Investigation of Fumed Silica/Aqueous NaCl Superdielectric Material
Abstract
:1. Introduction
2. Results
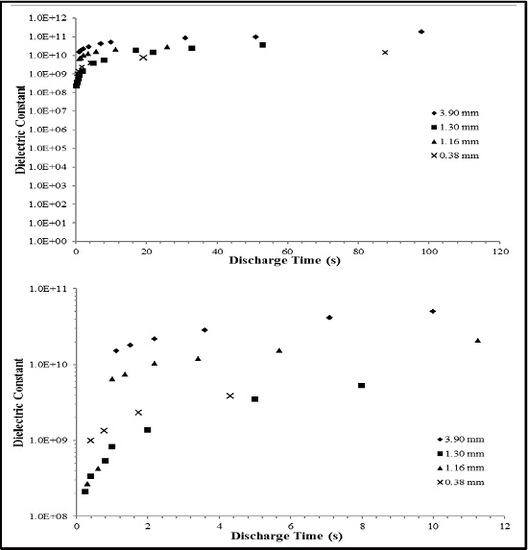
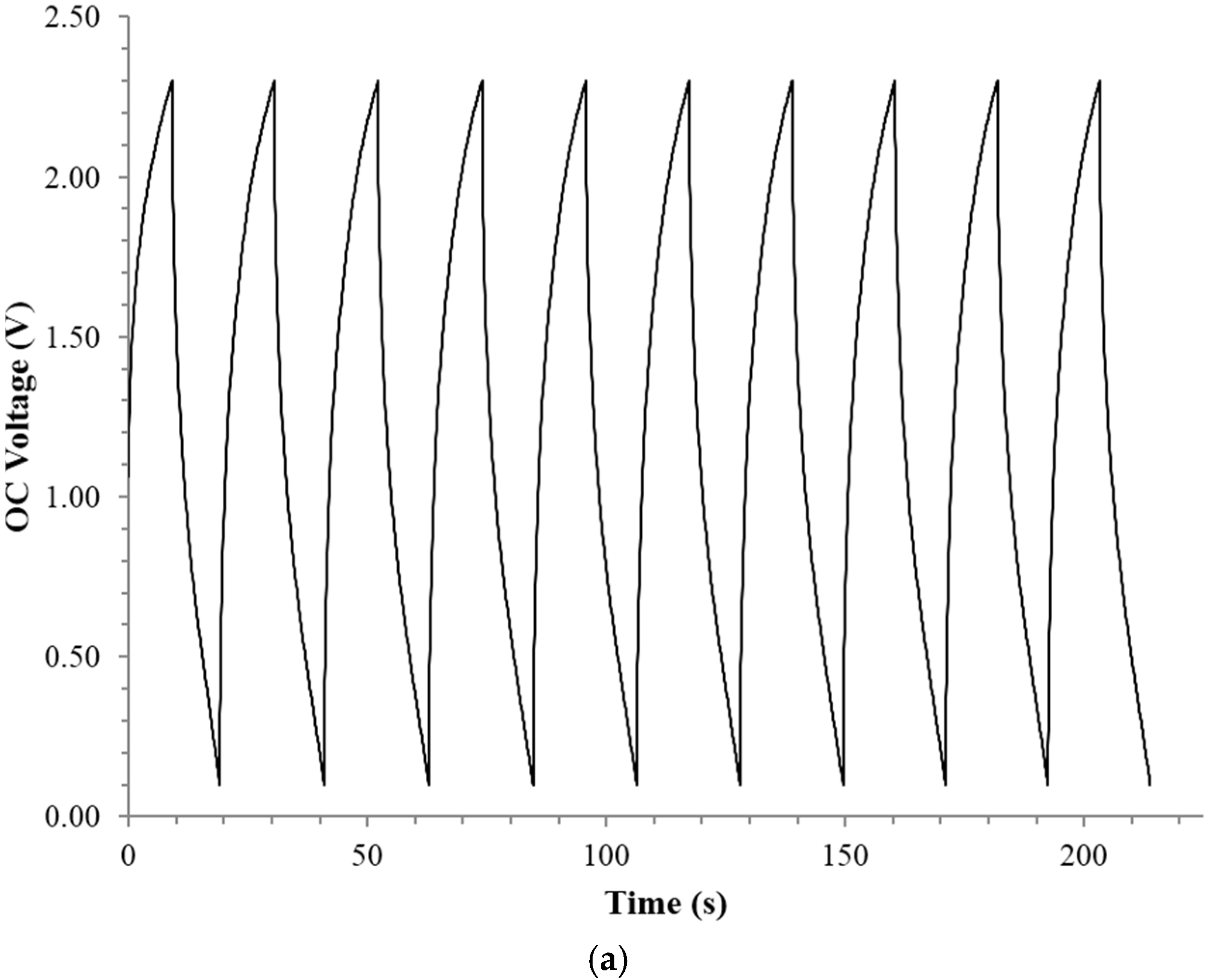
2.1. RC Time Constant
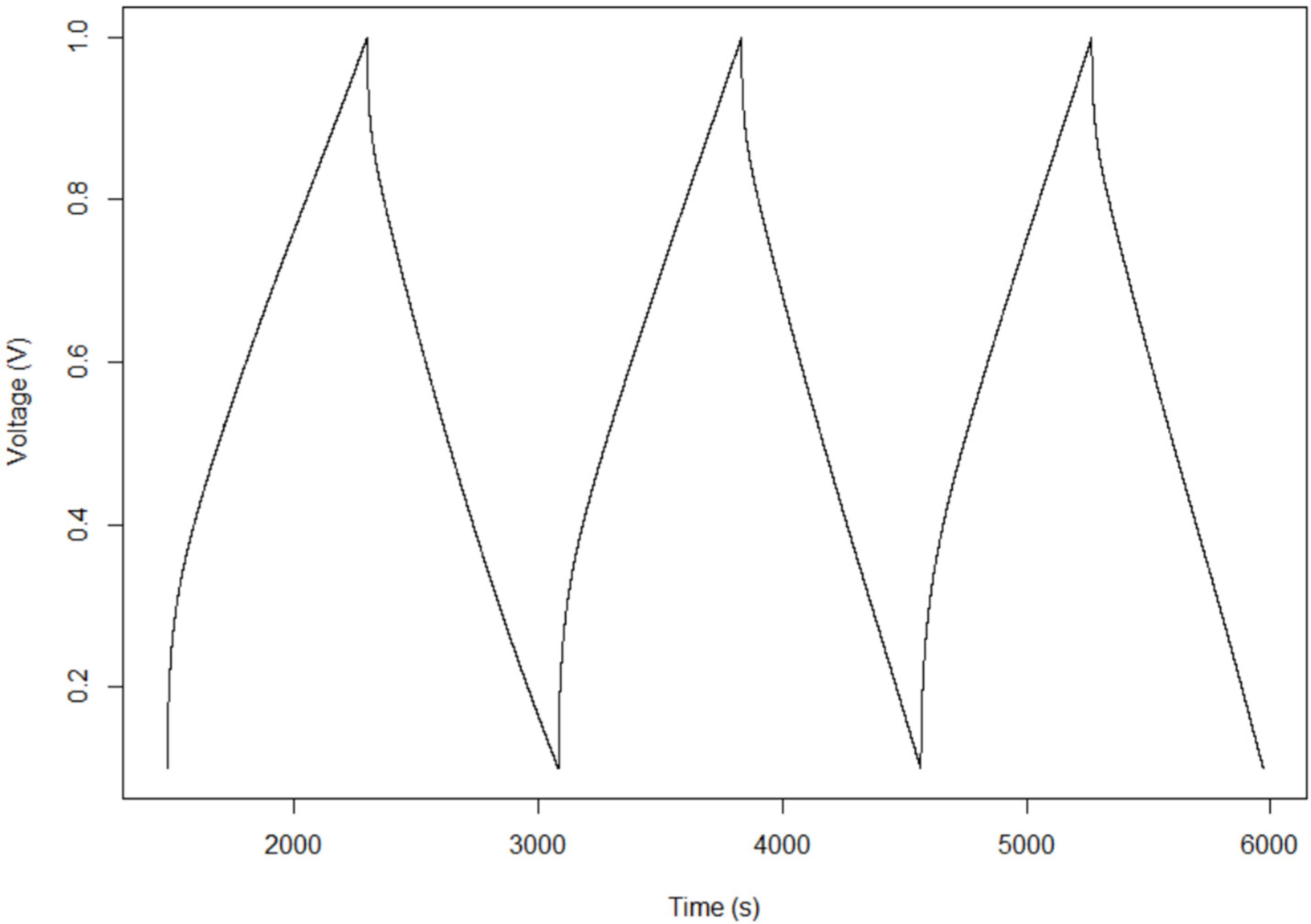
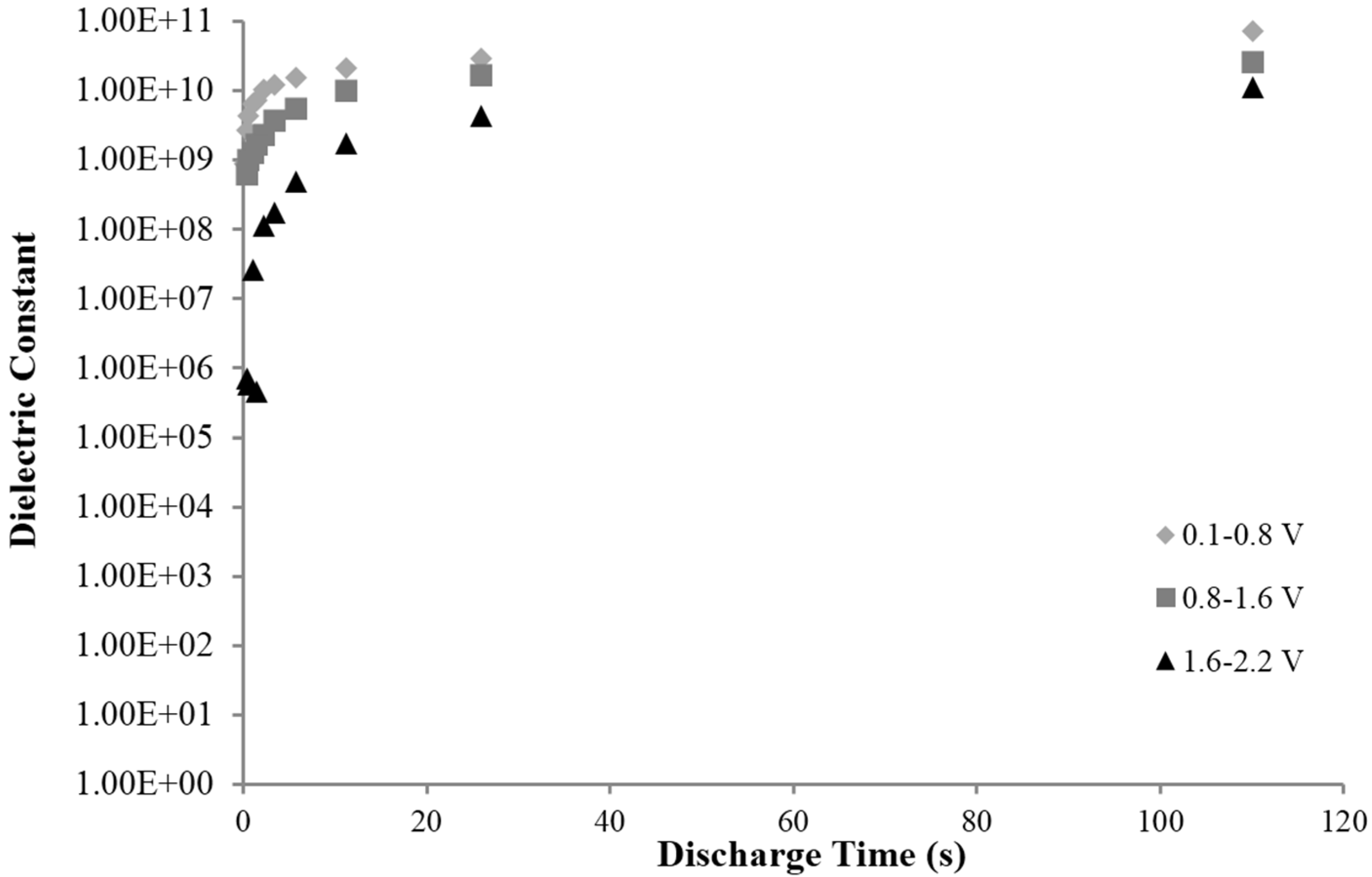
2.2. Constant Current
3. Discussion
3.1. Overview
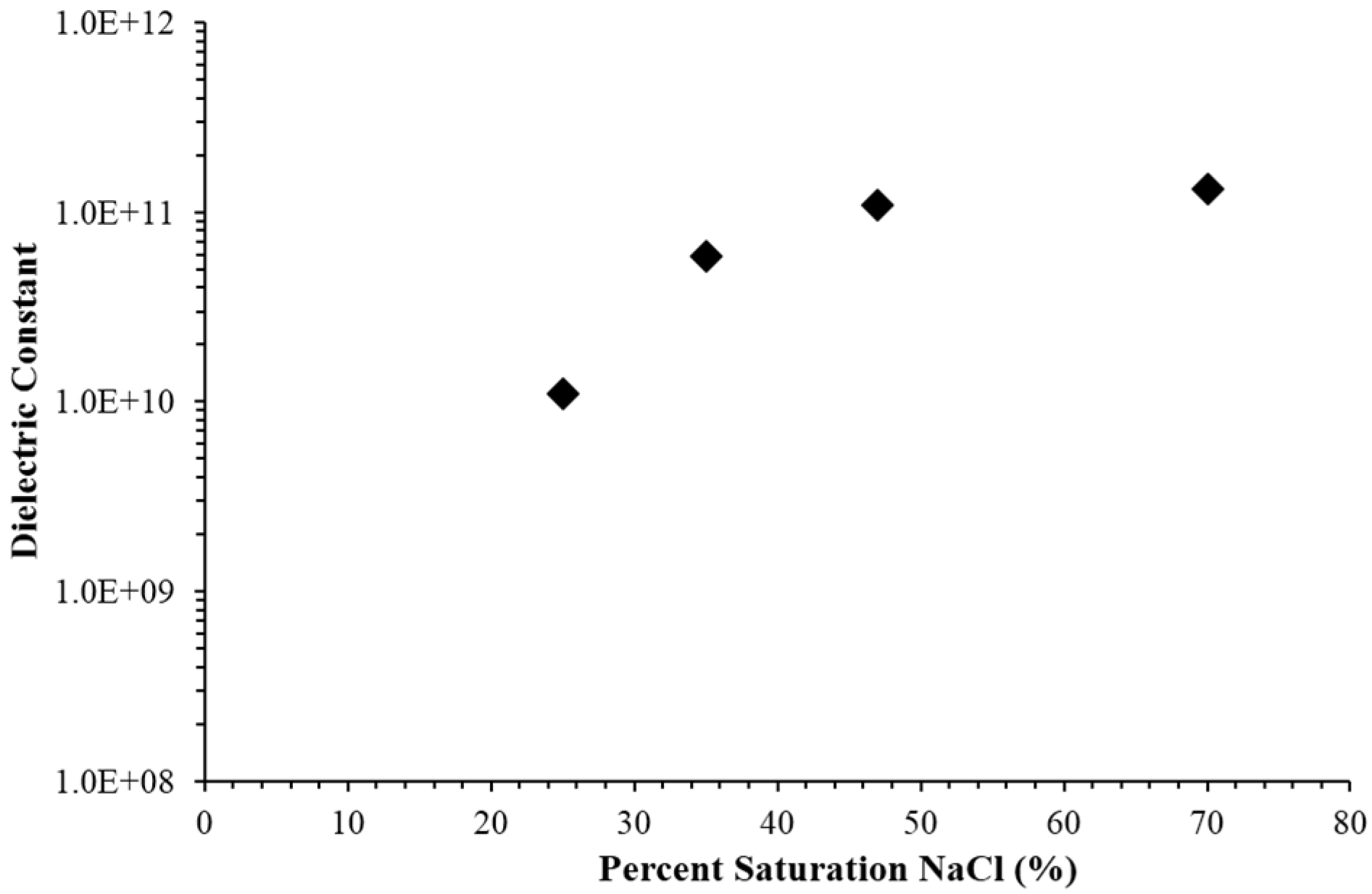
3.2. Empirical Findings
3.3. Model
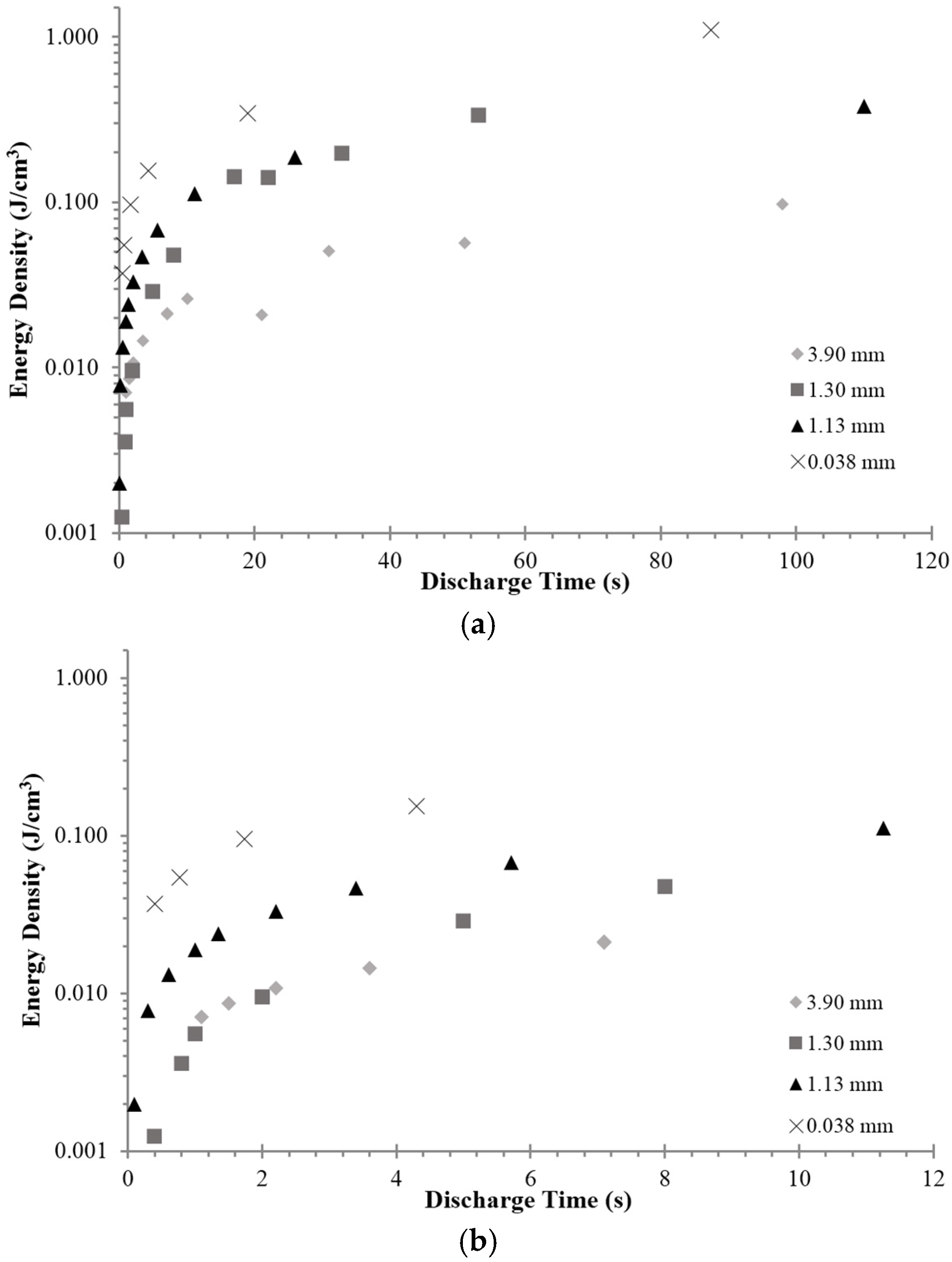
3.4. Energy Density
4. Materials and Methods
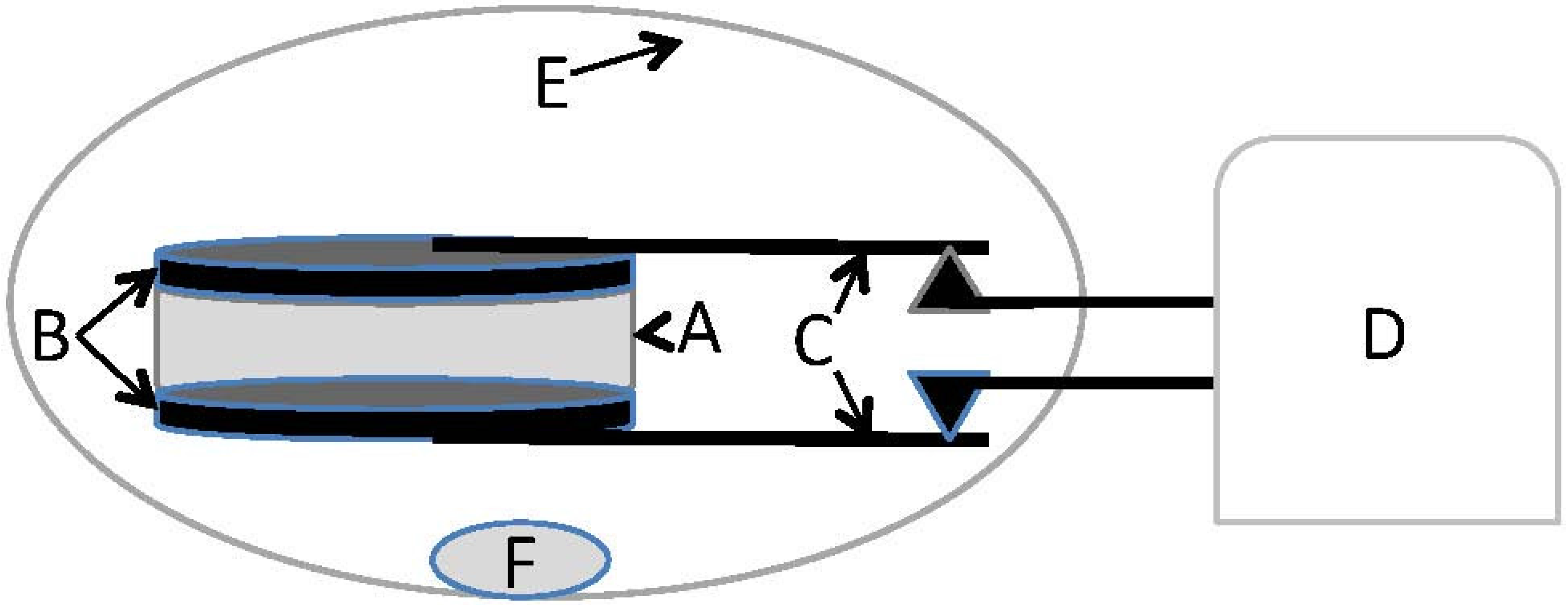
4.1. Capacitor
4.2. Measurement Methods
4.3. RC Time Constant
4.4. Constant Current
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, H.; Byamba-Ochir, N.; Shim, W.G.; Balathanigaimani, M.S.; Moon, H. High-performance super capacitors based on activated anthracite with controlled porosity. J. Power Sources 2015, 275, 668–674. [Google Scholar] [CrossRef]
- Sui, Z.Y.; Meng, Y.N.; Xiao, P.W.; Zhao, Z.Q.; Wei, Z.X.; Han, B.H. Nitrogen-doped grapheme aerogels as efficient supercapacitor electrodes and gas adsorbents. ACS Appl. Mater. Interfaces 2015, 7, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Murali, S.; Quarles, N.; Zhang, L.L.; Pottsa, J.R.; Tanb, Z.; Lub, Y.; Zhub, Y.; Ruoffa, R.S. Volumetric capacitance of compressed activated microwave-expanded graphite oxide (a-MEGO) electrodes. Nano Energy 2013, 2, 764–768. [Google Scholar] [CrossRef]
- Liu, C.; Yu, Z.; Neff, D.; Zhamu, D.N.; Jang, B.Z. Graphene-based supercapacitor with an ultrahigh energy density. Nano Lett. 2010, 10, 4863–4868. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Li, G.; Hou, Y.; Pan, Z.; Li, H.; Li, W.; Liu, M.; Ye, F.; Yang, X.; Zhang, Y. Vertically aligned carbon nanotubes on carbon nanofibers: A hierarchical three-dimensional carbon nanostructure for high-energy flexible supercapacitors. Chem. Mater. 2015, 27, 1194–1200. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Z.; Zhong, X.; Huang, X.; Weiss, N.O.; Huang, Y.; Duan, X. Holey graphene frameworks for highly efficient capacitive energy storage. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Ghidiu, M.; Lakatskaya, M.R.; Zhao, M.Q.; Gogotski, Y.; Barscum, M.W. Conductive two dimensional titanium carbide “clay” with high volumetric capacitance. Nature 2014. [Google Scholar] [CrossRef] [PubMed]
- Fromille, S.; Phillips, J. Super Dielectric Materials. Materials 2014, 7, 8197–8212. [Google Scholar] [CrossRef] [Green Version]
- Quintera, F.; Phillips, J. Super dielectrics composed of NaCl and H2O and porous alumina. J. Electro. Mater. 2015, 44, 1367–1376. [Google Scholar]
- Quintera, F.; Phillips, J. Tube-super dielectric materials: Electrostatic capacitors with energy density greater than 200 J·cm−3. Materials 2015, 8, 6208–6227. [Google Scholar]
- Pecharroman, C.; Esteban-Betegon, F.; Bartolome, F.; Lopez-Esteban, J.F.; Moya, J. New percolative BaTiO3-Ni composites with a high and frequency-independent dielectric constant (ϵr ≈ 80000). Adv. Mat. 2001, 13, 1541–1544. [Google Scholar] [CrossRef]
- Pecharroman, C.; Esteban-Betegon, F.; Jimenez, R. Electric field enhancement and conduction mechanisms in Ni/BaTiO3 percolative composites. Ferroelectrics 2010, 400, 81–88. [Google Scholar]
- Saha, S.K. Observation of giant dielectric constant in an assembly of ultrafine Ag particles. Phys. Rev. B 2004, 69, 125416–125420. [Google Scholar] [CrossRef]
- Jackson, J.D. Classical Electrodynamics, 2nd ed.; Wiley: New York, NY, USA, 1975. [Google Scholar]
- Bergman, D.J.; Imry, Y. Critical behavior of the complex dielectric constant near the percolation threshold of a heterogeneous material. Phys. Rev. Lett. 1977, 39, 1222–1225. [Google Scholar] [CrossRef]
- Efros, A.L.; Shklovskii, B.I. Critical behaviour of conductivity and dielectric constant near the metal-non-metal transition threshold. Phys. Stat. Sol. B 1976, 76, 475–485. [Google Scholar] [CrossRef]
- Efros, A.L. High volumetric capacitance near the insulator-metal percolation transition. Phys. Rev. B 2011, 84. [Google Scholar] [CrossRef]
- Samara, A.A.; Hammetter, W.F.; Venturini, E.L. Temperature and frequency dependences of the dielectric properties of YBa2Cu3O6+x (x ≊ 0). Phys.Rev. B 1990, 41, 8974–8980. [Google Scholar]
- Rey, C.M.; Mathias, H.; Testardi, L.R.; Skirius, S. High dielectric constant and nonlinear electric response in nonmetallic YBa2Cu3O6+δ. Phys. Rev. B 1992, 45, 10639–10646. [Google Scholar]
- Yang, Y.; Wang, X.; Liu, B. CaCu3Ti4O12 ceramics from different methods: microstructure and dielectric. J. Mat. Sci. 2014, 25, 146–151. [Google Scholar] [CrossRef]
- Lunkenheimer, P.; Krohns, S.; Riegg, S.; Ebbinghaus, S.G.; Reller, A.; Loidl, A. Colossal dielectric constants in transition-metal oxides. Eur. Phys. J. 2009, 180, 61–89. [Google Scholar] [CrossRef]
- Lunkenheimer, P.; Bobnar, V.; Pronin, A.V.; Ritus, A.I.; Volkov, A.A.; Loidl, A. Origin of apparent colossal dielectric constants. Phys. Rev. B 2002, 66. [Google Scholar] [CrossRef]
- Tang, H.; Sodano, H.A. Ultra high energy density nanocomposite capacitors with fast discharge using Ba0.2Sr0.8TiO3 nanowires. Nano Lett. 2013, 13, 1373–1379. [Google Scholar] [PubMed]
- Lunkenheimer, P.; Fichtl, R.; Ebbinghaus, S.G.; Loidl, A. Nonintrinsic origin of the colossal dielectric constants in CaCu3Ti4O12. Phys. Rev. B 2004, 70. [Google Scholar] [CrossRef]
- Krohns, S.; Lu, J.; Lunkenheimer, P.; Brize, V.; Autret-Lambert, C.; Gervais, M.; Gervais, F.; Bouree, F.; Porcher, F.; Loidl, A. Correlations of structural, magnetic, and dielectric properties of undoped and doped CaCu3Ti4O12. Eur. Phys. J. B 2009, 72, 173–182. [Google Scholar] [CrossRef]
- Kant, C.; Rudolf, T.; Mayr, F.; Krohns, S.; Lunkenheimer, P.; Ebbinghaus, S.G.; Loidl, A. Broadband dielectric response of CaCu3Ti4O12: From dc to the electronic transition regime. Phys. Rev. B 2008, 77. [Google Scholar] [CrossRef]
- Barsoukov, E.; MacDonald, J.R. Impedance Spectroscopy Theory, Experimental and Applications, 2nd ed.; Wiley: New York, NY, USA, 2005; p. 4. [Google Scholar]
- Raistrick, I.D.; Franceschetti, D.; MacDonald, J.R. Impedance Spectroscopy Emphasizing Solid Materials and Systems; MacDonald, J.R., Ed.; Wiley: New York, NY, USA, 1987; p. 27. [Google Scholar]
- Waser, R.; Lohse, O. Science and Technology of Integrated Ferroelectrics. In Proceedings of the International Symposium on Integrated Ferroelectrics, Colorado Springs, CO, USA, 11–14 March 2001; de Aranjo, C.P., Ramedi, R., Taylor, G.W., Eds.; CRC Press: Boca Raton, FL, USA, 2001; p. 501. [Google Scholar]
- Yun, L.; College of Physical and Mathematical Sciences, Australia National University, Canberra, Australia. Personal communication, 2015.
- Reynolds, G.J.; Krutzer, M.; Dubs, M.; Felzer, H.; Mamazza, R. Electrical properties of thin-film capacitors fabricated using high temperature sputtered modified barium titanate. Materials 2012, 5, 644–660. [Google Scholar] [CrossRef]
- Kim, Y.; Kathaperumal, M.; Chen, V.W.; Park, Y.; Fuentes-Hernandez, C.; Pan, M.J.; Kippelen, B.; Perry, J.W. Energy storage: Bilayer structure with ultrahigh energy/power density using hybrid sol-gel dielectric and charge-blocking monolayer. Adv. Energy Mat. 2015. [Google Scholar] [CrossRef]
- Parizi, S.S.; Mellinger, A.; Caruntu, G. Ferroelectric barium titanate nanocubes as capacitive building blocks for energy storage applications. ACS Appl. Mater. Interfaces 2014, 6, 17506–17517. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Ma, B.; Narayanan, M.; Liu, S.; Koritala, R.; Balachandran, U.; Shi, D. Lead lanthanum zirconate titanate ceramic thin films for energy storage. ACS Appl. Mater. Interfaces 2013, 5, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.; Clausen, B.; Dumesic, J.A. Iron pentacarbonyl decomposition over grafoil. Production of small metallic iron particles. J. Phys. Chem. 1980, 84, 1814–1822. [Google Scholar] [CrossRef]
- Phillips, J.; Dumesic, J.A. Iron pentacarbonyl decomposition over grafoil: II. Effect of sample outgassing on decomposition kinetics. Appl. of Surf. Sci. 1981, 7, 215–230. [Google Scholar] [CrossRef]
- Jenkins, N.R. Optimal Superdielectric Materials. M.S. Thesis, Naval Postgraduate School, Monterey, CA, USA, September 2015. [Google Scholar]

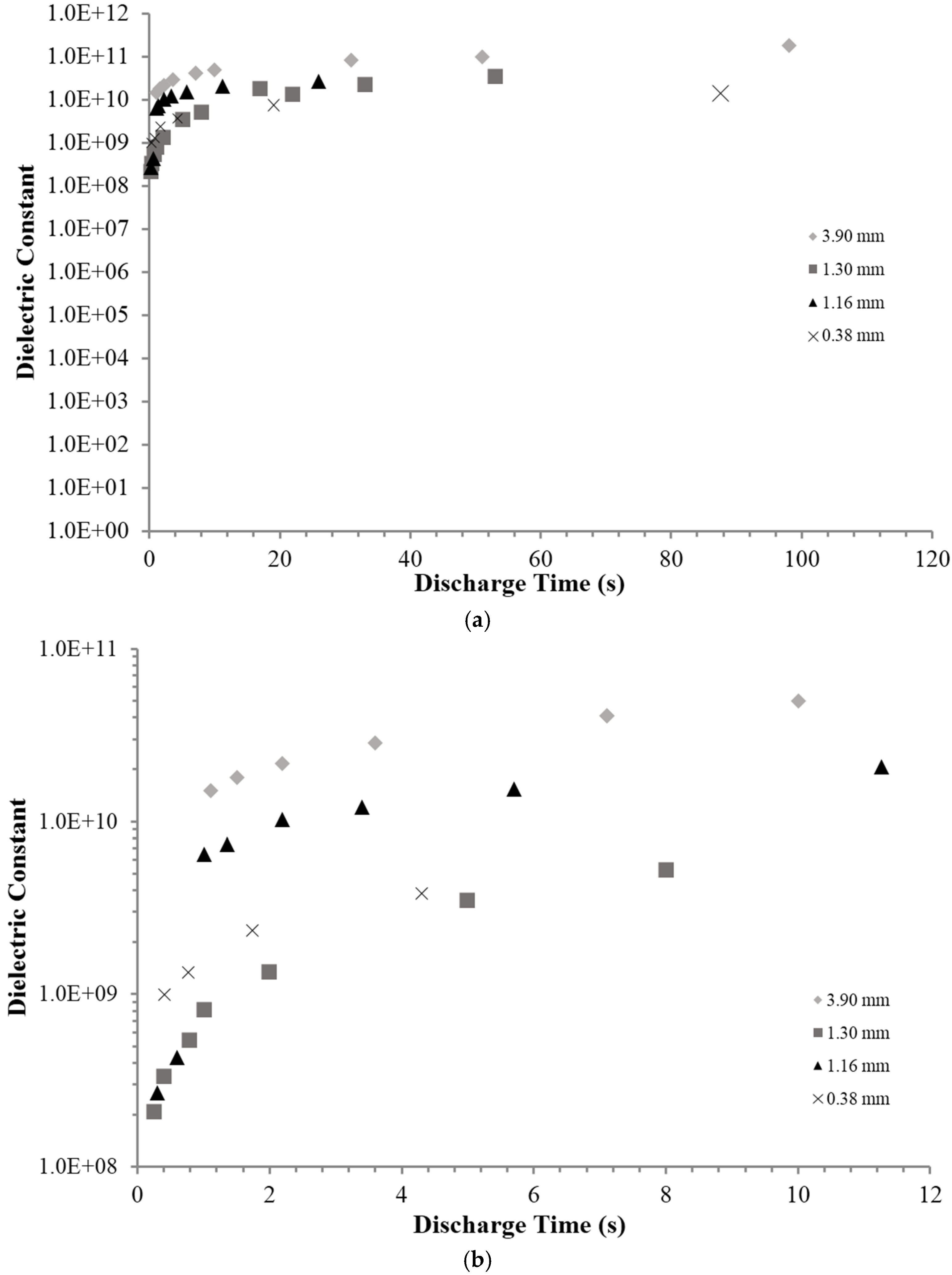



| Capacitor | Dielectric Layer Thickness * (mm) (±0.05) | MAX/MIN Current ** Applied (mAmps) | Dielectric Constant, <0.8 V At DT of 10 s (±10%) |
|---|---|---|---|
| 1 | 3.90 | 70/10 | 5.5 × 1010 |
| 2 | 1.30 | 90/8 | 7.0 × 109 |
| 3 | 1.15 | 110/10 | 1.05 × 1010 |
| 4 | 0.38 | 100/10 | 6.0 × 109 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jenkins, N.; Petty, C.; Phillips, J. Investigation of Fumed Silica/Aqueous NaCl Superdielectric Material. Materials 2016, 9, 118. https://doi.org/10.3390/ma9020118
Jenkins N, Petty C, Phillips J. Investigation of Fumed Silica/Aqueous NaCl Superdielectric Material. Materials. 2016; 9(2):118. https://doi.org/10.3390/ma9020118
Chicago/Turabian StyleJenkins, Natalie, Clayton Petty, and Jonathan Phillips. 2016. "Investigation of Fumed Silica/Aqueous NaCl Superdielectric Material" Materials 9, no. 2: 118. https://doi.org/10.3390/ma9020118