Preparation and Properties of Melamine Urea-Formaldehyde Microcapsules for Self-Healing of Cementitious Materials
Abstract
:1. Introduction
2. Experiments
2.1. Materials
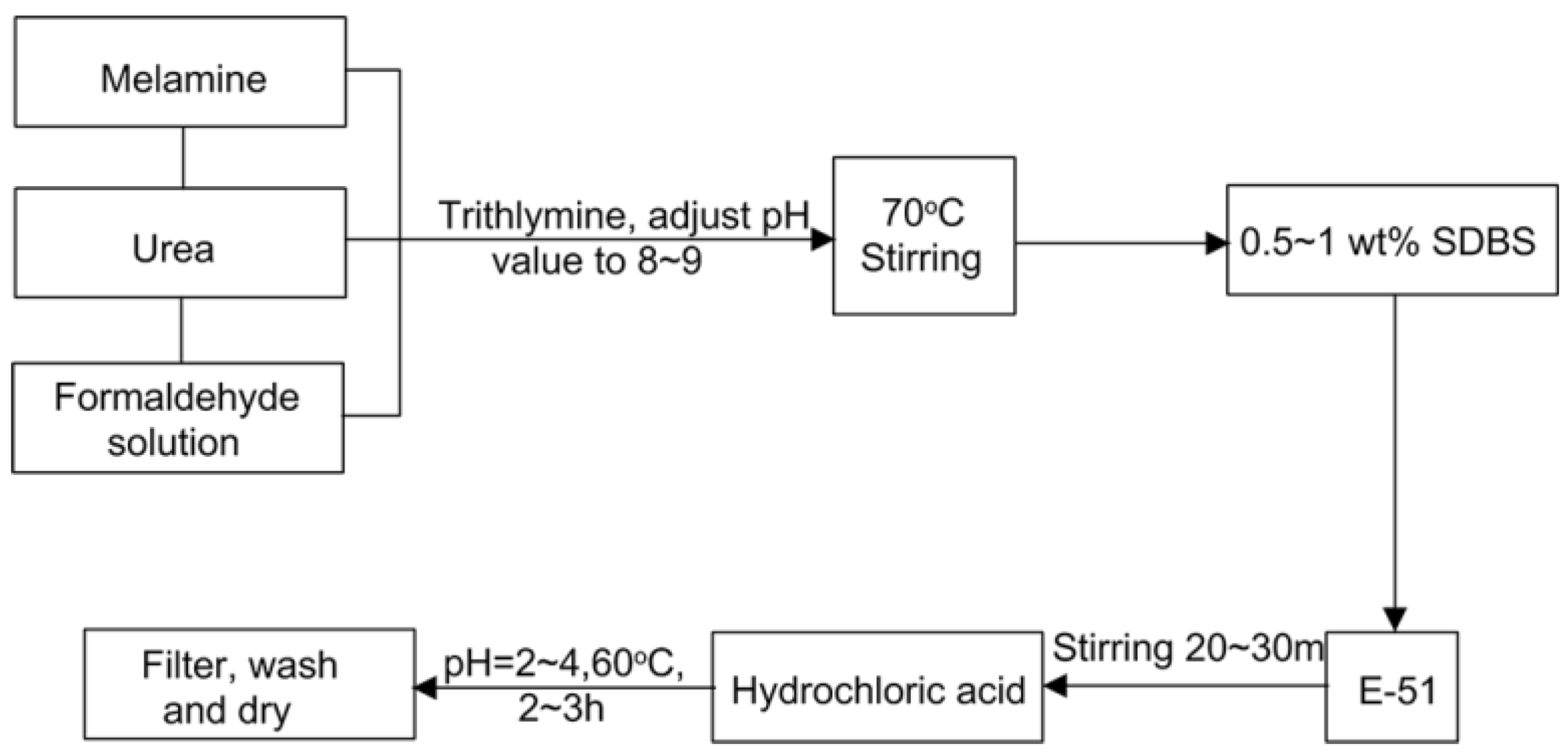
2.2. Preparation of Microcapsules
2.3. Test Procedure
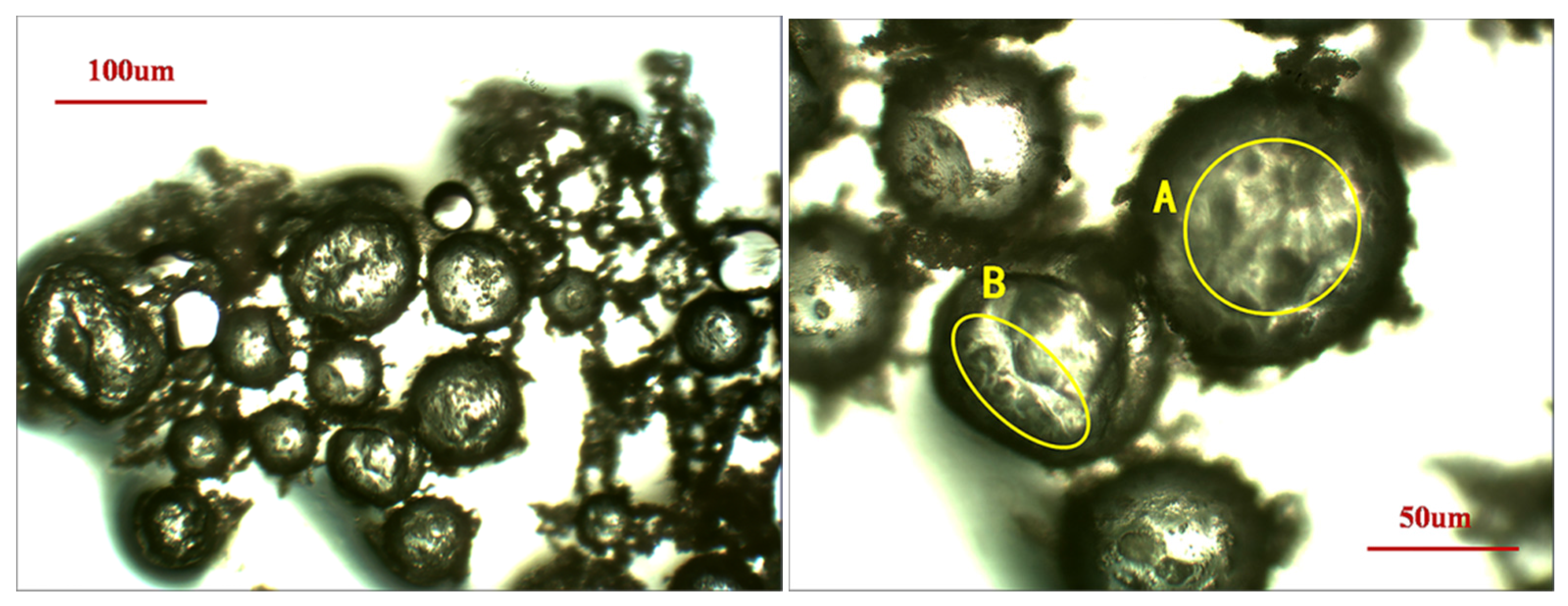
2.3.1. Morphology
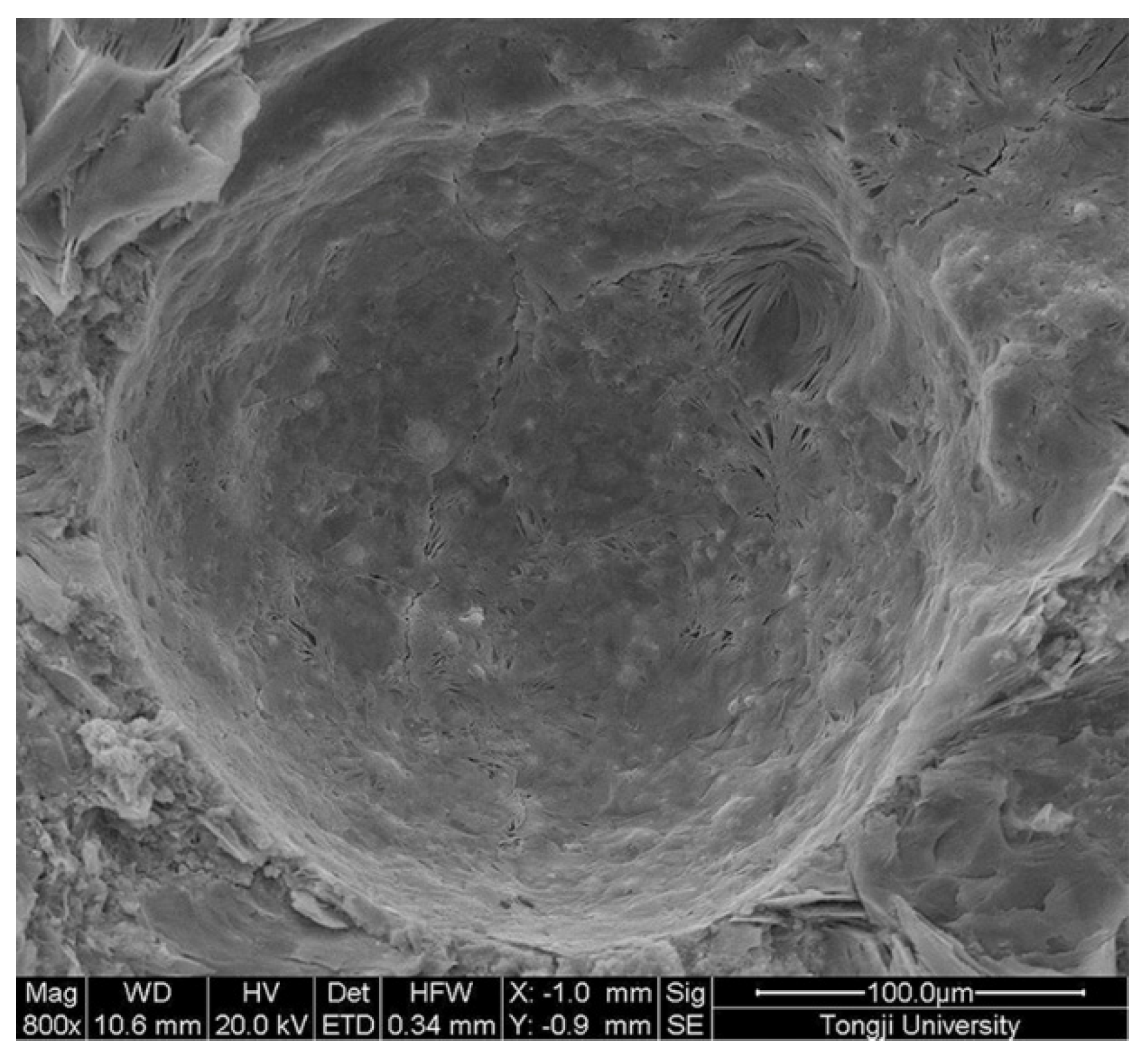
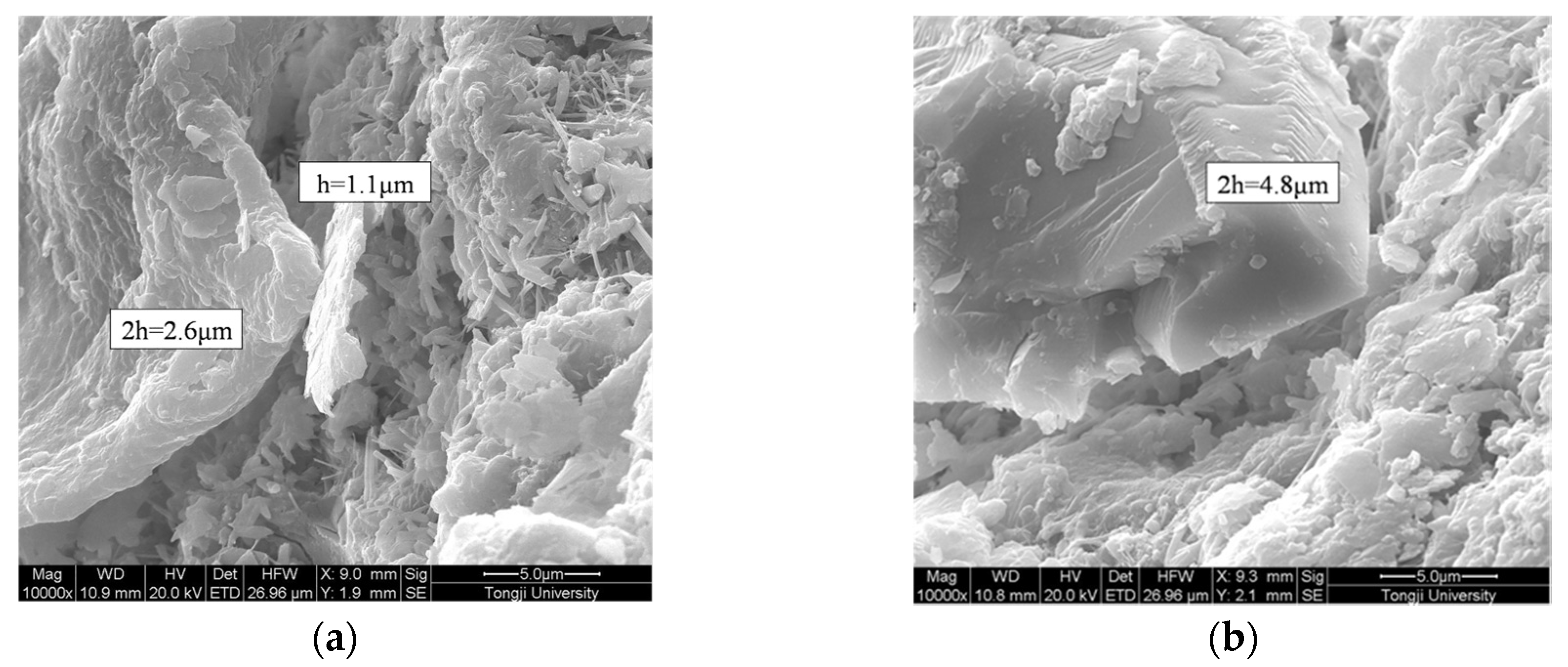
2.3.2. Shell Thickness Measurement
2.3.3. Size Distribution
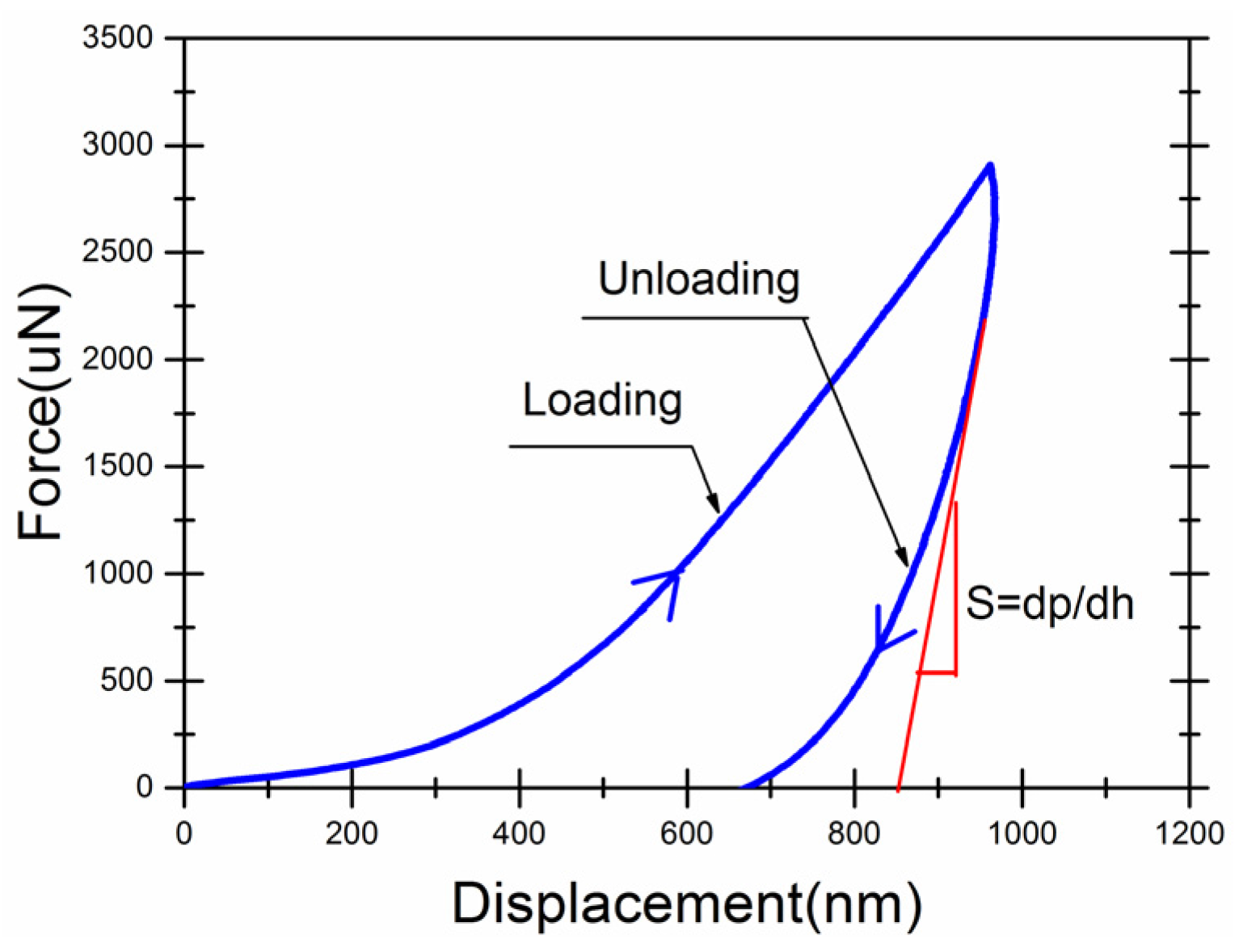
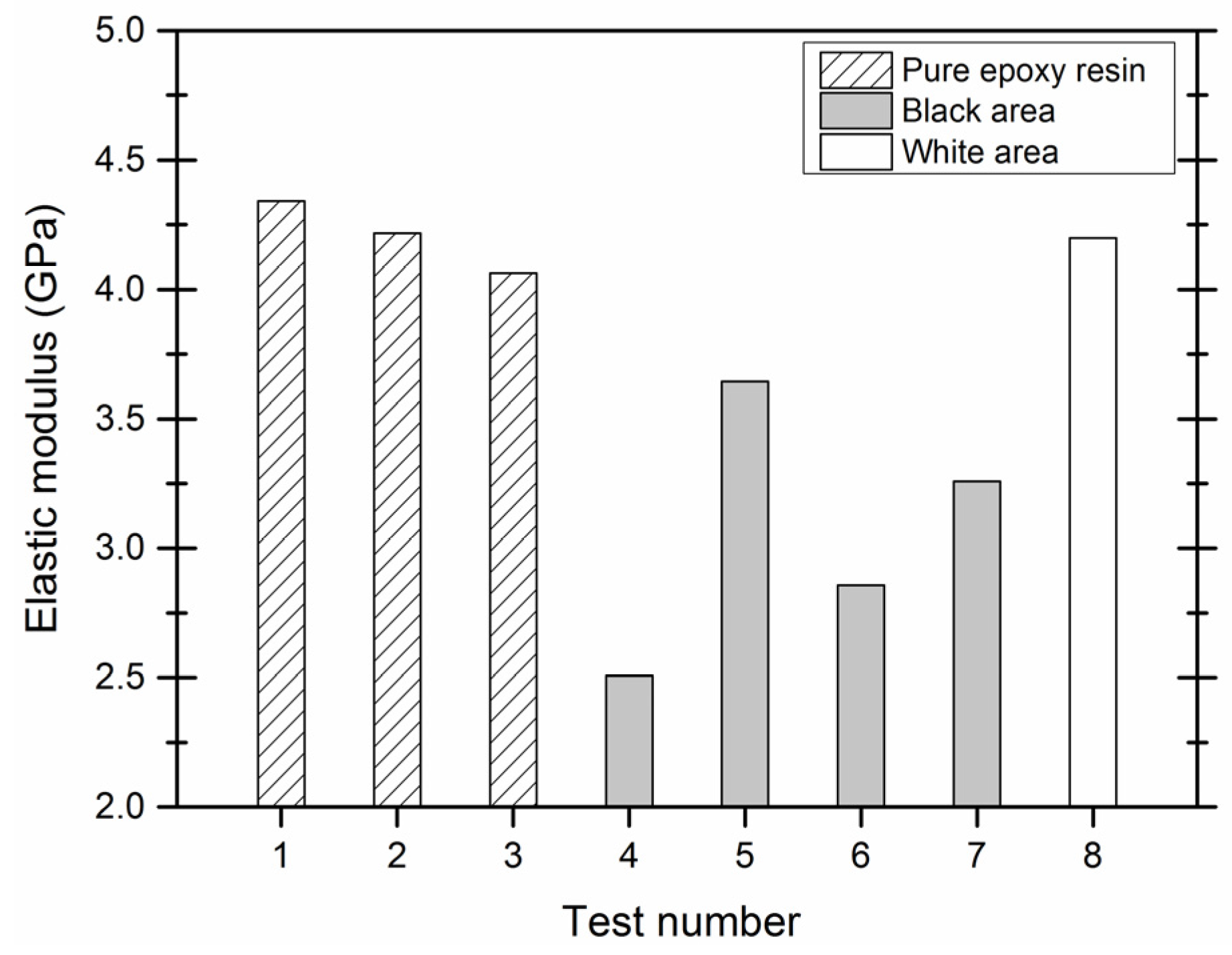
2.3.4. Mechanical Properties of the Shell
- a certain amount of epoxy resin was placed in a mold;
- dry microcapsule powder was mixed into the epoxy resin and stirred;
- a layer of microcapsule powder was deposited at the bottom of another mold, and the epoxy resin-dry microcapsule mixture was poured over the deposited layer under vacuum.
2.3.5. Chemical Structure of the Shell
2.3.6. Strength Restoration of Self-Healing Specimens
3. Results and Discussion
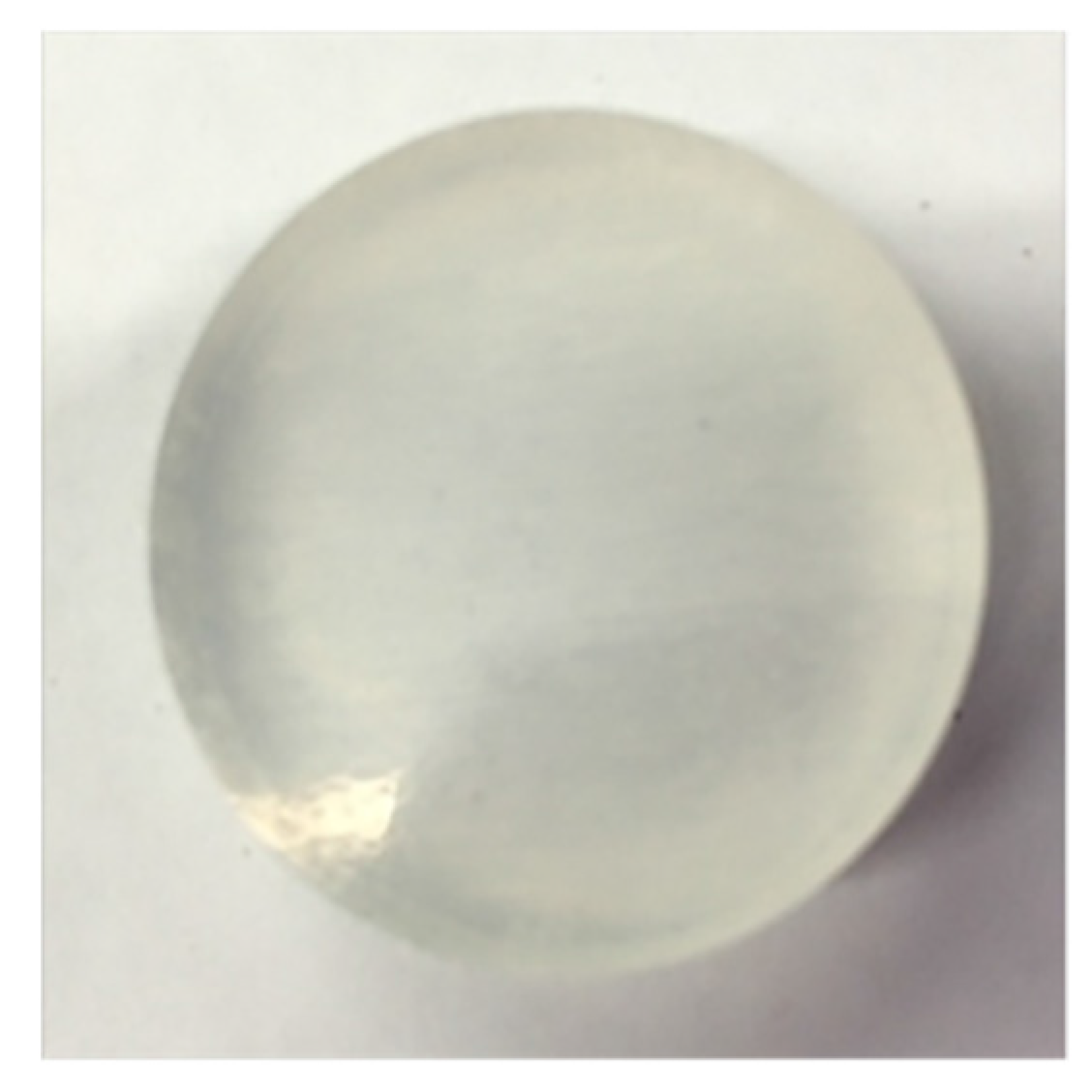
3.1. Morphology
3.2. Particle Size and Distribution
3.3. Mechanical Properties of Microcapsules
3.4. Chemical Structure of the Capsule Wall
4. Self-Healing Efficiency of Cement-Based Material by Microcapsules Prepared
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mehta, P.K. Concrete technology for sustainable development. Concr. Int. 1999, 21, 47–53. [Google Scholar]
- Pattanaik, S.C. Causes, Evaluation and Repair of Cracks in Concrete Structures. ACI Mater. J. 2011, 81, 211–230. [Google Scholar]
- Gerard, B.; Reinhardt, H.W.; Breysse, B. Measured transport in cracked concrete. In RILEM Report no.16: Penetration and Permeability of Concrete; Reinhardt, H.W., Ed.; E&FN Spon: London, UK, 1997; pp. 265–324. [Google Scholar]
- Picandet, V.; Khelidj, A.; Bellegou, H. Crack effects on gas and water permeability of concretes. Cem. Concr. Res. 2009, 39, 537–547. [Google Scholar] [CrossRef]
- White, S.R.; Sottos, N.R.; Moore, J.; Geubelle, P.; Kessler, M.; Brown, E.; Suresh, S.; Viswanathan, S. Autonomic healing of polymers composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Diesendruck, C.E.; Sottos, N.R.; Moore, J.S.; White, S.R. Biomimetic self-healing. Angew. Chem. Int. Ed. 2015, 54, 10428–10447. [Google Scholar] [CrossRef] [PubMed]
- Blaiszik, B.J.; Kramer, S.L.B.; Olugebefola, S.C.; Moore, J.S.; Sottos, N.R.; White, S.R. Self-Healing polymers and composites. Annu. Rev. Mater. Res. 2010, 40, 179–211. [Google Scholar] [CrossRef]
- Thakur, V.K.; Kessler, M.R. Self-healing polymer nanocomposite materials: A review. Polymer 2015, 69, 369–383. [Google Scholar] [CrossRef]
- Dry, C.M. Matrix cracking repair and filling using active and passive modes for smart timed release of chemicals from fibers into cement matrices. Smart Mater. Struct. 1994, 3, 118–123. [Google Scholar] [CrossRef]
- Tittelboom, K.V.; Belie, N.D. Self-healing in cementitious materials—A review. Materials 2013, 6, 2182–2217. [Google Scholar] [CrossRef]
- Breugel, K.V. Is there a market for self-healing cement-based materials? In Proceedings of the 1st International Conference on Self Healing Materials, Noordwijk aan Zee, The Netherlands, 18–20 April 2007.
- Neville, A. Autogenous healing—A concrete miracle? Concr. Int. 2002, 24, 76–82. [Google Scholar]
- Wu, M.; Johannesson, B.; Geiker, M. A review: Self-healing in cementitious materials and engineered cementitious composite as a self-healing material. Constr. Build. Mater. 2012, 28, 571–583. [Google Scholar] [CrossRef]
- Ghosh, S.K. Self-healing materials: Fundamentals, design strategies, and applications. In Self-healing Materials: Fundamentals, Design Strategies, and Applications; Wiley-VCH: Weinheim, Germany, 2009; pp. 1–28. [Google Scholar]
- Schlangen, E.; Sangadji, S. Addressing infrastructure durability and sustainability by self healing mechanisms—Recent advances in self healing concrete and asphalt. Proc. Eng. 2013, 54, 39–57. [Google Scholar] [CrossRef]
- Tang, W.; Kardani, O.; Cui, H. Robust evaluation of self-healing efficiency in cementitious materials—A review. Constr. Build. Mater. 2015, 81, 233–247. [Google Scholar] [CrossRef]
- Jonkers, H.M.; Thijssen, A.; Muyzer, G.; Copuroglu, O.; Schlangen, E. Application of bacteria as self-healing agent for the development of sustainable concrete. Ecol. Eng. 2010, 36, 230–235. [Google Scholar] [CrossRef]
- Jonkers, H.M. Self healing concrete: A biological approach. In Self Healing Materials: An Alternative Approach to 20 Centuries of Materials Science; van der Zwaag, S., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 195–204. [Google Scholar]
- Jonkers, H.M.; Schlangen, E. Towards a sustainable bacterially-mediated self healing concrete. In Proceedings of the 2nd International Conference on Self Healing Materials, Chicago, IL, USA, 28 June–1 July 2009.
- Van Tittelboom, K.; De Belie, N.; De Muynck, W.; Verstraete, W. Use of bacteria to repair cracks in concrete. Cem. Concr. Res. 2010, 40, 157–166. [Google Scholar] [CrossRef]
- De Muynck, W.; De Belie, N.; Verstraete, W. Microbial carbonate precipitation in construction materials: A review. Ecol. Eng. 2010, 36, 118–136. [Google Scholar] [CrossRef]
- Wang, J.; Van Tittelboom, K.; De Belie, N.; Verstraete, W. Use of silica gel or polyurethane immobilized bacteria for self-healing concrete. Constr. Build. Mater. 2012, 26, 532–540. [Google Scholar] [CrossRef]
- Li, V.C.; Herbert, E.N. Robust self-healing concrete for sustainable infrastructure. J. Adv. Concr. Technol. 2012, 10, 207–218. [Google Scholar] [CrossRef]
- Huang, H.; Ye, G.; Damidot, D. Characterization and quantification of self-healing behaviors of microcracks due to further hydration in cement paste. Cem. Concr. Res. 2013, 52, 71–81. [Google Scholar] [CrossRef]
- Sahmaran, M.; Keskin, S.B.; Ozerkan, G.; Yaman, I.O. Self-healing of mechanically-loaded self consolidating concretes with high volumes of fly ash. Cem. Concr. Compos. 2008, 30, 872–879. [Google Scholar] [CrossRef]
- Sahmaran, M.; Yildirim, G.; Erdem, T.K. Self-healing capability of cementitious composites incorporating different supplementary cementitious materials. Cem. Concr. Compo. 2013, 35, 89–101. [Google Scholar] [CrossRef]
- Hosoda, A.; Kishi, T.; Arita, H.; Takakuwa, Y. Self healing of crack and water permeability of expansive concrete. In Proceedings of the 1st International Conference on Self Healing Materials, Noordwijk aan Zee, The Netherlands, 18–20 April 2007.
- Kishi, T.; Ahn, T.H.; Hosoda, A.; Suzuki, S.; Takaoka, H. Self-healing behaviour by cementitious recrystallization of cracked concrete incorporating expansive agent. In Proceedings of the 1st International Conference on Self Healing Materials, Noordwijk aan Zee, The Netherlands, 18–20 April 2007.
- Ahn, T.H.; Kishi, T. Crack Self-healing Behavior of Cementitious Composites Incorporating Various Mineral Admixtures. J. Adv. Concr. Technol. 2010, 8, 171–186. [Google Scholar] [CrossRef]
- Granger, S.; Loukili, A.; Pijaudier-Cabot, G.; Chanvillard, G. Experimental characterization of the self-healing of cracks in an ultra high performance cementitious material: Mechanical tests and acoustic emission analysis. Cem. Concr. Res. 2007, 37, 519–527. [Google Scholar] [CrossRef]
- Na, S.H.; Hama, Y.; Taniguchi, M.; Katsura, O.; Sagawa, T.; Zakaria, M. Experimental investigation on reaction rate and self-healing ability in fly ash blended cement mixtures. J. Adv. Concr. Technol. 2012, 10, 240–253. [Google Scholar] [CrossRef]
- Sisomphon, K.; Copuroglu, O.; Koenders, E.A.B. Self-healing of surface cracks in mortars with expansive additive and crystalline additive. Cem. Concr. Compos. 2012, 34, 566–574. [Google Scholar] [CrossRef]
- Yang, Z.; Hollar, J.; He, X.; Shi, X. Laboratory assessment of a self-healing cementitious composite. J. Transp. Res. Board 2010, 13, 9–17. [Google Scholar] [CrossRef]
- Yang, Z.; Hollar, J.; He, X.; Shi, X. A self-healing cementitious composite using oil core/silica gel shell microcapsules. Cem. Concr. Compos. 2011, 33, 506–512. [Google Scholar] [CrossRef]
- Feng, X.; Ni, Z.; Han, N.; Dong, B.; Du, X.; Huang, Z.; Zhang, M. Self-healing mechanism of a novel cementitious composite using microcapsules. In Proceedings of the 1st International Conference on Durability of Concrete Structures, Hangzhou, China, 26–27 November 2008.
- Kaltzakorta, I.; Erkizia, E. Silica microcapsules encapsulating epoxy compounds for self-healing cementitious materials. In Proceedings of the 3rd International Conference on Self Healing Materials, Bath, UK, 27–29 June 2011.
- Behzadnasab, M.; Esfandeh, M.; Mirabedini, S.M.; Zohuriaan-Mehr, M.J.; Farnood, R.R. Preparation and characterization of linseed oil-filled urea–formaldehyde microcapsules and their effect on mechanical properties of an epoxy-based coating. Colloid. Surface. A. 2014, 457, 16–26. [Google Scholar] [CrossRef]
- Blaiszik, B.J.; Caruso, M.M.; Mcilroy, D.A.; Moore, J.S.; White, S.R.; Sottos, N.R. Microcapsules filled with reactive solutions for self-healing materials. Polymer 2009, 50, 990–997. [Google Scholar] [CrossRef]
- Jin, H.; Mangun, C.L.; Stradley, D.S.; Moore, J.S.; Sottos, N.R.; White, S.R. Self-healing thermoset using encapsulated epoxy-amine healing chemistry. Polymer 2012, 53, 581–587. [Google Scholar] [CrossRef]
- Jin, H.; Mangun, C.L.; Griffin, A.S.; Moore, J.S.; Sottos, N.R.; White, S.R. Thermally stable autonomic healing in epoxy using a dual-microcapsule system. Adv. Mater. 2014, 26, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.; Rahamtullah; Kumar, D.; Rajagopal, C.; Kumar Roy, P. Influence of microcapsule shell material on the mechanical behavior of epoxy composites for self-healing applications. J. Appl. Polym. Sci. 2014, 131, 338–347. [Google Scholar] [CrossRef]
- Yuan, L.; Gu, A.; Liang, G. Preparation and properties of poly (urea–formaldehyde) microcapsules filled with epoxy resins. Mater. Chem. Phys. 2008, 110, 417–425. [Google Scholar] [CrossRef]
- Nishiwaki, T. Fundamental study on development of intelligent concrete with self-healing capability. Master Thesis, Tohoku University, Sendai, Japan, 1997. [Google Scholar]
- Huang, H.; Ye, G. Application of sodium silicate solution as self-healing agent in cementitious materials. In Proceedings of the International RILEM Conference on Advances in Construction Materials through Science and Engineering, Hong Kong, China, 31 October 2011.
- Pelletier, M.; Brown, R.; Shukla, A.; Bose, A. Self-healing Concrete with a Microencapsulated Healing Agent. Available online: http://energetics.chm.uri.edu/system/files/Self%20healing%20concrete%20-7-11/pdf (accessed on 17 May 2013).
- Alansari, M.; Hassan, M.M.; Asadi, S.; Mostavi, E. Evaluation of self-healing mechanisms in concrete with double-walled sodium silicate microcapsules. J. Mater. Civil. Eng. 2015, 27, 4015–4035. [Google Scholar]
- Flinn, J.E.; Nack, H. Advances in microencapsulation techniques. Battelle Tech. Rev. 1967, 16, 2–8. [Google Scholar]
- James Gilford, B.S., III. Microencapsulation of Self-healing Concrete Properties. Master thesis, Texas A&M University, State of Texas, College Station, TX, USA, 2012. [Google Scholar]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Ahangari, M.G.; Fereidoon, A.; Jahanshahi, M.; Sharifi, N. Effect of nanoparticles on the micromechanical and surface properties of poly(urea–formaldehyde) composite microcapsules. Composites: Part B 2014, 56, 450–455. [Google Scholar] [CrossRef]
- Sorelli, L.; Constantinides, G.; Ulm, F.J.; Toutlemonde, F. The nano-mechanical signature of ultra high performance concrete by statistical nanoindentation techniques. Cem. Concr. Res. 2008, 38, 1447–1456. [Google Scholar] [CrossRef]
- Zhang, M.A. Study on Microcapsule Based Self-healing Method and Mechanism for Cemetitious Composites. PhD Thesis, Changsha ZhongNan University, Changsha, China, 2013. [Google Scholar]

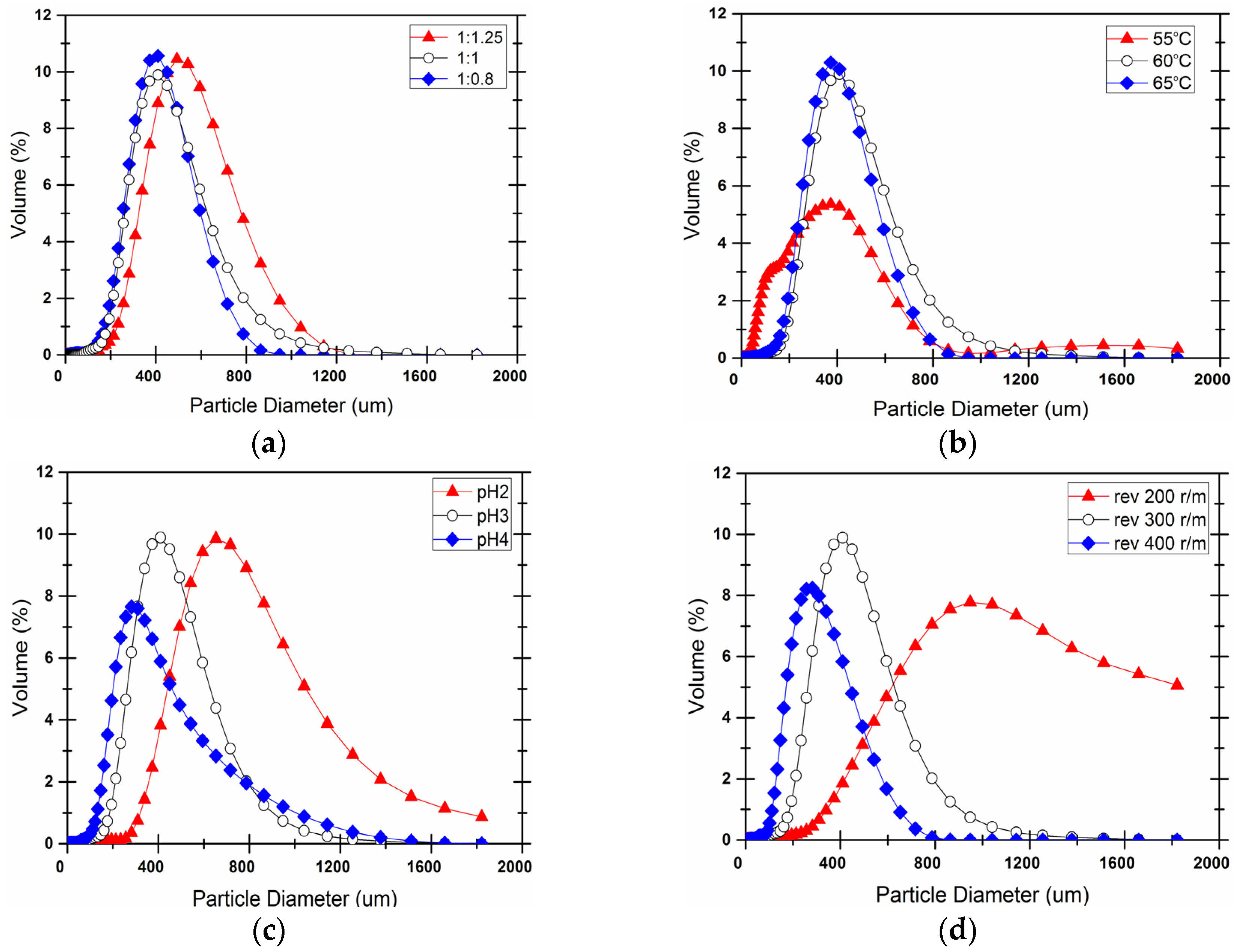

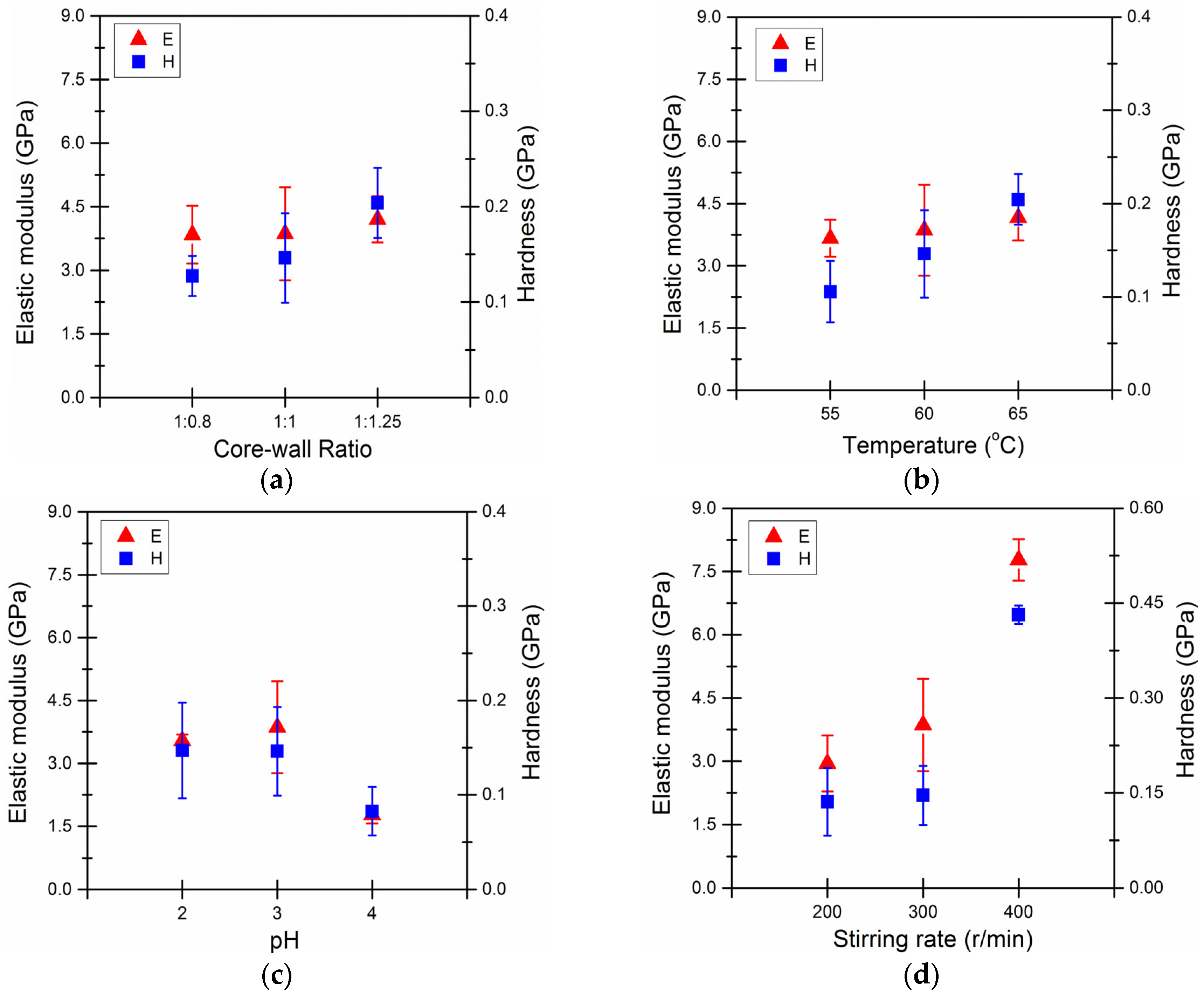
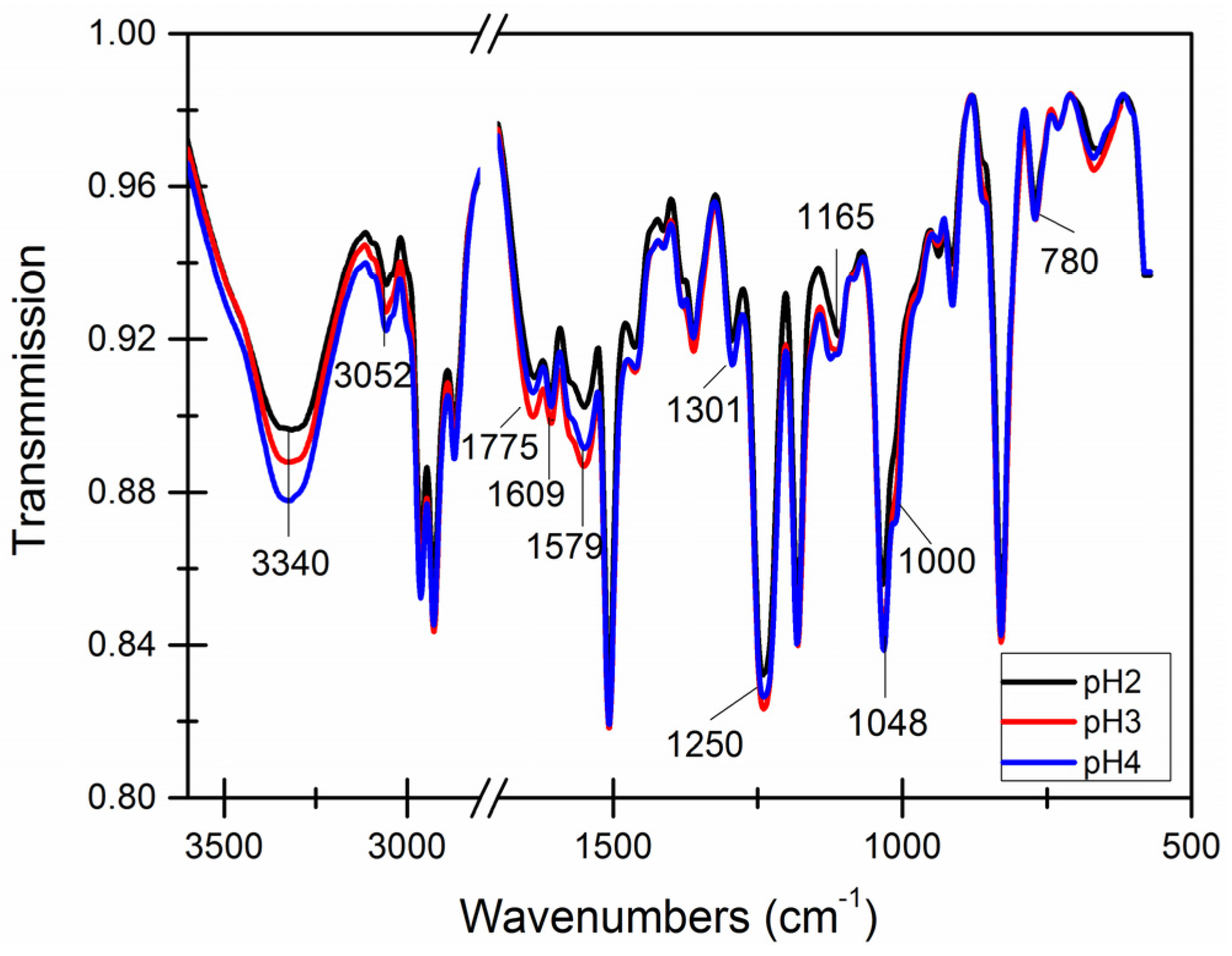
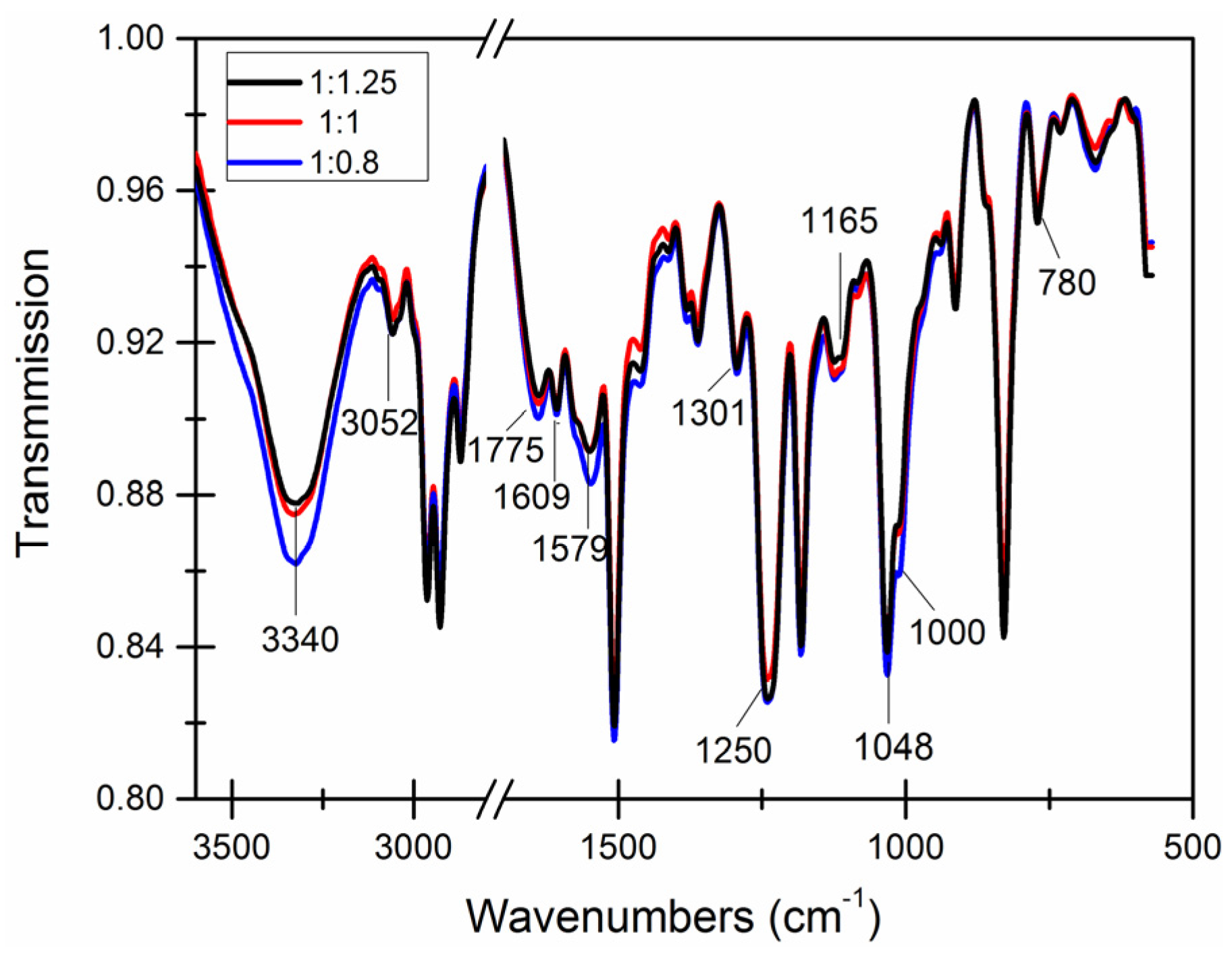
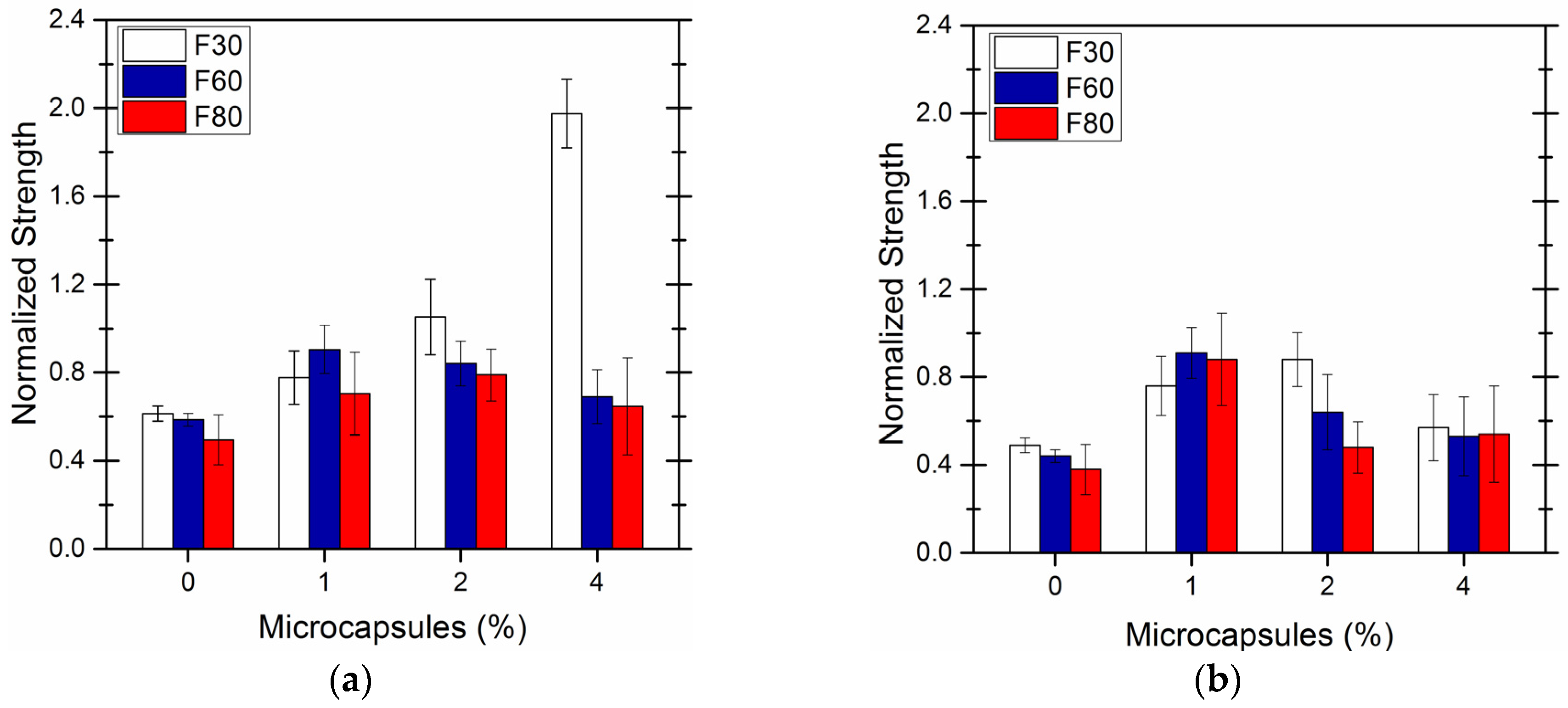

| Core-Wall Ratio | Reaction Temperature (°C) | pH Value | Stirring (r/min) |
|---|---|---|---|
| 1:0.8 | 60 | 3 | 300 |
| 1:1 | 60 | 3 | 300 |
| 1:1.25 | 60 | 3 | 300 |
| 1:1 | 55 | 3 | 300 |
| 1:1 | 60 | 3 | 300 |
| 1:1 | 65 | 3 | 300 |
| 1:1 | 60 | 2 | 300 |
| 1:1 | 60 | 3 | 300 |
| 1:1 | 60 | 4 | 300 |
| 1:1 | 60 | 3 | 200 |
| 1:1 | 60 | 3 | 300 |
| 1:1 | 60 | 3 | 400 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Zhu, X.; Zhao, N.; Jiang, Z. Preparation and Properties of Melamine Urea-Formaldehyde Microcapsules for Self-Healing of Cementitious Materials. Materials 2016, 9, 152. https://doi.org/10.3390/ma9030152
Li W, Zhu X, Zhao N, Jiang Z. Preparation and Properties of Melamine Urea-Formaldehyde Microcapsules for Self-Healing of Cementitious Materials. Materials. 2016; 9(3):152. https://doi.org/10.3390/ma9030152
Chicago/Turabian StyleLi, Wenting, Xujing Zhu, Nan Zhao, and Zhengwu Jiang. 2016. "Preparation and Properties of Melamine Urea-Formaldehyde Microcapsules for Self-Healing of Cementitious Materials" Materials 9, no. 3: 152. https://doi.org/10.3390/ma9030152





