Nanotextured Shrink Wrap Superhydrophobic Surfaces by Argon Plasma Etching
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of SH Surfaces via Ar Gas Plasma Treatment
2.2. Surface Characterization
2.3. Contact Angle and Contact Angle Hysteresis
2.4. Hydrophilic Patterning
2.5. Protein Capture and Detection
2.6. Microfluidic Wicking in Superhydrophilic Channels
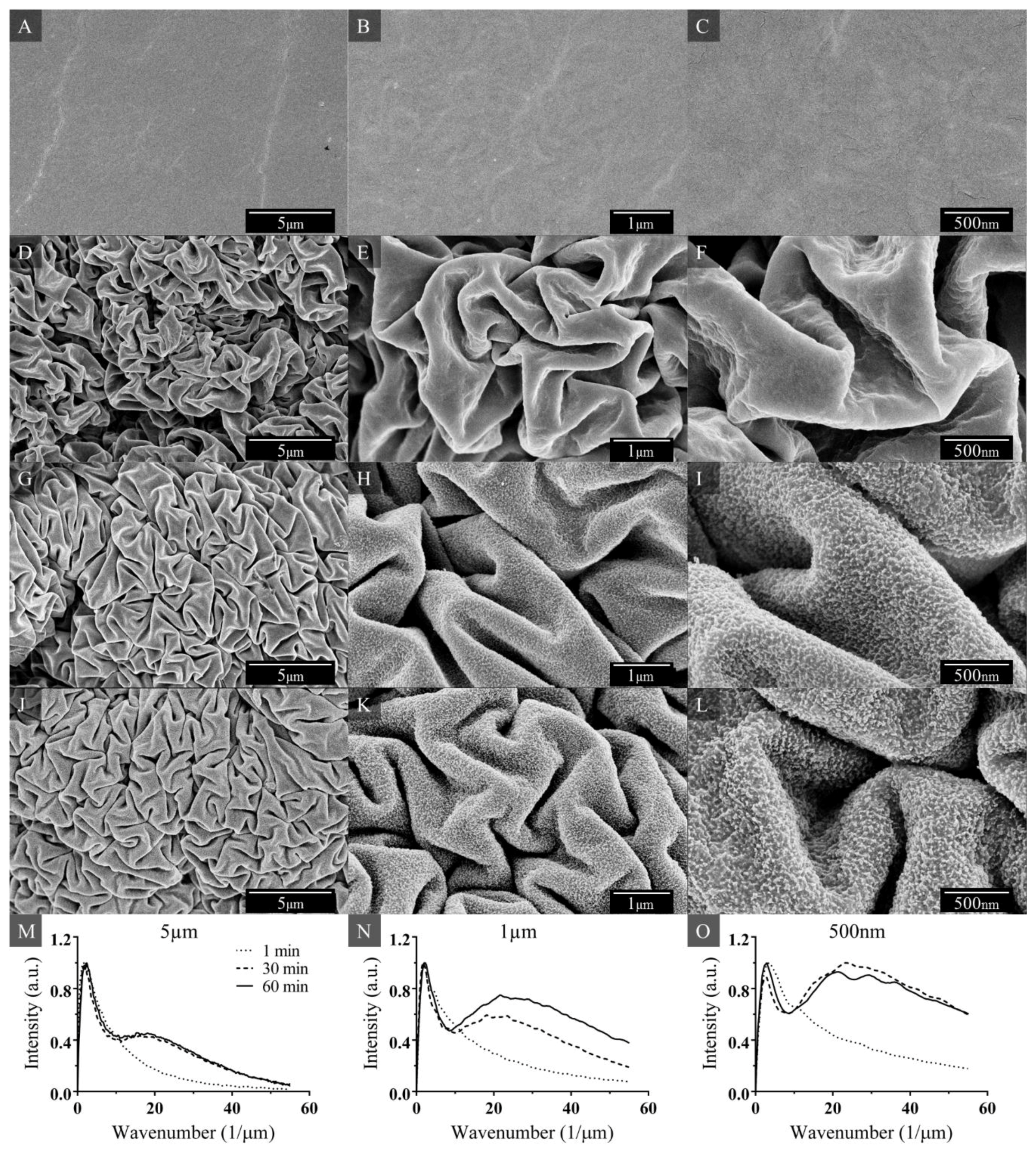
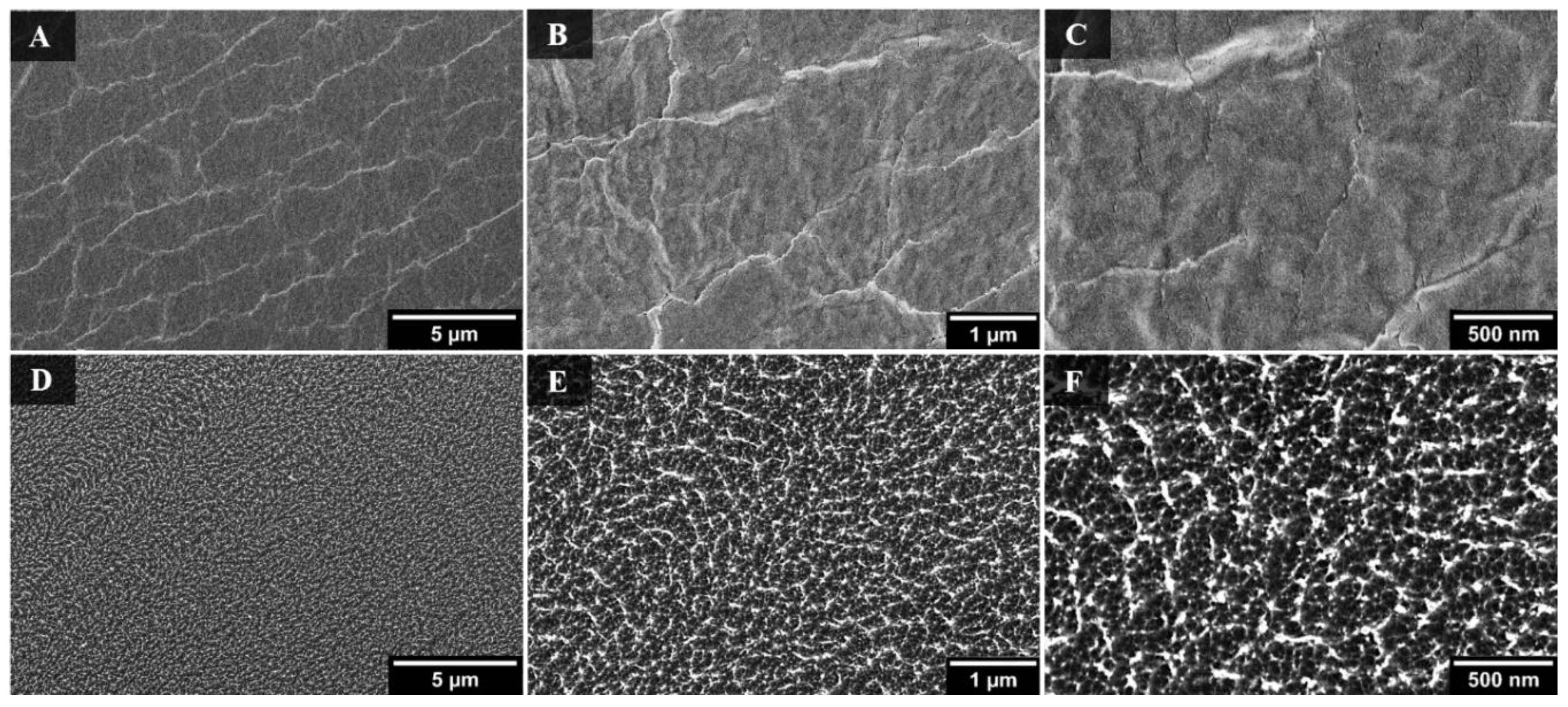
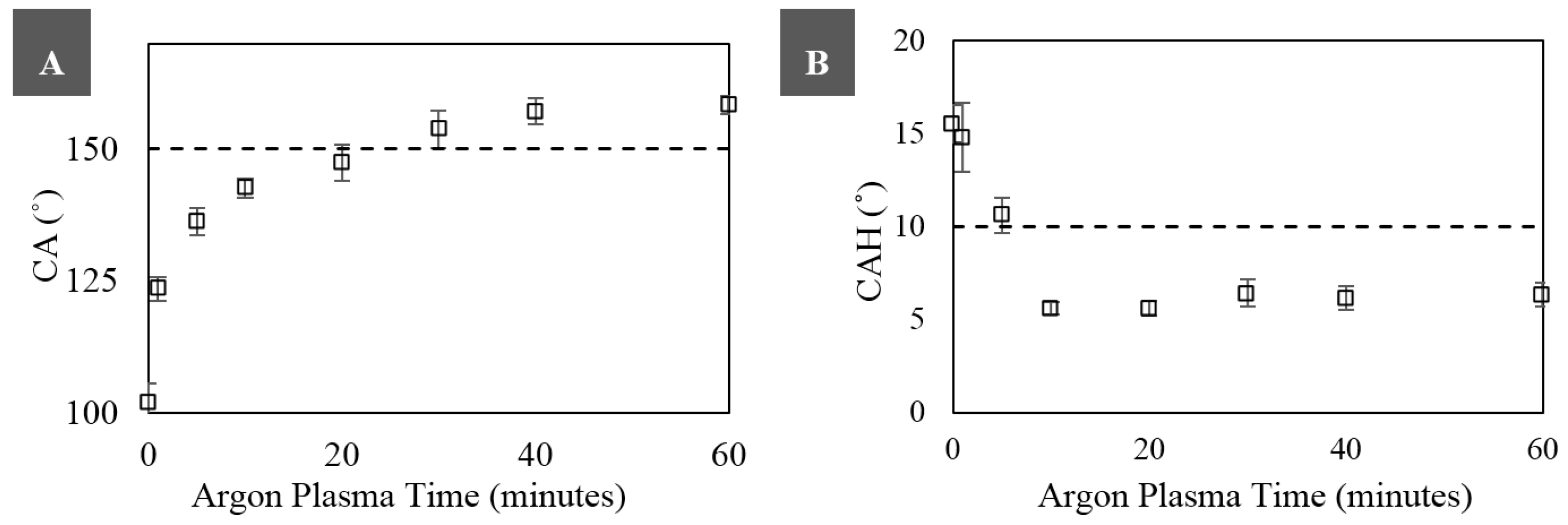
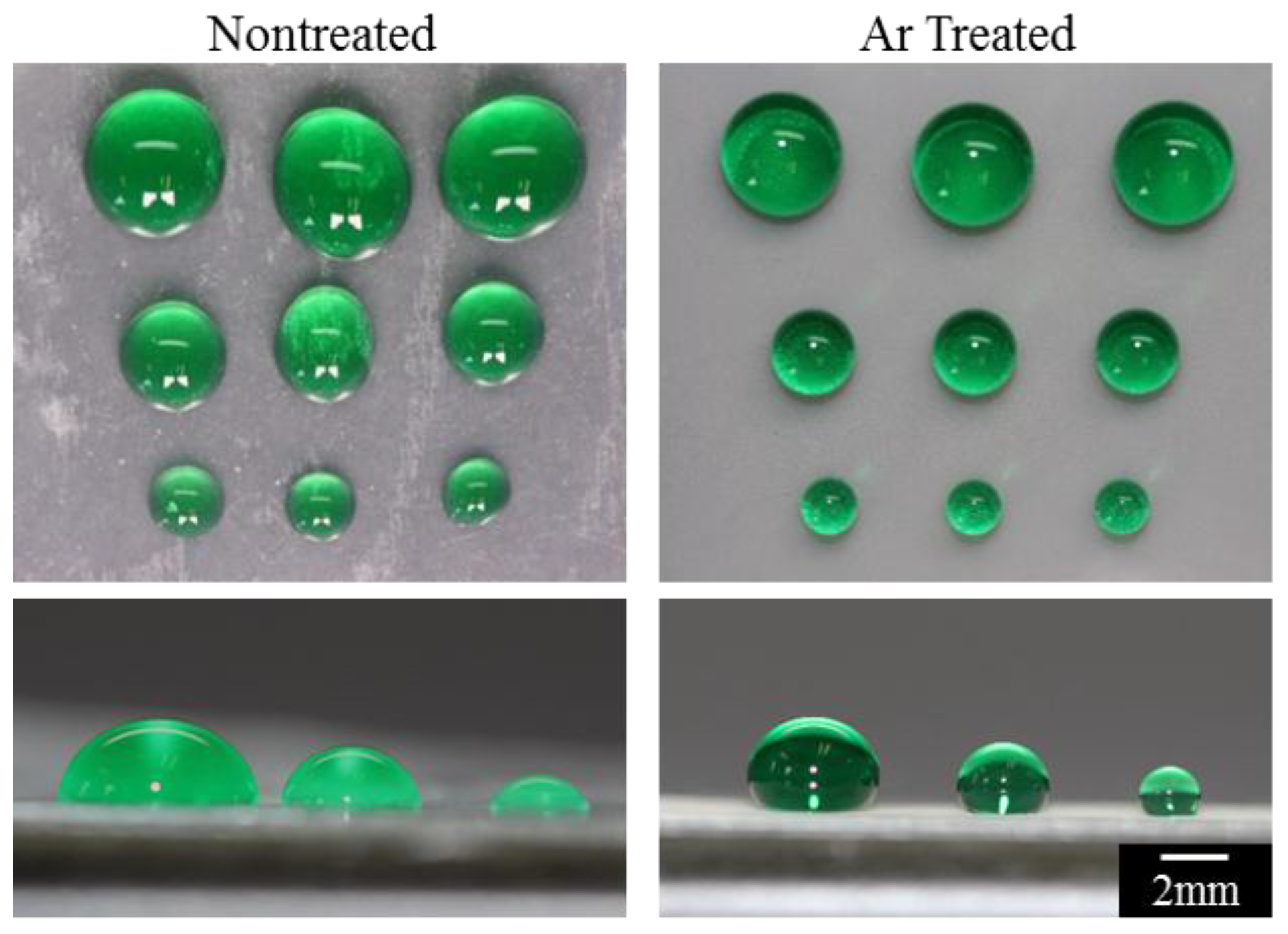
3. Results and Discussion
3.1. Nanotextured Superhydrophobic Wrinkle Topology
3.2. Superhydrophobicity
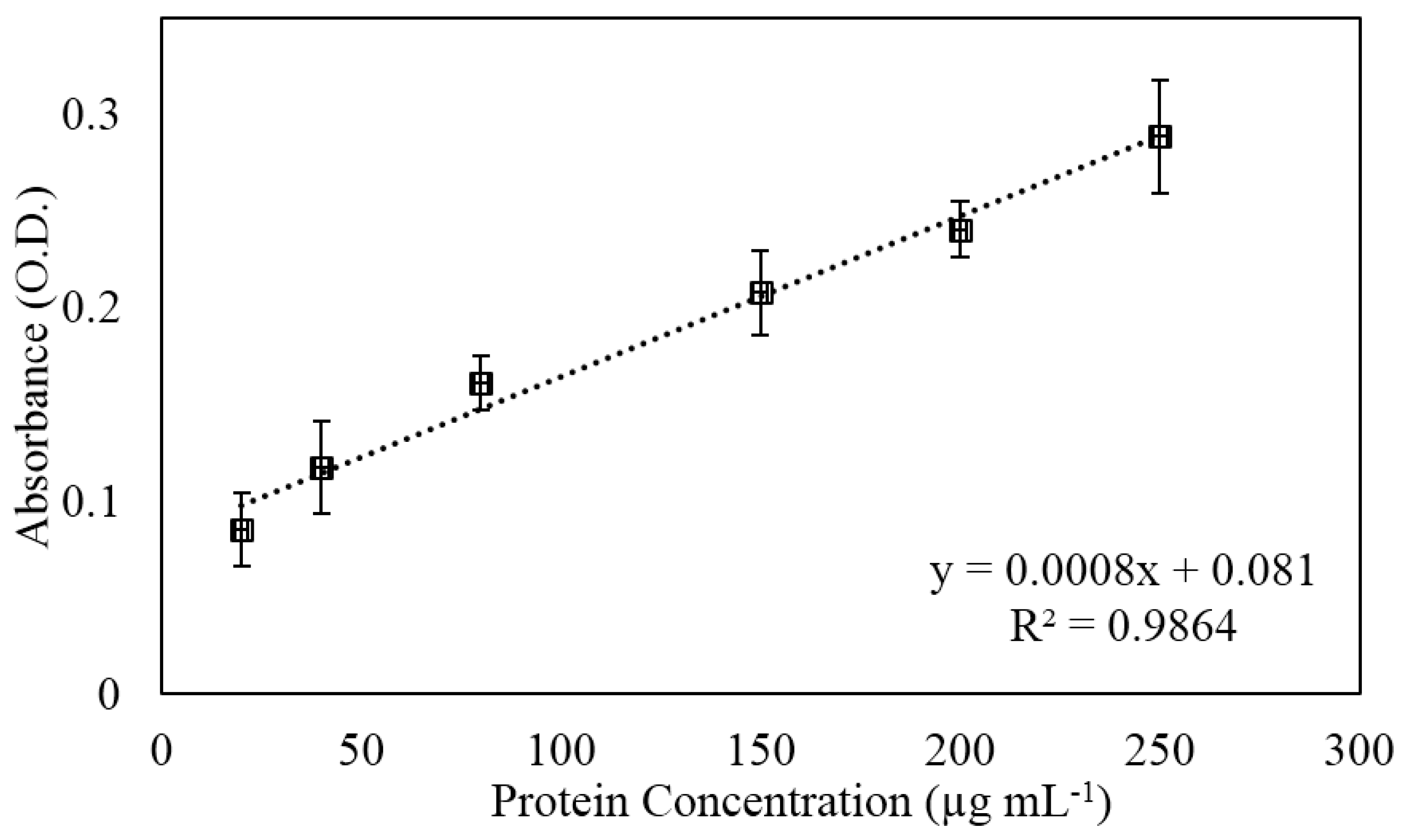
3.3. Protein Capture and Detection
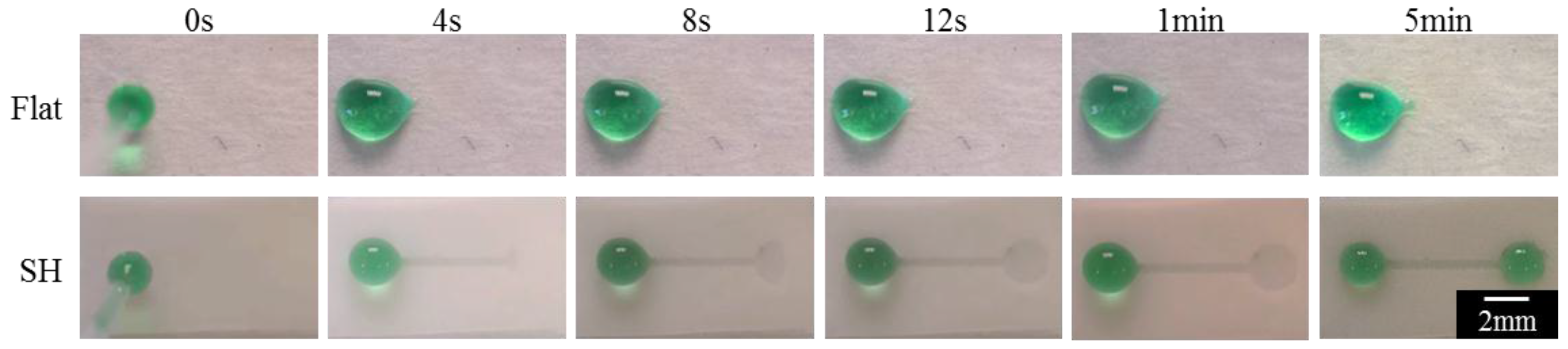
3.4. Open-Channel Microfluidic Wicking
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhai, L.; Berg, M.C.; Cebeci, F.C.; Kim, Y.; Milwid, J.M.; Rubner, M.F.; Cohen, R.E. Patterned superhydrophobic surfaces: Toward a synthetic mimic of the namib desert beetle. Nano Lett. 2006, 6, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.T.; Rodak, D.; Wong, C.; Hayden, C. Effects of micro- and nano-structures on the self-cleaning behaviour of lotus leaves. Nanotechnology 2006, 17. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Si, Y.; Guo, Z. Superhydrophobic nanocoatings: From materials to fabrications and to applications. Nanoscale 2015, 7, 5922–5946. [Google Scholar] [CrossRef] [PubMed]
- Darmanin, T.; Guittard, F. Recent advances in the potential applications of bioinspired superhydrophobic materials. J. Mater. Chem. A 2014, 2, 16319–16359. [Google Scholar] [CrossRef]
- Dong, H.; Cheng, M.; Zhang, Y.; Wei, H.; Shi, F. Extraordinary drag-reducing effect of a superhydrophobic coating on a macroscopic model ship at high speed. J. Mater. Chem. A 2013, 1, 5886–5891. [Google Scholar] [CrossRef]
- McLane, J.; Wu, C.; Khine, M. Enhanced detection of protein in urine by droplet evaporation on a superhydrophobic plastic. Adv. Mater. Interfaces 2015, 2. [Google Scholar] [CrossRef]
- Yao, L.; He, J. Recent progress in antireflection and self-cleaning technology–From surface engineering to functional surfaces. Prog. Mater. Sci. 2014, 61, 94–143. [Google Scholar] [CrossRef]
- Bruce, R.L.; Engelmann, S.; Lin, T.; Kwon, T.; Phaneuf, R.J.; Oehrlein, G.S.; Long, B.K.; Willson, C.G.; Végh, J.J.; Nest, D. Study of ion and vacuum ultraviolet-induced effects on styrene-and ester-based polymers exposed to argon plasma. J. Vacuum Sci. Technol. B 2009, 27, 1142–1155. [Google Scholar] [CrossRef]
- Nokes, J.M. Roll-to-Roll, Shrink-Induced Superhydrophobic Surfaces for Antibacterial Applications, Enhanced Point-of-Care Detection, and Blood Anticoagulation. Ph.D. Thesis, University of California, Irvine, Irvine, CA, USA, 4 September 2015. [Google Scholar]
- Shiu, J.-Y.; Kuo, C.-W.; Chen, P. Fabrication of tunable superhydrophobic surfaces. Proc. SPIE 2004. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- McHale, G.; Shirtcliffe, N.; Newton, M. Contact-angle hysteresis on super-hydrophobic surfaces. Langmuir 2004, 20, 10146–10149. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.F.; Wang, J.N.; Sanderson, K.D. Organic-inorganic composite nanocoatings with superhydrophobicity, good transparency, and thermal stability. ACS Nano 2010, 4, 2201–2209. [Google Scholar] [CrossRef] [PubMed]
- Erbil, H.Y.; Demirel, A.L.; Avcı, Y.; Mert, O. Transformation of a simple plastic into a superhydrophobic surface. Science 2003, 299, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Gomez, G.; Flendrig, L.M.; Cooper, J.M. Hysteresis and reversibility of a superhydrophobic photopatternable silicone elastomer. Langmuir 2010, 26, 7248–7253. [Google Scholar] [CrossRef] [PubMed]
- Öner, D.; McCarthy, T.J. Ultrahydrophobic surfaces. Effects of topography length scales on wettability. Langmuir 2000, 16, 7777–7782. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Pan, L.; Yang, M.; Peng, L.; Zong, S.; Shi, Y.; Yu, G. Multifunctional superhydrophobic surfaces templated from innately microstructured hydrogel matrix. Nano Lett. 2014, 14, 4803–4809. [Google Scholar] [CrossRef] [PubMed]
- Nosonovsky, M. Multiscale roughness and stability of superhydrophobic biomimetic interfaces. Langmuir 2007, 23, 3157–3161. [Google Scholar] [CrossRef] [PubMed]
- Jokinen, V.; Sainiemi, L.; Franssila, S. Complex droplets on chemically modified silicon nanograss. Adv. Mater. 2008, 20, 3453–3456. [Google Scholar] [CrossRef]
- Guo, S.S.; Sun, M.H.; Shi, J.; Liu, Y.J.; Huang, W.H.; Combellas, C.; Chen, Y. Patterning of hydrophilic micro arrays with superhydrophobic surrounding zones. Microelectron. Eng. 2007, 84, 1673–1676. [Google Scholar] [CrossRef]
- Cortese, B.; D’Amone, S.; Manca, M.; Viola, I.; Cingolani, R.; Gigli, G. Superhydrophobicity due to the hierarchical scale roughness of pdms surfaces. Langmuir 2008, 24, 2712–2718. [Google Scholar] [CrossRef] [PubMed]
- Genzer, J.; Efimenko, K. Creating long-lived superhydrophobic polymer surfaces through mechanically assembled monolayers. Science 2000, 290, 2130–2133. [Google Scholar] [CrossRef] [PubMed]
- Privett, B.J.; Youn, J.; Hong, S.A.; Lee, J.; Han, J.; Shin, J.H.; Schoenfisch, M.H. Antibacterial fluorinated silica colloid superhydrophobic surfaces. Langmuir 2011, 27, 9597–9601. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xia, J.; Lei, W.; Wang, B. Superhydrophobic surface based on a coral-like hierarchical structure of zno. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Raichur, A.M.; Basu, M.J. Adsorption of fluoride onto mixed rare earth oxides. Sep. Purif. Technol. 2001, 24, 121–127. [Google Scholar] [CrossRef]
- Jayadev, S.; Pegan, J.; Dyer, D.; McLane, J.; Lim, J.; Khine, M. Adaptive wettability-enhanced surfaces ordered on molded etched substrates using shrink film. Smart Mater. Struct. 2013, 22. [Google Scholar] [CrossRef]
- Fu, C.C.; Grimes, A.; Long, M.; Ferri, C.G.; Rich, B.D.; Ghosh, S.; Ghosh, S.; Lee, L.P.; Gopinathan, A.; Khine, M. Tunable nanowrinkles on shape memory polymer sheets. Adv. Mater. 2009, 21, 4472–4476. [Google Scholar] [CrossRef]
- Huntington, M.D.; Engel, C.J.; Hryn, A.J.; Odom, T.W. Polymer nanowrinkles with continuously tunable wavelengths. ACS Appl. Mater. Interfaces 2013, 5, 6438–6442. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; McCarthy, T.J. Contact angle hysteresis explained. Langmuir 2006, 22, 6234–6237. [Google Scholar] [CrossRef] [PubMed]
- France, R.; Short, R. Plasma treatment of polymers effects of energy transfer from an argon plasma on the surface chemistry of poly (styrene), low density poly (ethylene), poly (propylene) and poly (ethylene terephthalate). J. Chem. Soc. Faraday Trans. 1997, 93, 3173–3178. [Google Scholar] [CrossRef]
- Guruvenket, S.; Rao, G.M.; Komath, M.; Raichur, A.M. Plasma surface modification of polystyrene and polyethylene. Appl. Surf. Sci. 2004, 236, 278–284. [Google Scholar] [CrossRef]
- Efimenko, K.; Rackaitis, M.; Manias, E.; Vaziri, A.; Mahadevan, L.; Genzer, J. Nested self-similar wrinkling patterns in skins. Nat. Mater. 2005, 4, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-K.; Engel, C.J.; Huntington, M.D.; Hu, J.; Odom, T.W. Controlled three-dimensional hierarchical structuring by memory-based, sequential wrinkling. Nano Lett. 2015, 15, 5624–5629. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, H.; Maaranen, J.; Suvanto, M.; Pakkanen, T. Solvent-assisted and thermal wrinklings of argon plasma treated polystyrene coatings on silicon substrate. Surf. Coat. Technol. 2014, 238, 133–138. [Google Scholar] [CrossRef]
- Bruce, R.L.; Weilnboeck, F.; Lin, T.; Phaneuf, R.J.; Oehrlein, G.S.; Long, B.K.; Willson, C.G.; Vegh, J.J.; Nest, D.; Graves, D.B. Relationship between nanoscale roughness and ion-damaged layer in argon plasma exposed polystyrene films. J. Appl. Phys. 2010, 107. [Google Scholar] [CrossRef]
- Tran, P.A.; Webster, T.J. Understanding the wetting properties of nanostructured selenium coatings: The role of nanostructured surface roughness and air-pocket formation. Int. J. Nanomed. 2013, 8, 2001–2009. [Google Scholar]
- Wang, Z.M. Nanodroplets; Springer: Beijing, China, 2013. [Google Scholar]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Giordano, B.C.; Burgi, D.S.; Hart, S.J.; Terray, A. On-line sample pre-concentration in microfluidic devices: A review. Anal. Chim. Acta 2012, 718, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Trietsch, S.; Hankemeier, T.; Van der Linden, H. Lab-on-a-chip technologies for massive parallel data generation in the life sciences: A review. Chemom. Intell. Lab. Syst. 2011, 108, 64–75. [Google Scholar] [CrossRef]
- Khare, K.; Zhou, J.; Yang, S. Tunable open-channel microfluidics on soft poly (dimethylsiloxane)(pdms) substrates with sinusoidal grooves. Langmuir 2009, 25, 12794–12799. [Google Scholar] [CrossRef] [PubMed]
- Seemann, R.; Brinkmann, M.; Kramer, E.J.; Lange, F.F.; Lipowsky, R. Wetting morphologies at microstructured surfaces. Proc. Nat. Acad. Sci. USA 2005, 102, 1848–1852. [Google Scholar] [CrossRef] [PubMed]
- Tsougeni, K.; Papageorgiou, D.; Tserepi, A.; Gogolides, E. “Smart” polymeric microfluidics fabricated by plasma processing: Controlled wetting, capillary filling and hydrophobic valving. Lab Chip 2010, 10, 462–469. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nokes, J.M.; Sharma, H.; Tu, R.; Kim, M.Y.; Chu, M.; Siddiqui, A.; Khine, M. Nanotextured Shrink Wrap Superhydrophobic Surfaces by Argon Plasma Etching. Materials 2016, 9, 196. https://doi.org/10.3390/ma9030196
Nokes JM, Sharma H, Tu R, Kim MY, Chu M, Siddiqui A, Khine M. Nanotextured Shrink Wrap Superhydrophobic Surfaces by Argon Plasma Etching. Materials. 2016; 9(3):196. https://doi.org/10.3390/ma9030196
Chicago/Turabian StyleNokes, Jolie M., Himanshu Sharma, Roger Tu, Monica Y. Kim, Michael Chu, Ali Siddiqui, and Michelle Khine. 2016. "Nanotextured Shrink Wrap Superhydrophobic Surfaces by Argon Plasma Etching" Materials 9, no. 3: 196. https://doi.org/10.3390/ma9030196




