Bone Regeneration Using a Mixture of Silicon-Substituted Coral HA and β-TCP in a Rat Calvarial Bone Defect Model
Abstract
:1. Introduction
2. Results
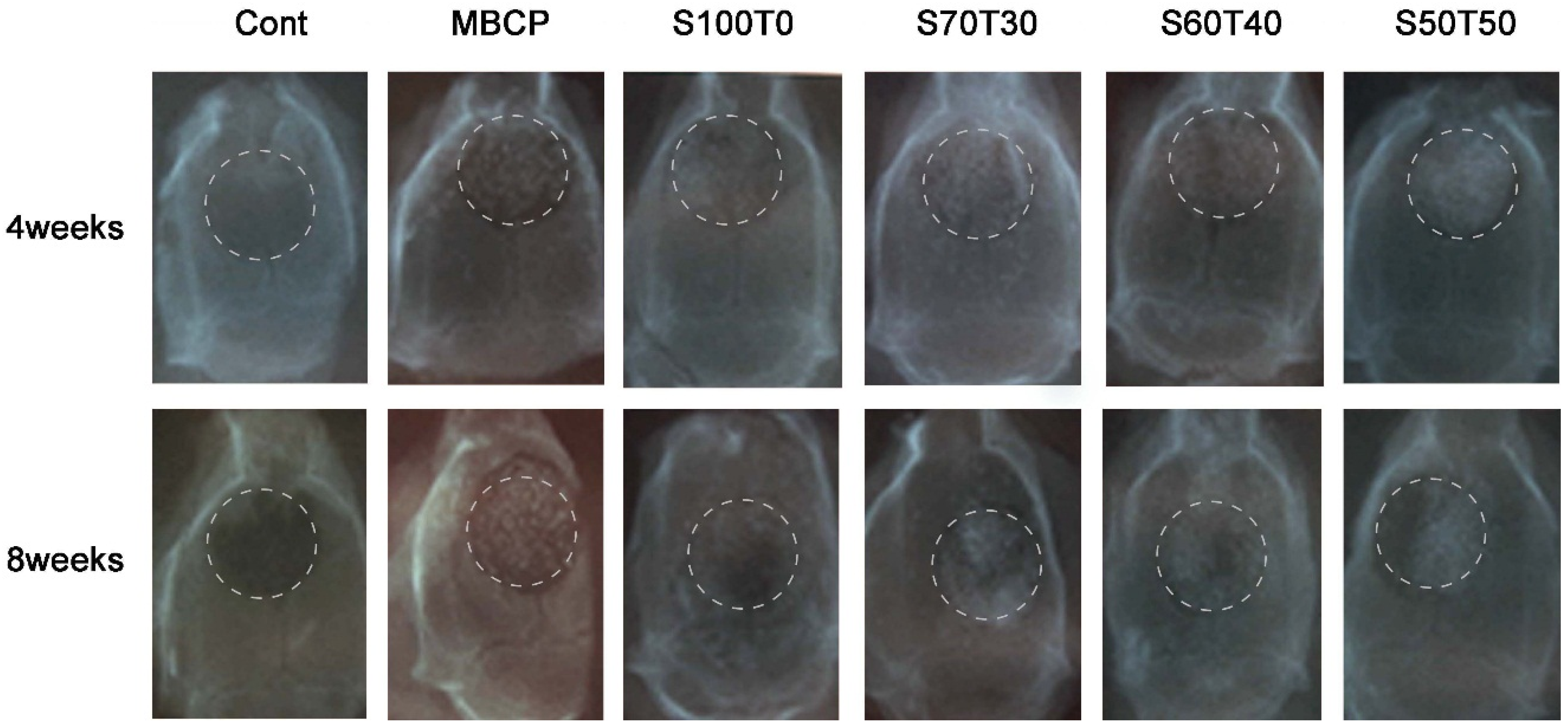
2.1. Radiological Findings after Four Weeks and Eight Weeks
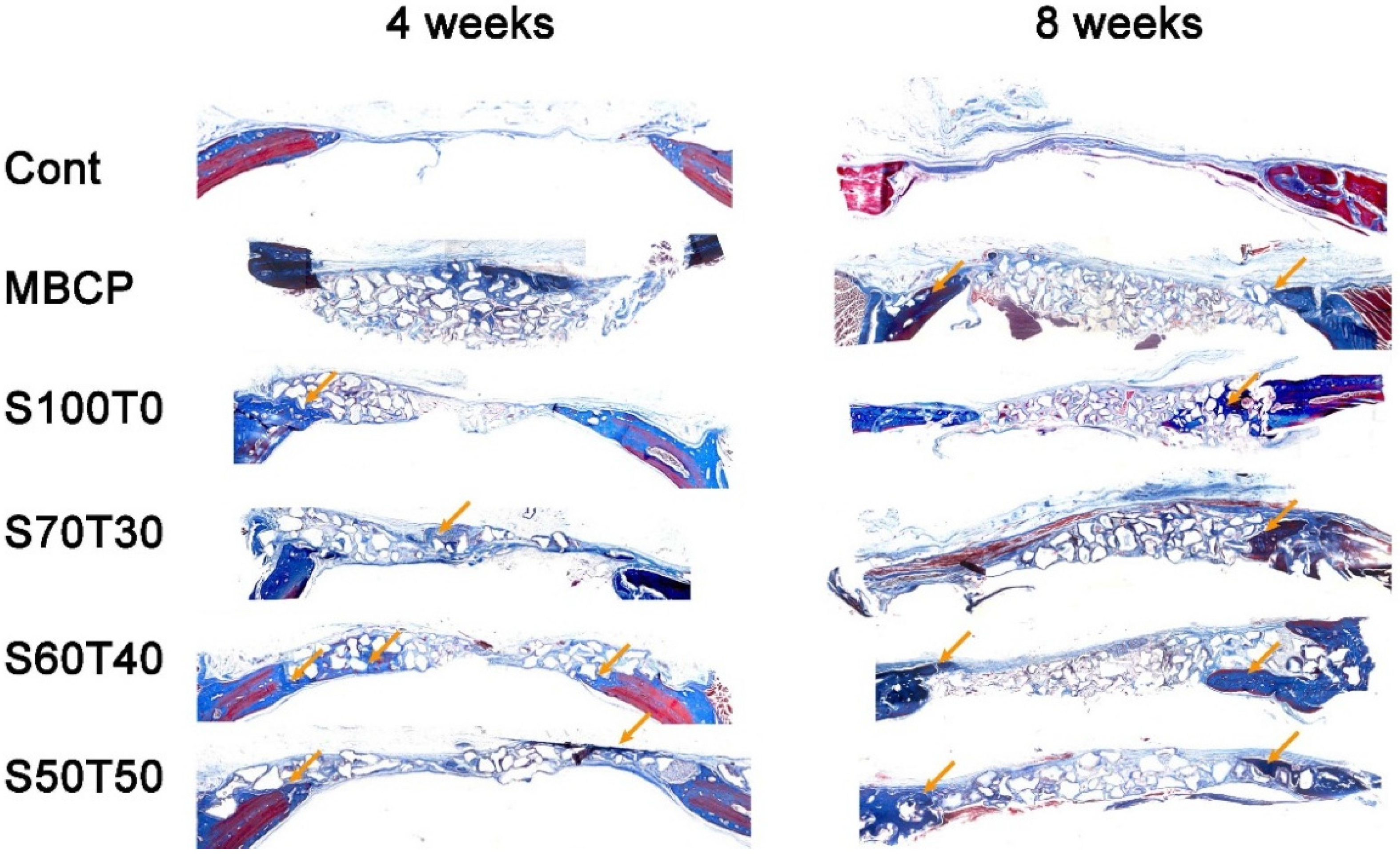
2.2. Histological Findings at Four Weeks and Eight Weeks
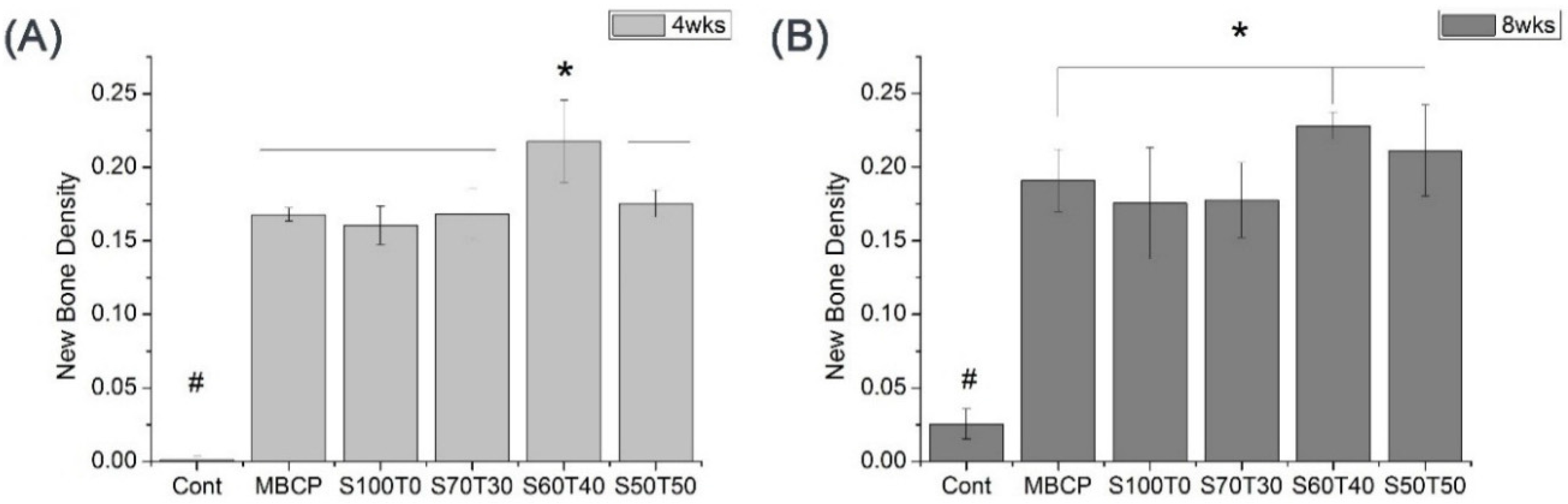
2.3. Quantified Results of Histological Slides (Four Weeks and Eight Weeks)
3. Discussion
4. Experimental Section
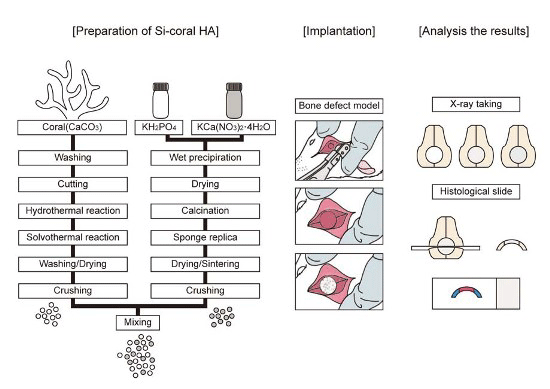
4.1. Preparation of Si-Substituted Coral HA and β-TCP Mixture
4.1.1. Preparation and Characterization of Si-Substituted Coral HA
4.1.2. Synthesis of β-TCP
4.1.3. Si-Substituted Coral HA and β-TCP Mixture
4.2. Materials for Animal Experiments
| Groups | Label | Pure HA | Si-Substituted Coral HA | β-TCP |
|---|---|---|---|---|
| Blank control group | Cont | - | - | - |
| Control product group | MBCP | 60 | - | 40 |
| Experimental groups | S100T0 | - | 100 | 0 |
| S70T30 | - | 70 | 30 | |
| S60T40 | - | 60 | 40 | |
| S50T50 | - | 50 | 50 |
4.3. In Vivo Determination of Bone Regeneration of Bone Defect Model
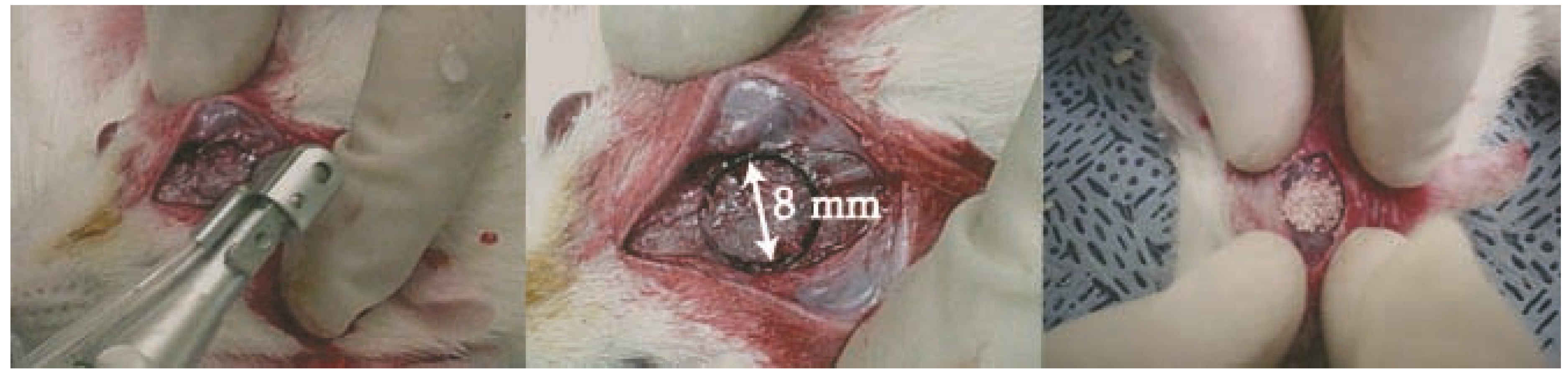
4.4. Histological Evaluation
4.5. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Le Nihouannen, D.; Saffarzadeh, A.; Aguado, E.; Goyenvalle, E.; Gauthier, O.; Moreau, F.; Pilet, P.; Spaethe, R.; Daculsi, G.; Layrolle, P. Osteogenic properties of calcium phosphate ceramics and fibrin glue based composites. J. Mater. Sci. Mater. Med. 2007, 18, 225–235. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.; Hussain, R. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- Guillemin, G.; Patat, J.L.; Fournie, J.; Chetail, M. The use of coral as a bone graft substitute. J. Biomed. Mater. Res. 1987, 21, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Ha, S.M.; Choi, Y.M. Porous Composite Comprising Silicon-Substituted Hydroxyapatite and Beta-Tricalcium Phosphate, and Process for Preparing the Same. U.S. Patent US2011/0185946, 2011. [Google Scholar]
- Sivakumar, M.; Kumar, T.S.S.; Shantha, K.L.; Rao, K.P. Development of hydroxyapatite derived from Indian coral. Biomaterials 1996, 17, 1709–1714. [Google Scholar] [CrossRef]
- Schwarz, K. A bound form of silicon in glycosaminoglycans and polyuronides. Proc. Natl. Acad. Sci. USA 1973, 70, 1608–1612. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, E.M. Silicon: A possible factor in bone calcification. Sci. N.Y. 1970, 167, 279–280. [Google Scholar] [CrossRef]
- Tanizawa, Y.; Suzuki, T. Effects of silicate ions on the formation and transformation of calcium phosphates in neutral aqueous solutions. J. Chem. Soc. Faraday Trans. 1995, 91, 3499–3503. [Google Scholar] [CrossRef]
- Damen, J.J.M.; Ten Cate, J.M. Silica-induced precipitation of calcium phosphate in the presence of inhibitors of hydroxyapatite formation. J. Dent. Res. 1992, 71, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Jung, U.-W.; Lee, I.-S.; Kim, C.S.; Lee, Y.-K.; Choi, S.-H. Resolution of surgically created three-wall intrabony defects in implants using three different biomaterials: An in vivo study. Clin. Oral Implants Res. 2011, 22, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Pietak, A.M.; Reid, J.W.; Stott, M.J.; Sayer, M. Silicon substitution in the calcium phosphate bioceramics. Biomaterials 2007, 28, 4023–4032. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.E.; Patel, N.; Skepper, J.N.; Best, S.M.; Bonfield, W. Effect of sintered silicate-substituted hydroxyapatite on remodelling processes at the bone-implant interface. Biomaterials 2004, 25, 3303–3314. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.-H.; Ahn, J.-S.; Lee, J.-I.; Ahn, K.-J.; Yun, P.-Y.; Kim, Y.-K. A prospective study on the effectiveness of newly developed autogenous tooth bone graft material for sinus bone graft procedure. J. Adv. Prosthodont. 2014, 6, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.E.; Best, S.M.; Bonfield, W. Ultrastructural comparison of hydroxyapatite and silicon-substituted hydroxyapatite for biomedical applications. J. Biomed. Mater. Res. Part A 2004, 68, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.E.; Patel, N.; Skepper, J.N.; Best, S.M.; Bonfield, W. Comparison of in vivo dissolution processes in hydroxyapatite and silicon-substituted hydroxyapatite bioceramics. Biomaterials 2003, 24, 4609–4620. [Google Scholar] [CrossRef]
- Dupoirieux, L.; Pourquier, D.; Picot, M.C.; Neves, M. Comparative study of three different membranes for guided bone regeneration of rat cranial defects. Int. J. Oral Maxillofac. Surg. 2001, 30, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Toker, H.; Ozdemir, H.; Ozer, H.; Eren, K. A comparative evaluation of the systemic and local alendronate treatment in synthetic bone graft: A histologic and histomorphometric study in a rat calvarial defect model. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, S146–S152. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Weir, M.D.; Lippens, E.; Mehta, M.; Wang, P.; Duda, G.N.; Kim, W.S.; Mooney, D.J.; Xu, H.H.K. Bone regeneration via novel macroporous CPC scaffolds in critical-sized cranial defects in rats. Dent. Mater. 2014, 30, e199–e207. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, P.; Chen, W.; Weir, M.D.; Bao, C.; Xu, H.H.K. Human embryonic stem cells and macroporous calcium phosphate construct for bone regeneration in cranial defects in rats. Acta Biomater. 2014, 10, 4484–4493. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.P.; Hollinger, J.O. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin. Orthop. Relat. Res. 1986, 205, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Handschel, J.; Wiesmann, H.P.; Stratmann, U.; Kleinheinz, J.; Meyer, U.; Joos, U. TCP is hardly resorbed and not osteoconductive in a non-loading calvarial model. Biomaterials 2002, 23, 1689–1695. [Google Scholar] [CrossRef]
- Fleckenstein, K.B.; Cuenin, M.F.; Peacock, M.E.; Billman, M.A.; Swiec, G.D.; Buxton, T.B.; Singh, B.B.; McPherson, J.C., III. Effect of a hydroxyapatite tricalcium phosphate alloplast on osseous repair in the rat calvarium. J. Periodontol. 2006, 77, 39–45. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roh, J.; Kim, J.-Y.; Choi, Y.-M.; Ha, S.-M.; Kim, K.-N.; Kim, K.-M. Bone Regeneration Using a Mixture of Silicon-Substituted Coral HA and β-TCP in a Rat Calvarial Bone Defect Model. Materials 2016, 9, 97. https://doi.org/10.3390/ma9020097
Roh J, Kim J-Y, Choi Y-M, Ha S-M, Kim K-N, Kim K-M. Bone Regeneration Using a Mixture of Silicon-Substituted Coral HA and β-TCP in a Rat Calvarial Bone Defect Model. Materials. 2016; 9(2):97. https://doi.org/10.3390/ma9020097
Chicago/Turabian StyleRoh, Jiyeon, Ji-Youn Kim, Young-Muk Choi, Seong-Min Ha, Kyoung-Nam Kim, and Kwang-Mahn Kim. 2016. "Bone Regeneration Using a Mixture of Silicon-Substituted Coral HA and β-TCP in a Rat Calvarial Bone Defect Model" Materials 9, no. 2: 97. https://doi.org/10.3390/ma9020097