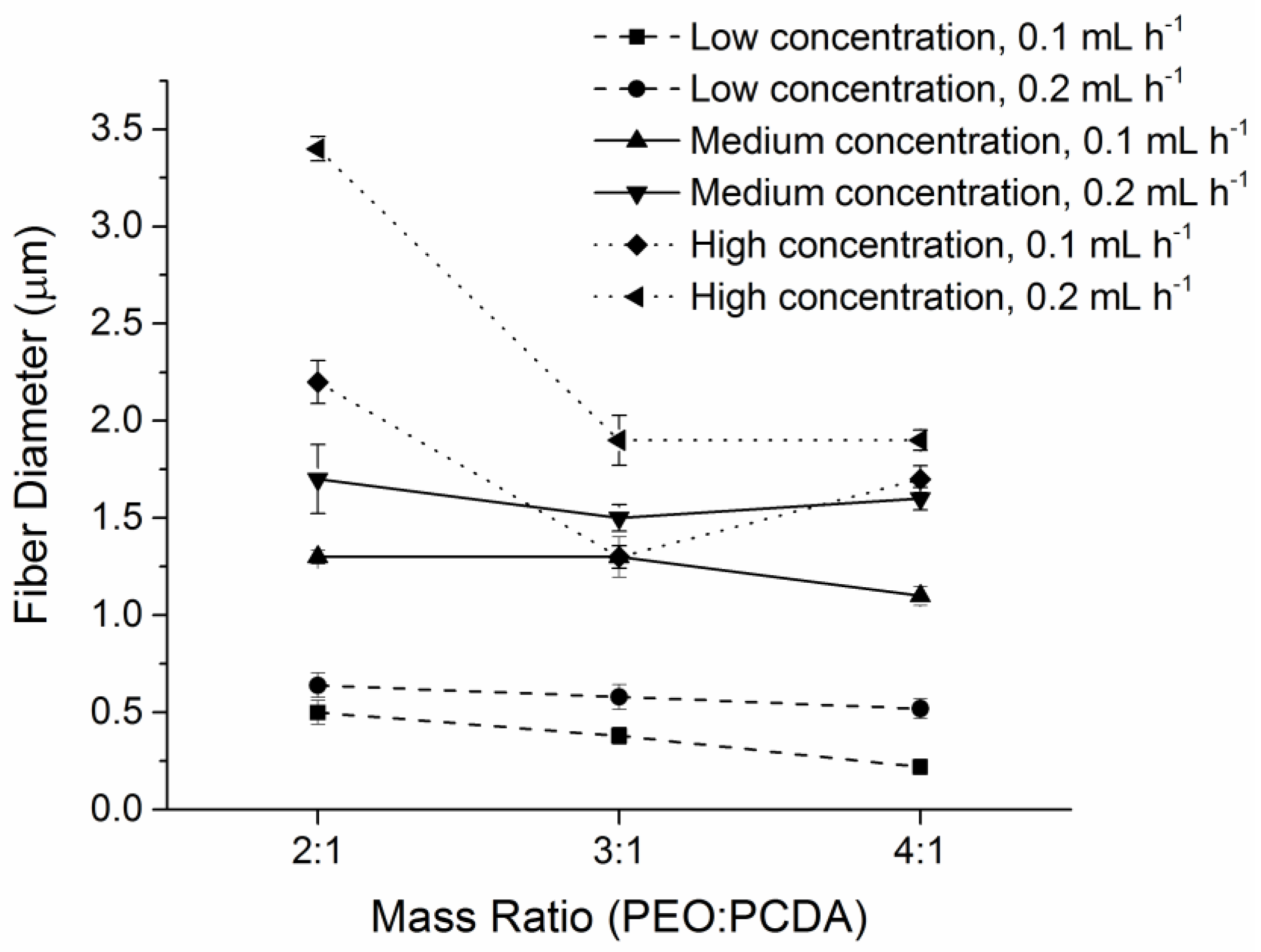
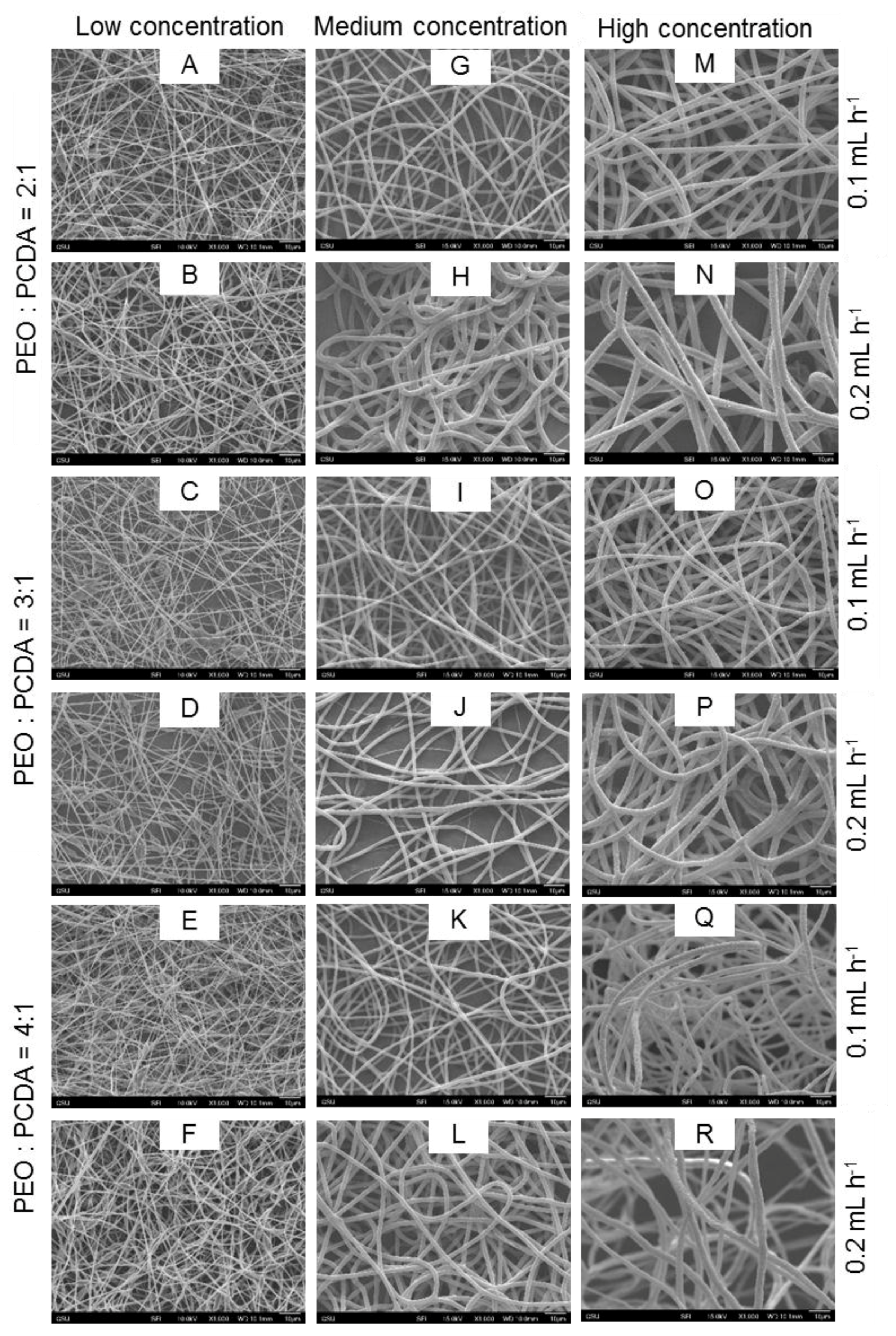

| PEO:PCDA (w/w) | Polymer Concentration (wt. %) | Injection Speed (mL·h−1) | Fiber Identification (ID) Number |
|---|---|---|---|
| 2:1 | 1.5 | 0.1 | #1 |
| 0.2 | #2 | ||
| 3.75 | 0.1 | #3 | |
| 0.2 | #4 | ||
| 7.5 | 0.1 | #5 | |
| 0.2 | #6 | ||
| 3:1 | 1.33 | 0.1 | #7 |
| 0.2 | #8 | ||
| 3.34 | 0.1 | #9 | |
| 0.2 | #10 | ||
| 6.67 | 0.1 | #11 | |
| 0.2 | #12 | ||
| 4:1 | 1.25 | 0.1 | #13 |
| 0.2 | #14 | ||
| 3.13 | 0.1 | #15 | |
| 0.2 | #16 | ||
| 6.25 | 0.1 | #17 | |
| 0.2 | #18 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, A.K.M.M.; Yapor, J.P.; Reynolds, M.M.; Li, Y.V. Study of Polydiacetylene-Poly (Ethylene Oxide) Electrospun Fibers Used as Biosensors. Materials 2016, 9, 202. https://doi.org/10.3390/ma9030202
Alam AKMM, Yapor JP, Reynolds MM, Li YV. Study of Polydiacetylene-Poly (Ethylene Oxide) Electrospun Fibers Used as Biosensors. Materials. 2016; 9(3):202. https://doi.org/10.3390/ma9030202
Chicago/Turabian StyleAlam, A K M Mashud, Janet P. Yapor, Melissa M. Reynolds, and Yan Vivian Li. 2016. "Study of Polydiacetylene-Poly (Ethylene Oxide) Electrospun Fibers Used as Biosensors" Materials 9, no. 3: 202. https://doi.org/10.3390/ma9030202






