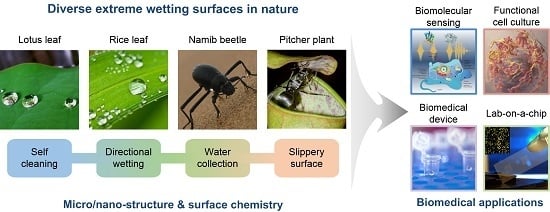
Bio-Inspired Extreme Wetting Surfaces for Biomedical Applications
Abstract
:1. Introduction
2. Design and Fabrication of Bio-Inspired Extreme Wetting Surfaces
2.1. Non-Wettable Superhydrophobic Surfaces
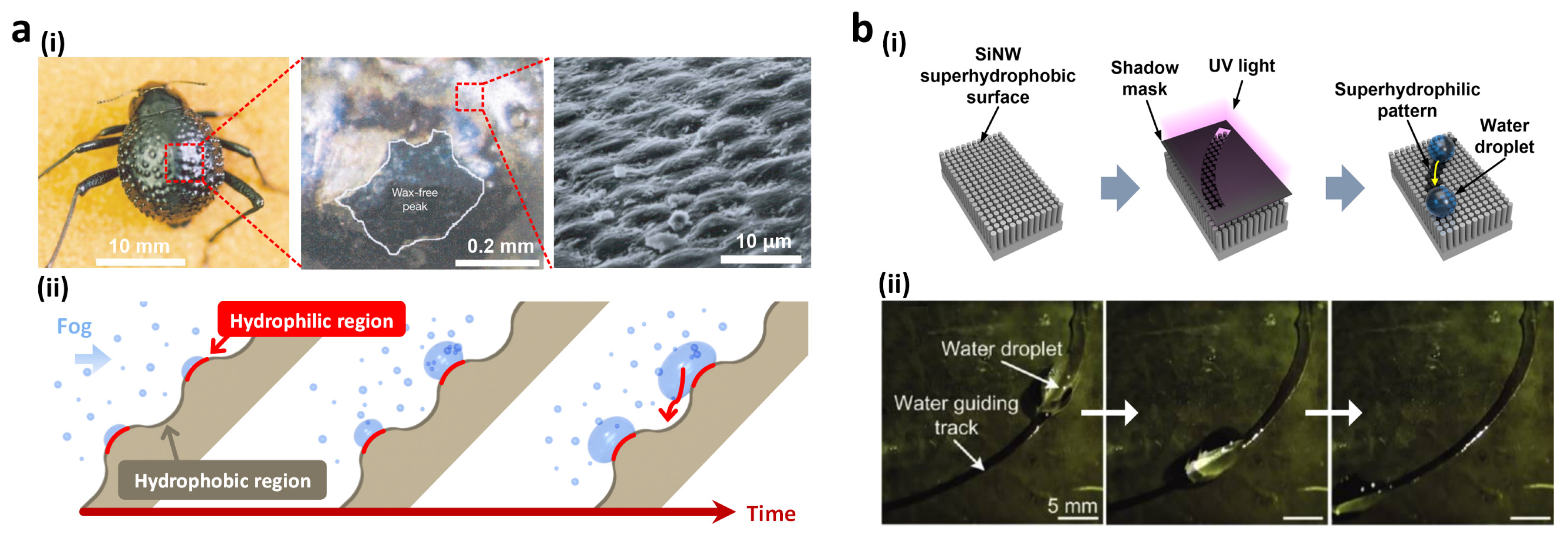
2.2. Patterned Wettabiltiy for Water Collection
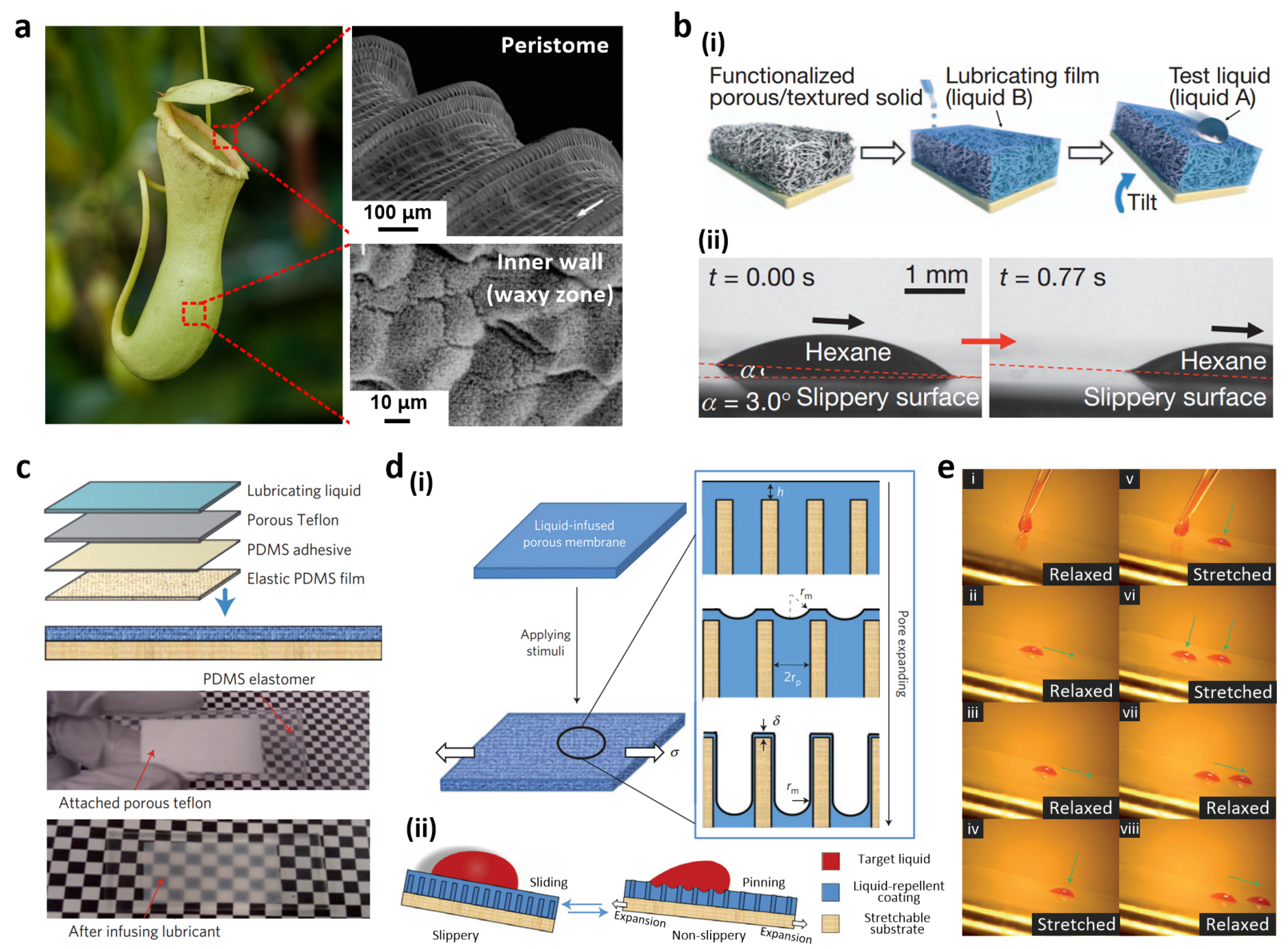
2.3. Liquid Slippery Surfaces
2.4. Wettability Switchable Superhydrophobic Surfaces
3. Biomedical Applications of Bio-Inspired Extreme Wetting Surfaces
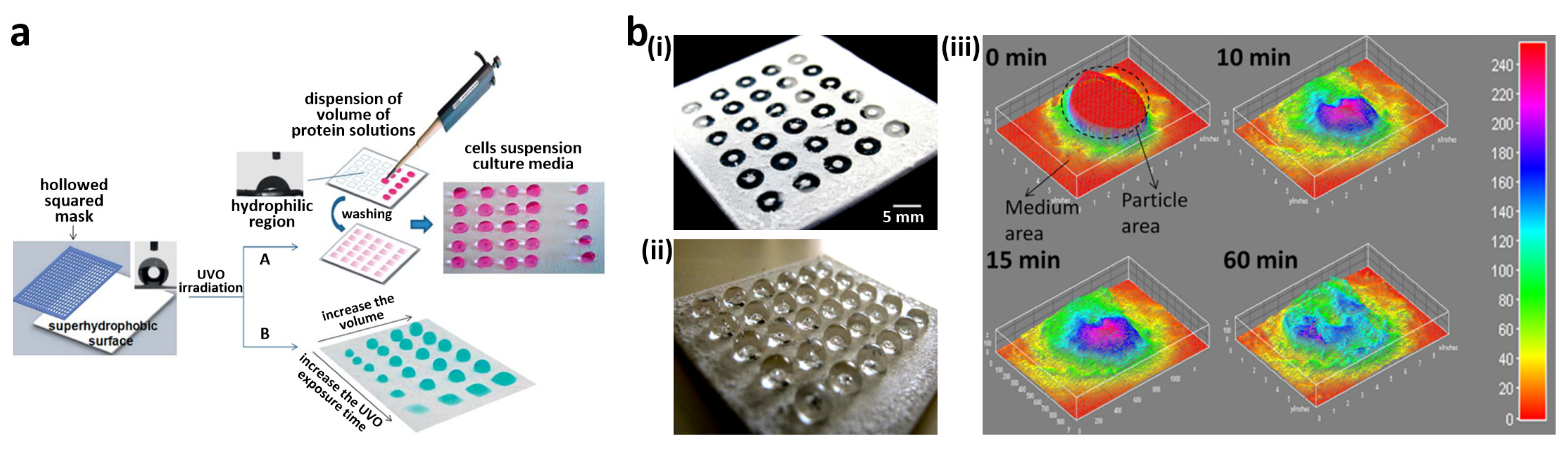
3.1. Cell Patterning for Cellular Interaction Studies
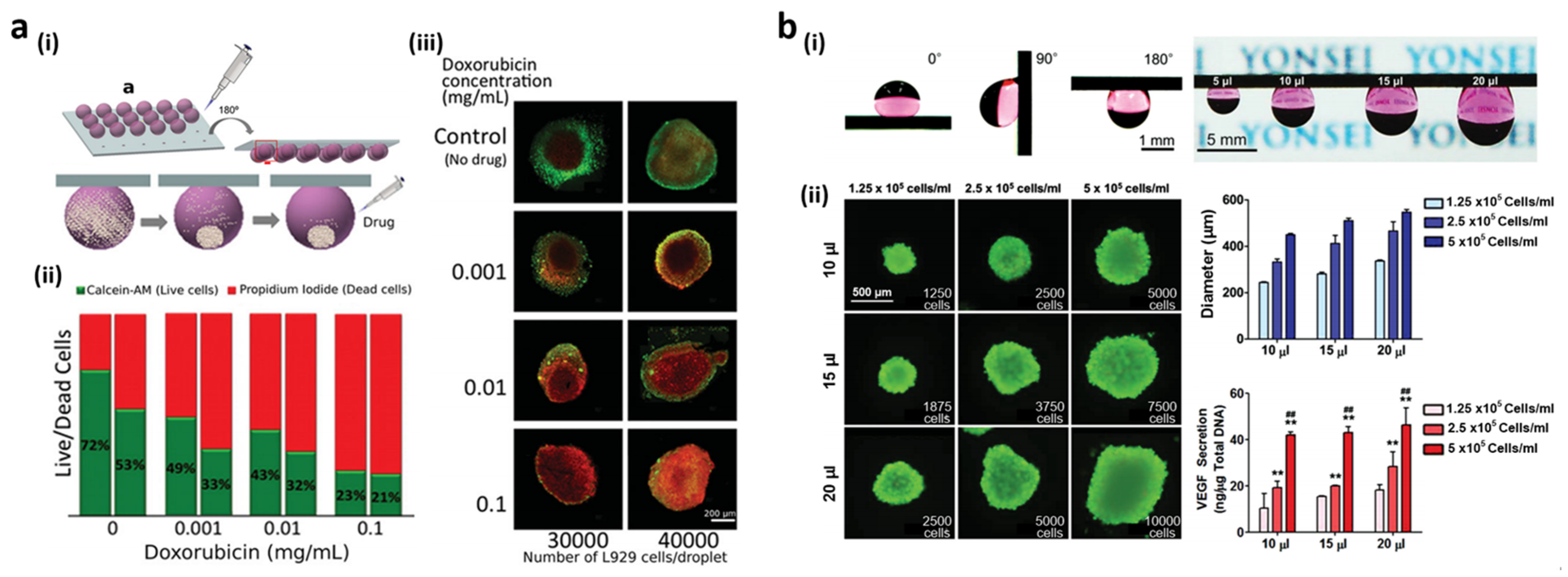
3.2. Functional Cell Spheroid Culture
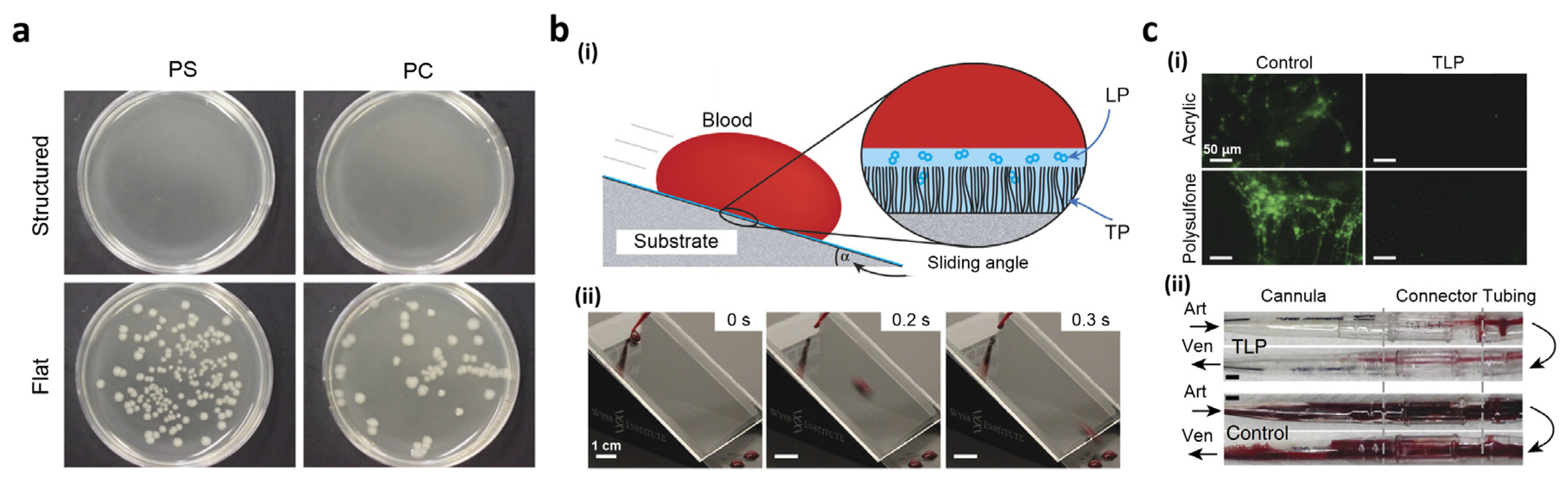
3.3. Biomedical Devices
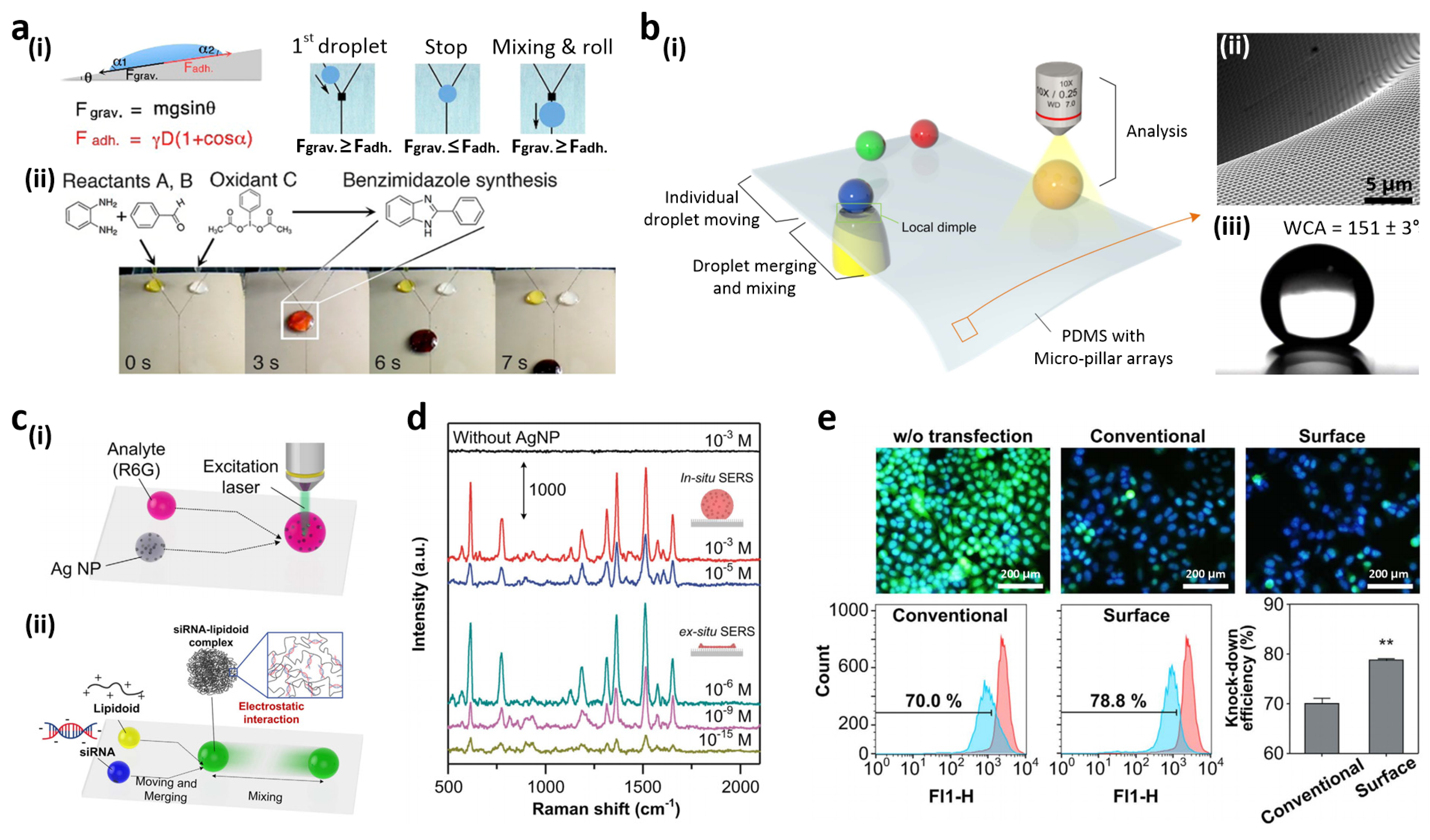
3.4. Open-Channel, Droplet-Based Lab-on-a-Chip
3.5. High-Throughput Cell Assay
4. Conclusions and Future Prospects
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, K.; Jiang, L. Bio-inspired design of multiscale structures for function integration. Nano Today 2011, 6, 155–175. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Xia, H.; Kim, E.; Sun, H.-B. Recent developments in superhydrophobic surfaces with unique structural and functional properties. Soft Matter 2012, 8, 11217–11231. [Google Scholar] [CrossRef]
- Liu, X.; Liang, Y.; Zhou, F.; Liu, W. Extreme wettability and tunable adhesion: Biomimicking beyond nature? Soft Matter 2012, 8, 2070–2086. [Google Scholar] [CrossRef]
- Bixler, G.D.; Theiss, A.; Bhushan, B.; Lee, S.C. Anti-fouling properties of microstructured surfaces bio-inspired by rice leaves and butterfly wings. J. Colloid Interface Sci. 2014, 419, 114–133. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Song, Y.; Jiang, L. Applications of bio-inspired special wettable surfaces. Adv. Mater. 2011, 23, 719–734. [Google Scholar] [CrossRef] [PubMed]
- A Chinese nano-society? Nat. Mater. 2005, 4, 355.
- Zorba, V.; Chen, X.; Mao, S.S. Superhydrophilic TiO2 surface without photocatalytic activation. Appl. Phys. Lett. 2010, 96. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, D.; Qiu, R. Liquid/solid contact mode of super-hydrophobic film in aqueous solution and its effect on corrosion resistance. Corros. Sci. 2012, 54, 77–84. [Google Scholar] [CrossRef]
- Bai, H.; Wang, L.; Ju, J.; Sun, R.; Zheng, Y.; Jiang, L. Efficient water collection on integrative bioinspired surfaces with star-shaped wettability patterns. Adv. Mater. 2014, 26, 5025–5030. [Google Scholar] [CrossRef] [PubMed]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Parker, A.R.; Lawrence, C.R. Water capture by a desert beetle. Nature 2001, 414, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Bohn, H.F.; Federle, W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proc. Natl. Acad. Sci. USA 2004, 101, 14138–14143. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, T.; Saito, N.; Takai, O. Correlation of cell adhesive behaviors on superhydrophobic, superhydrophilic, and micropatterned superhydrophobic/superhydrophilic surfaces to their surface chemistry. Langmuir 2010, 26, 8147–8154. [Google Scholar] [CrossRef] [PubMed]
- Geyer, F.L.; Ueda, E.; Liebel, U.; Grau, N.; Levkin, P.A. Superhydrophobic-superhydrophilic micropatterning: Towards genome-on-a-chip cell microarrays. Angew. Chem. Int. Ed. 2011, 50, 8424–8427. [Google Scholar] [CrossRef] [PubMed]
- Piret, G.; Galopin, E.; Coffinier, Y.; Boukherroub, R.; Legrand, D.; Slomianny, C. Culture of mammalian cells on patterned superhydrophilic/superhydrophobic silicon nanowire arrays. Soft Matter 2011, 7, 8642–8649. [Google Scholar] [CrossRef]
- Oliveira, M.B.; Neto, A.I.; Correia, C.R.; Rial-Hermida, M.I.; Alvarez-Lorenzo, C.; Mano, J.F. Superhydrophobic chips for cell spheroids high-throughput generation and drug screening. ACS Appl. Mater. Interfaces 2014, 6, 9488–9495. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.I.; Correia, C.R.; Custódio, C.A.; Mano, J.F. Biomimetic miniaturized platform able to sustain arrays of liquid droplets for high-throughput combinatorial tests. Adv. Funct. Mater. 2014, 24, 5096–5103. [Google Scholar] [CrossRef]
- Neto, A.I.; Custodio, C.A.; Song, W.; Mano, J.F. High-throughput evaluation of interactions between biomaterials, proteins and cells using patterned superhydrophobic substrates. Soft Matter 2011, 7, 4147–4151. [Google Scholar] [CrossRef] [Green Version]
- Ueda, E.; Levkin, P.A. Emerging applications of superhydrophilic-superhydrophobic micropatterns. Adv. Mater. 2013, 25, 1234–1247. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.B.; Mano, J.F. High-throughput screening for integrative biomaterials design: Exploring advances and new trends. Trends Biotechnol. 2014, 32, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J.; Reifler, F.A.; Fortunato, G.; Gerhardt, L.-C.; Seeger, S. A simple, one-step approach to durable and robust superhydrophobic textiles. Adv. Funct. Mater. 2008, 18, 3662–3669. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, X.; Li, B.; Sun, P.; Yang, J.; Xu, H.; Liu, Y. Superhydrophobic and ultraviolet-blocking cotton textiles. ACS Appl. Mater. Interfaces 2011, 3, 1277–1281. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wu, S.-Z.; Chen, Q.-D.; Zhang, Y.-L.; Yao, J.; Yao, X.; Niu, L.-G.; Wang, J.-N.; Jiang, L.; Sun, H.-B. Curvature-driven reversible in situ switching between pinned and roll-down superhydrophobic states for water droplet transportation. Adv. Mater. 2011, 23, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Liu, T.; Chen, S.; Liu, T.; Cheng, S. Structure stability and corrosion inhibition of super-hydrophobic film on aluminum in seawater. Appl. Surf. Sci. 2008, 255, 2978–2984. [Google Scholar] [CrossRef]
- Grignard, B.; Vaillant, A.; de Coninck, J.; Piens, M.; Jonas, A.M.; Detrembleur, C.; Jerome, C. Electrospinning of a functional perfluorinated block copolymer as a powerful route for imparting superhydrophobicity and corrosion resistance to aluminum substrates. Langmuir 2011, 27, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhang, F.; Wang, L.; Xu, S.; Guo, X. In situ crystallized zirconium phenylphosphonate films with crystals vertically to the substrate and their hydrophobic, dielectric, and anticorrosion properties. Langmuir 2010, 26, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Szunerits, S.; Xu, W.; Boukherroub, R. Preparation of superhydrophobic coatings on zinc as effective corrosion barriers. ACS Appl. Mater. Interfaces 2009, 1, 1150–1153. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Young, T. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Neinhuis, C.; Barthlott, W. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Bot. 1997, 79, 667–677. [Google Scholar] [CrossRef]
- Kim, T.; Tahk, D.; Lee, H.H. Wettability-controllable super water- and moderately oil-repellent surface fabricated by wet chemical etching. Langmuir 2009, 25, 6576–6579. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Lee, S.; Han, H.; Chung, Y.; Lee, J.; Kim, S.-D.; Kim, Y.-W.; Lim, S.; Lee, T. Reversible wettability control of silicon nanowire surfaces: From superhydrophilicity to superhydrophobicity. Thin Solid Films 2013, 527, 179–185. [Google Scholar] [CrossRef]
- Piret, G.; Coffinier, Y.; Roux, C.; Melnyk, O.; Boukherroub, R. Biomolecule and nanoparticle transfer on patterned and heterogeneously wetted superhydrophobic silicon nanowire surfaces. Langmuir 2008, 24, 1670–1672. [Google Scholar] [CrossRef] [PubMed]
- Coffinier, Y.; Piret, G.; Das, M.R.; Boukherroub, R. Effect of surface roughness and chemical composition on the wetting properties of silicon-based substrates. C. R. Chim. 2013, 16, 65–72. [Google Scholar] [CrossRef]
- Dupré, M.; Enjalbal, C.; Cantel, S.; Martinez, J.; Megouda, N.; Hadjersi, T.; Boukherroub, R.; Coffinier, Y. Investigation of silicon-based nanostructure morphology and chemical termination on laser desorption ionization mass spectrometry performance. Anal. Chem. 2012, 84, 10637–10644. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.N.; Dufour, R.; Thomy, V.; Senez, V.; Boukherroub, R.; Coffinier, Y. Fabrication of superhydrophobic and highly oleophobic silicon-based surfaces via electroless etching method. Appl. Surf. Sci. 2014, 295, 38–43. [Google Scholar] [CrossRef]
- Yu, L.-Y.; Shen, H.-M.; Xu, Z.-L. PVDF-TiO2 composite hollow fiber ultrafiltration membranes prepared by TiO2 sol-gel method and blending method. J. Appl. Polym. Sci. 2009, 113, 1763–1772. [Google Scholar] [CrossRef]
- He, G.; Wang, K. The super hydrophobicity of ZnO nanorods fabricated by electrochemical deposition method. Appl. Surf. Sci. 2011, 257, 6590–6594. [Google Scholar] [CrossRef]
- Thomas, Y.R.J.; Benayad, A.; Schroder, M.; Morin, A.; Pauchet, J. New method for super hydrophobic treatment of gas diffusion layers for proton exchange membrane fuel cells using electrochemical reduction of diazonium salts. ACS Appl. Mater. Interfaces 2015, 7, 15068–15077. [Google Scholar] [CrossRef] [PubMed]
- Tadanaga, K.; Morinaga, J.; Matsuda, A.; Minami, T. Superhydrophobic-superhydrophilic micropatterning on flowerlike alumina coating film by the sol-gel method. Chem. Mater. 2000, 12, 590–592. [Google Scholar] [CrossRef]
- Kim, H.-C.; Kreller, C.R.; Tran, K.A.; Sisodiya, V.; Angelos, S.; Wallraff, G.; Swanson, S.; Miller, R.D. Nanoporous thin films with hydrophilicity-contrasted patterns. Chem. Mater. 2004, 16, 4267–4272. [Google Scholar] [CrossRef]
- Kavalenka, M.N.; Hopf, A.; Schneider, M.; Worgull, M.; Hölscher, H. Wood-based microhaired superhydrophobic and underwater superoleophobic surfaces for oil/water separation. RSC Adv. 2014, 4, 31079–31083. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Zheng, Y.; Huang, Z.; Feng, L.; Jiang, L. Stable, superhydrophobic, and conductive polyaniline/polystyrene films for corrosive environments. Adv. Funct. Mater. 2006, 16, 568–574. [Google Scholar] [CrossRef]
- Sarkar, M.K.; Bal, K.; He, F.; Fan, J. Design of an outstanding super-hydrophobic surface by electro-spinning. Appl. Surf. Sci. 2011, 257, 7003–7009. [Google Scholar] [CrossRef]
- Nuraje, N.; Khan, W.S.; Lei, Y.; Ceylan, M.; Asmatulu, R. Superhydrophobic electrospun nanofibers. J. Mater. Chem. A 2013, 1, 1929–1946. [Google Scholar] [CrossRef]
- Tadanaga, K.; Katata, N.; Minami, T. Formation process of super-water-repellent Al2O3 coating films with high transparency by the sol-gel method. J. Am. Ceram. Soc. 1997, 80, 3213–3216. [Google Scholar] [CrossRef]
- Shirtcliffe, N.J.; McHale, G.; Newton, M.I.; Perry, C.C. Intrinsically Superhydrophobic organosilica sol-gel foams. Langmuir 2003, 19, 5626–5631. [Google Scholar] [CrossRef]
- Tsai, M.-Y.; Hsu, C.-C.; Chen, P.-H.; Lin, C.-S.; Chen, A. Surface modification on a glass surface with a combination technique of sol-gel and air brushing processes. Appl. Surf. Sci. 2011, 257, 8640–8646. [Google Scholar] [CrossRef]
- Mahadik, S.; Mahadik, D.B.; Kavale, M.S.; Parale, V.G.; Wagh, P.B.; Barshilia, H.; Gupta, S.; Hegde, N.D.; Rao, A.V. Thermally stable and transparent superhydrophobic sol-gel coatings by spray method. J. Sol Gel Sci. Technol. 2012, 63, 580–586. [Google Scholar] [CrossRef]
- Latthe, S.S.; Terashima, C.; Nakata, K.; Sakai, M.; Fujishima, A. Development of sol-gel processed semi-transparent and self-cleaning superhydrophobic coatings. J. Mater. Chem. A 2014, 2, 5548–5553. [Google Scholar] [CrossRef]
- Buck, M.E.; Schwartz, S.C.; Lynn, D.M. Superhydrophobic thin films fabricated by reactive layer-by-layer assembly of azlactone-functionalized polymers. Chem. Mater. 2010, 22, 6319–6327. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; He, J. A self-templated etching route to surface-rough silica nanoparticles for superhydrophobic coatings. ACS Appl. Mater. Interfaces 2011, 3, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Manna, U.; Carter, M.C.D.; Lynn, D.M. “Shrink-to-fit” superhydrophobicity: Thermally-induced microscale wrinkling of thin hydrophobic multilayers fabricated on flexible shrink-wrap substrates. Adv. Mater. 2013, 25, 3085–3089. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Dilworth, Z.R.; Hsiao, E.; Barnette, A.L.; Marino, M.; Kim, J.H.; Kang, J.-G.; Jung, T.-H.; Kim, S.H. One-step production of superhydrophobic coatings on flat substrates via atmospheric rf plasma process using non-fluorinated hydrocarbons. ACS Appl. Mater. Interfaces 2011, 3, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Salapare, H.S., III; Guittard, F.; Noblin, X.; Taffin de Givenchy, E.; Celestini, F.; Ramos, H.J. Stability of the hydrophilic and superhydrophobic properties of oxygen plasma-treated poly(tetrafluoroethylene) surfaces. J. Colloid Interface Sci. 2013, 396, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Kessler, F.; Kühn, S.; Radtke, C.; Weibel, D.E. Controlling the surface wettability of poly(sulfone) films by UV-assisted treatment: Benefits in relation to plasma treatment. Polym. Int. 2013, 62, 310–318. [Google Scholar] [CrossRef]
- Balu, B.; Breedveld, V.; Hess, D.W. Fabrication of “roll-off” and “sticky” superhydrophobic cellulose surfaces via plasma processing. Langmuir 2008, 24, 4785–4790. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhao, Y.; Zhai, J. A lotus-leaf-like superhydrophobic surface: A porous microsphere/nanofiber composite film prepared by electrohydrodynamics. Angew. Chem. Int. Ed. 2004, 116, 4438–4441. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.J.; Heo, S.H.; Li, C.C.; Choi, Y.K.; Cai, W.P.; Cho, S.O. Superhydrophobic bionic surfaces with hierarchical microsphere/SWCNT composite arrays. Langmuir 2007, 23, 2169–2174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-X.; Chen, Y.-X.; Guo, Z.; Liu, H.-L.; Wang, D.-P.; Huang, X.-J. Bioinspired multifunctional hetero-hierarchical micro/nanostructure tetragonal array with self-cleaning, anticorrosion, and concentrators for the SERS detection. ACS Appl. Mater. Interfaces 2013, 5, 10633–10642. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhang, Y.; Xi, J.; Zhu, Y.; Wang, N.; Xia, F.; Jiang, L. Petal effect: A superhydrophobic state with high adhesive force. Langmuir 2008, 24, 4114–4119. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Luo, C.; Xu, L.; Ji, H.; Ouyang, Q.; Yu, D.; Chen, Y. Artificial lotus leaf by nanocasting. Langmuir 2005, 21, 8978–8981. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Gao, X.; Zhuang, H.; Huang, J.; Lin, C.; Jiang, L. Designing superhydrophobic porous nanostructures with tunable water adhesion. Adv. Mater. 2009, 21, 3799–3803. [Google Scholar] [CrossRef]
- Bixler, G.D.; Bhushan, B. Bioinspired rice leaf and butterfly wing surface structures combining shark skin and lotus effects. Soft Matter 2012, 8, 11271–11284. [Google Scholar] [CrossRef]
- Malvadkar, N.A.; Hancock, M.J.; Sekeroglu, K.; Dressick, W.J.; Demirel, M.C. An engineered anisotropic nanofilm with unidirectional wetting properties. Nat. Mater. 2010, 9, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lu, Q.; Li, M.; Li, X. Anisotropic wetting characteristics on submicrometer-scale periodic grooved surface. Langmuir 2007, 23, 6212–6217. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Brueck, S.R.J. Strongly anisotropic wetting on one-dimensional nanopatterned surfaces. Nano Lett. 2008, 8, 2819–2824. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, R.; Sun, Y.; Lin, D.; Sun, Z.; Pan, W.; Downs, P. Biomimetic nanofiber patterns with controlled wettability. Soft Matter 2008, 4, 2429–2433. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Y.; Xu, H.; Wang, Z.; Zhang, X. Mimicking biological structured surfaces by phase-separation micromolding. Langmuir 2009, 25, 4365–4369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, J.; Ren, Z.; Li, X.; Zhang, X.; Zhu, D.; Wang, T.; Tian, T.; Yang, B. Morphology and wettability control of silicon cone arrays using colloidal lithography. Langmuir 2009, 25, 7375–7382. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-Z.; Wu, D.; Yao, J.; Chen, Q.-D.; Wang, J.-N.; Niu, L.-G.; Fang, H.-H.; Sun, H.-B. One-step preparation of regular micropearl arrays for two-direction controllable anisotropic wetting. Langmuir 2010, 26, 12012–12016. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, J.-N.; Wu, S.-Z.; Chen, Q.-D.; Zhao, S.; Zhang, H.; Sun, H.-B.; Jiang, L. Three-level biomimetic rice-leaf surfaces with controllable anisotropic sliding. Adv. Funct. Mater. 2011, 21, 2927–2932. [Google Scholar] [CrossRef]
- Kang, S.M.; Lee, C.; Kim, H.N.; Lee, B.J.; Lee, J.E.; Kwak, M.K.; Suh, K.-Y. Directional oil sliding surfaces with hierarchical anisotropic groove microstructures. Adv. Mater. 2013, 25, 5756–5761. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Gao, X.; Jiang, L. Directional adhesion of superhydrophobic butterfly wings. Soft Matter 2007, 3, 178–182. [Google Scholar] [CrossRef]
- Seo, J.; Lee, S.; Lee, J.; Lee, T. Guided transport of water droplets on superhydrophobic-hydrophilic patterned Si nanowires. ACS Appl. Mater. Interfaces 2011, 3, 4722–4729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jin, M.; Liu, Z.; Tryk, D.A.; Nishimoto, S.; Murakami, T.; Fujishima, A. Superhydrophobic TiO2 surfaces: Preparation, photocatalytic wettability conversion, and superhydrophobic-superhydrophilic patterning. J. Phys. Chem. C 2007, 111, 14521–14529. [Google Scholar] [CrossRef]
- Garrod, R.P.; Harris, L.G.; Schofield, W.C.E.; McGettrick, J.; Ward, L.J.; Teare, D.O.H.; Badyal, J.P.S. Mimicking a stenocara beetle’s back for microcondensation using plasmachemical patterned superhydrophobic-superhydrophilic surfaces. Langmuir 2007, 23, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, L.; Shankar, S.S.; Fragouli, D.; Athanassiou, A.; Cingolani, R.; Pompa, P.P. Microscale patterning of hydrophobic/hydrophilic surfaces by spatially controlled galvanic displacement reactions. Langmuir 2009, 25, 6019–6023. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, Z.; Men, X.; Yang, J.; Xu, X. Rapid formation of superhydrophobic surfaces with fast response wettability transition. ACS Appl. Mater. Interfaces 2010, 2, 3636–3641. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; You, I.; Cho, W.K.; Shon, H.K.; Lee, T.G.; Choi, I.S.; Karp, J.M.; Lee, H. One-step modification of superhydrophobic surfaces by a mussel-inspired polymer coating. Angew. Chem. Int. Ed. 2010, 49, 9401–9404. [Google Scholar] [CrossRef] [PubMed]
- Piret, G.; Desmet, R.; Diesis, E.; Drobecq, H.; Segers, J.; Rouanet, C.; Debrie, A.-S.; Boukherroub, R.; Locht, C.; Melnyk, O. Chips from chips: Application to the study of antibody responses to methylated proteins. J. Proteome Res. 2010, 9, 6467–6478. [Google Scholar] [CrossRef] [PubMed]
- Piret, G.; Drobecq, H.; Coffinier, Y.; Melnyk, O.; Boukherroub, R. Matrix-free laser desorption/ionization mass spectrometry on silicon nanowire arrays prepared by chemical etching of crystalline silicon. Langmuir 2010, 26, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Suzuki, H.; Su, Y.; Tang, Z.; Zhang, L.; Yomo, T. Bio-inspired three-dimensional self-patterning of functional coatings for PDMS microfluidics. Soft Matter 2013, 9, 3473–3477. [Google Scholar] [CrossRef]
- Zhai, L.; Berg, M.C.; Cebeci, F.Ç.; Kim, Y.; Milwid, J.M.; Rubner, M.F.; Cohen, R.E. Patterned superhydrophobic surfaces: Toward a synthetic mimic of the namib desert beetle. Nano Lett. 2006, 6, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Li, J.S.; Ueda, E.; Nallapaneni, A.; Li, L.X.; Levkin, P.A. Printable superhydrophilic-superhydrophobic micropatterns based on supported lipid layers. Langmuir 2012, 28, 8286–8291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, J.; Hedhili, M.N.; Yang, X.; Wang, P. Inkjet printing for direct micropatterning of a superhydrophobic surface: Toward biomimetic fog harvesting surfaces. J. Mater. Chem. A 2015, 3, 2844–2852. [Google Scholar] [CrossRef]
- Beutel, R.G.; Gorb, S.N. Ultrastructure of attachment specializations of hexapods (arthropoda): Evolutionary patterns inferred from a revised ordinal phylogeny. J. Zool. Syst. Evol. Res. 2001, 39, 177–207. [Google Scholar] [CrossRef]
- Gorb, E.; Kastner, V.; Peressadko, A.; Arzt, E.; Gaume, L.; Rowe, N.; Gorb, S. Structure and properties of the glandular surface in the digestive zone of the pitcher in the carnivorous plant nepenthes ventrata and its role in insect trapping and retention. J. Exp. Biol. 2004, 207, 2947–2963. [Google Scholar] [CrossRef] [PubMed]
- Bauer, U.; Federle, W. The insect-trapping rim of nepenthes pitchers. Plant Signal. Behav. 2009, 4, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.-S.; Kang, S.H.; Tang, S.K.Y.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Hu, Y.; Grinthal, A.; Wong, T.-S.; Mahadevan, L.; Aizenberg, J. Adaptive fluid-infused porous films with tunable transparency and wettability. Nat. Mater. 2013, 12, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Li, J.; Mieszkin, S.; Di Fino, A.; Clare, A.S.; Callow, M.E.; Callow, J.A.; Grunze, M.; Rosenhahn, A.; Levkin, P.A. Slippery liquid-infused porous surfaces showing marine antibiofouling properties. ACS Appl. Mater. Interfaces 2013, 5, 10074–10080. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; Lu, W.; Xu, H.; Kim, P.; Kreder, M.J.; Alvarenga, J.; Aizenberg, J. Inhibition of ice nucleation by slippery liquid-infused porous surfaces (SLIPS). Phys. Chem. Chem. Phys. 2013, 15, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kleintschek, T.; Rieder, A.; Cheng, Y.; Baumbach, T.; Obst, U.; Schwartz, T.; Levkin, P.A. Hydrophobic liquid-infused porous polymer surfaces for antibacterial applications. ACS Appl. Mater. Interfaces 2013, 5, 6704–6711. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cheng, F.; Lv, J.; Zhao, Y.; Liu, M.; Jiang, L.; Yu, Y. Light-controlled quick switch of adhesion on a micro-arrayed liquid crystal polymer superhydrophobic film. Soft Matter 2012, 8, 3730–3733. [Google Scholar] [CrossRef]
- Seo, J.; Lee, S.; Han, H.; Jung, H.B.; Hong, J.; Song, G.; Cho, S.M.; Park, C.; Lee, W.; Lee, T. Gas-driven ultrafast reversible switching of super-hydrophobic adhesion on palladium-coated silicon nanowires. Adv. Mater. 2013, 25, 4139–4144. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, J.; He, H.; Wan, M.; Jiang, L. Reversible wettability switching of polyaniline-coated fabric, triggered by ammonia gas. Macromol. Rapid Commun. 2007, 28, 2230–2236. [Google Scholar] [CrossRef]
- Cheng, Z.; Feng, L.; Jiang, L. Tunable adhesive superhydrophobic surfaces for superparamagnetic microdroplets. Adv. Funct. Mater. 2008, 18, 3219–3225. [Google Scholar] [CrossRef]
- Li, C.; Guo, R.; Jiang, X.; Hu, S.; Li, L.; Cao, X.; Yang, H.; Song, Y.; Ma, Y.; Jiang, L. Reversible switching of water-droplet mobility on a superhydrophobic surface based on a phase transition of a side-chain liquid-crystal polymer. Adv. Mater. 2009, 21, 4254–4258. [Google Scholar] [CrossRef]
- Liu, X.; Ye, Q.; Yu, B.; Liang, Y.; Liu, W.; Zhou, F. Switching water droplet adhesion using responsive polymer brushes. Langmuir 2010, 26, 12377–12382. [Google Scholar] [CrossRef] [PubMed]
- Qing, G.; Sun, T. Chirality-triggered wettability switching on a smart polymer surface. Adv. Mater. 2011, 23, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cai, M.; Liang, Y.; Zhou, F.; Liu, W. Photo-regulated stick-slip switch of water droplet mobility. Soft Matter 2011, 7, 3331–3336. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Ju, J.; Cheng, F.; Liu, M.; Jiang, L.; Yu, Y. In situ fully light-driven switching of superhydrophobic adhesion. Adv. Funct. Mater. 2012, 22, 760–763. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Fei, B.; Hu, H.; Lai, C.; Xin, J.H. Bioinspired, stimuli-responsive, multifunctional superhydrophobic surface with directional wetting, adhesion, and transport of water. Adv. Funct. Mater. 2015, 25, 5047–5056. [Google Scholar] [CrossRef]
- Verplanck, N.; Coffinier, Y.; Thomy, V.; Boukherroub, R. Wettability switching techniques on superhydrophobic surfaces. Nanoscale Res. Lett. 2007, 2, 577–596. [Google Scholar] [CrossRef]
- Gras, S.L.; Mahmud, T.; Rosengarten, G.; Mitchell, A.; Kalantar-zadeh, K. Intelligent control of surface hydrophobicity. Chemphyschem 2007, 8, 2036–2050. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, Y.; Liu, X.; Zhou, F.; Liu, W.; Xue, Q. Towards a tunable and switchable water adhesion on a TiO2 nanotube film with patterned wettability. Chem. Commun. 2009, 7018–7020. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.M.; Shi, J.; Oramas, E.; Santos, J.L.; Tomás, H.; Mano, J.F. Bioinspired superhydrophobic poly(l-lactic acid) surfaces control bone marrow derived cells adhesion and proliferation. J. Biomed. Mater. Res. A 2009, 91, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.M.; Song, W.; Alves, N.M.; Mano, J.F. Chemical modification of bioinspired superhydrophobic polystyrene surfaces to control cell attachment/proliferation. Soft Matter 2011, 7, 8932–8941. [Google Scholar] [CrossRef]
- Ballester-Beltran, J.; Rico, P.; Moratal, D.; Song, W.; Mano, J.F.; Salmeron-Sanchez, M. Role of superhydrophobicity in the biological activity of fibronectin at the cell-material interface. Soft Matter 2011, 7, 10803–10811. [Google Scholar] [CrossRef]
- Auad, P.; Ueda, E.; Levkin, P.A. Facile and multiple replication of superhydrophilic-superhydrophobic patterns using adhesive tape. ACS Appl. Mater. Interfaces 2013, 5, 8053–8057. [Google Scholar] [CrossRef] [PubMed]
- Efremov, A.N.; Stanganello, E.; Welle, A.; Scholpp, S.; Levkin, P.A. Micropatterned superhydrophobic structures for the simultaneous culture of multiple cell types and the study of cell-cell communication. Biomaterials 2013, 34, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.M.; Alves, N.M.; Mano, J.F. Cell interactions with superhydrophilic and superhydrophobic surfaces. J. Adhes. Sci. Technol. 2014, 28, 843–863. [Google Scholar] [CrossRef]
- Li, H.; Yang, Q.; Li, G.; Li, M.; Wang, S.; Song, Y. Splitting a droplet for femtoliter liquid patterns and single cell isolation. ACS Appl. Mater. Interfaces 2015, 7, 9060–9065. [Google Scholar] [CrossRef] [PubMed]
- Galopin, E.; Piret, G.; Szunerits, S.; Lequette, Y.; Faille, C.; Boukherroub, R. Selective adhesion of bacillus cereus spores on heterogeneously wetted silicon nanowires. Langmuir 2010, 26, 3479–3484. [Google Scholar] [CrossRef] [PubMed]
- Marcon, L.; Addad, A.; Coffinier, Y.; Boukherroub, R. Cell micropatterning on superhydrophobic diamond nanowires. Acta Biomater. 2013, 9, 4585–4591. [Google Scholar] [CrossRef] [PubMed]
- Salgado, C.L.; Oliveira, M.B.; Mano, J.F. Combinatorial cell-3D biomaterials cytocompatibility screening for tissue engineering using bioinspired superhydrophobic substrates. Integr. Biol. 2012, 4, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Minchinton, A.I.; Tannock, I.F. Drug penetration in solid tumours. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Frith, J.E.; Thomson, B.; Genever, P.G. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng. Part C. Methods 2010, 16, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Meli, L.; Jordan, E.T.; Clark, D.S.; Linhardt, R.J.; Dordick, J.S. Influence of a three-dimensional, microarray environment on human cell culture in drug screening systems. Biomaterials 2012, 33, 9087–9096. [Google Scholar] [CrossRef] [PubMed]
- Breslin, S.; O’Driscoll, L. Three-dimensional cell culture: the missing link in drug discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Tofilon, P.J.; Buckley, N.; Deen, D.F. Effect of cell-cell interactions on drug sensitivity and growth of drug-sensitive and -resistant tumor cells in spheroids. Science 1984, 226, 862–864. [Google Scholar] [CrossRef] [PubMed]
- Bartosh, T.J.; Ylöstalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. 2010, 107, 13724–13729. [Google Scholar] [CrossRef] [PubMed]
- Torisawa, Y.; Takagi, A.; Nashimoto, Y.; Yasukawa, T.; Shiku, H.; Matsue, T. A multicellular spheroid array to realize spheroid formation, culture, and viability assay on a chip. Biomaterials 2007, 28, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Brophy, C.M.; Luebke-Wheeler, J.L.; Amiot, B.P.; Khan, H.; Remmel, R.P.; Rinaldo, P.; Nyberg, S.L. Rat hepatocyte spheroids formed by rocked technique maintain differentiated hepatocyte gene expression and function. Hepatology 2009, 49, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, A.Y.; Torisawa, Y.; Tung, Y.-C.; Sud, S.; Taichman, R.S.; Pienta, K.J.; Takayama, S. Microfluidic system for formation of pc-3 prostate cancer co-culture spheroids. Biomaterials 2009, 30, 3020–3027. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.F.; No, D.Y.; Choi, Y.Y.; Kim, D.S.; Chung, B.G.; Lee, S.-H. Concave microwell based size-controllable hepatosphere as a three-dimensional liver tissue model. Biomaterials 2011, 32, 8087–8096. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-Z.; Chang, H.-Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008, 3, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.-C.; Hsiao, A.Y.; Allen, S.G.; Torisawa, Y.; Ho, M.; Takayama, S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 2011, 136, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Cavnar, S.P.; Salomonsson, E.; Luker, K.E.; Luker, G.D.; Takayama, S. Transfer, imaging, and analysis plate for facile handling of 384 hanging drop 3D tissue spheroids. J. Lab. Autom. 2014, 19, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Yang, K.; Hwang, Y.H.; Byun, Y.; Lee, D.Y.; Cho, S.-W.; Lee, H. Spheroform: Therapeutic spheroid-forming nanotextured surfaces inspired by desert beetle physosterna cribripes. Adv. Healthc. Mater. 2015, 4, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Lee, J.S.; Lee, K.; Kim, D.; Yang, K.; Shin, S.; Mahata, C.; Jung, H.B.; Lee, W.; Cho, S.-W.; et al. Switchable water-adhesive, superhydrophobic palladium-layered silicon nanowires potentiate the angiogenic efficacy of human stem cell spheroids. Adv. Mater. 2014, 26, 7043–7050. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, S.; Huttunen-Saarivirta, E.; Nikkanen, J.-P.; Raulio, M.; Priha, O.; Laakso, J.; Storgårds, E.; Levänen, E. Antibacterial properties and chemical stability of superhydrophobic silver-containing surface produced by sol-gel route. Colloids Surf. A Physicochem. Eng. Asp. 2014, 453, 149–161. [Google Scholar] [CrossRef]
- Wang, Z.; Ou, J.; Wang, Y.; Xue, M.; Wang, F.; Pan, B.; Li, C.; Li, W. Anti-bacterial superhydrophobic silver on diverse substrates based on the mussel-inspired polydopamine. Surf. Coat. Technol. 2015, 280, 378–383. [Google Scholar] [CrossRef]
- Heinonen, S.; Nikkanen, J.-P.; Laakso, J.; Raulio, M.; Priha, O.; Levänen, E. Bacterial growth on a superhydrophobic surface containing silver nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2013, 47. [Google Scholar] [CrossRef]
- Berendjchi, A.; Khajavi, R.; Yazdanshenas, M. Fabrication of superhydrophobic and antibacterial surface on cotton fabric by doped silica-based sols with nanoparticles of copper. Nanoscale Res. Lett. 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Ma, B.; Pan, T.; Chen, S.; Sun, J. Silver-nanoparticle-colored cotton fabrics with tunable colors and durable antibacterial and self-healing superhydrophobic properties. Adv. Funct. Mater. 2016, 26, 569–576. [Google Scholar] [CrossRef]
- Shateri Khalil-Abad, M.; Yazdanshenas, M.E. Superhydrophobic antibacterial cotton textiles. J. Colloid Interface Sci. 2010, 351, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.; Yang, Y.; Zhang, W.; Lv, H.; Yang, F.; Lin, C.; Tang, P. Inhibitory effect of super-hydrophobicity on silver release and antibacterial properties of super-hydrophobic Ag/TiO2 nanotubes. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015. [Google Scholar] [CrossRef] [PubMed]
- Lichter, J.A.; Van Vliet, K.J.; Rubner, M.F. Design of antibacterial surfaces and interfaces: Polyelectrolyte multilayers as a multifunctional platform. Macromolecules 2009, 42, 8573–8586. [Google Scholar] [CrossRef]
- Song, K.; Gao, A.; Cheng, X.; Xie, K. Preparation of the superhydrophobic nano-hybrid membrane containing carbon nanotube based on chitosan and its antibacterial activity. Carbohydr. Polym. 2015, 130, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; You, W.; Shen, Q.; Wang, X.; Sheng, J.; Cheng, D.; Cao, X.; Wu, C. Preparation of lotus-leaf-like antibacterial film based on mesoporous silica microcapsule-supported Ag nanoparticles. RSC Adv. 2014, 4, 2793–2796. [Google Scholar] [CrossRef]
- Privett, B.J.; Youn, J.; Hong, S.A.; Lee, J.; Han, J.; Shin, J.H.; Schoenfisch, M.H. Antibacterial fluorinated silica colloid superhydrophobic surfaces. Langmuir 2011, 27, 9597–9601. [Google Scholar] [CrossRef] [PubMed]
- Freschauf, L.R.; McLane, J.; Sharma, H.; Khine, M. Shrink-induced superhydrophobic and antibacterial surfaces in consumer plastics. PLoS One 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Fadeeva, E.; Truong, V.K.; Stiesch, M.; Chichkov, B.N.; Crawford, R.J.; Wang, J.; Ivanova, E.P. Bacterial retention on superhydrophobic titanium surfaces fabricated by femtosecond laser ablation. Langmuir 2011, 27, 3012–3019. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Dong, L.; Liu, T.; Yin, Y. Investigations on reducing microbiologically-influenced corrosion of aluminum by using super-hydrophobic surfaces. Electrochim. Acta 2010, 55, 5281–5285. [Google Scholar] [CrossRef]
- Ye, W.; Shi, Q.; Hou, J.; Jin, J.; Fan, Q.; Wong, S.-C.; Xu, X.; Yin, J. Superhydrophobic coating of elastomer on different substrates using a liquid template to construct a biocompatible and antibacterial surface. J. Mater. Chem. B 2014, 2, 7186–7191. [Google Scholar] [CrossRef]
- Leslie, D.C.; Waterhouse, A.; Berthet, J.B.; Valentin, T.M.; Watters, A.L.; Jain, A.; Kim, P.; Hatton, B.D.; Nedder, A.; Donovan, K.; et al. Bioinspired omniphobic surface coating on medical devices prevents thrombosis and biofouling. Nat. Biotechnol. 2014, 32, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Grinthal, A.; Aizenberg, J. Mobile interfaces: Liquids as a perfect structural material for multifunctional, antifouling surfaces. Chem. Mater. 2014, 26, 698–708. [Google Scholar] [CrossRef]
- Epstein, A.K.; Wong, T.-S.; Belisle, R.A.; Boggs, E.M.; Aizenberg, J. Liquid-infused structured surfaces with exceptional anti-biofouling performance. Proc. Natl. Acad. Sci. 2012, 109, 13182–13187. [Google Scholar] [CrossRef] [PubMed]
- Balu, B.; Berry, A.D.; Hess, D.W.; Breedveld, V. Patterning of superhydrophobic paper to control the mobility of micro-liter drops for two-dimensional lab-on-paper applications. Lab Chip 2009, 9, 3066–3075. [Google Scholar] [CrossRef] [PubMed]
- Mertaniemi, H.; Jokinen, V.; Sainiemi, L.; Franssila, S.; Marmur, A.; Ikkala, O.; Ras, R.H.A. Superhydrophobic tracks for low-friction, guided transport of water droplets. Adv. Mater. 2011, 23, 2911–2914. [Google Scholar] [CrossRef] [PubMed]
- You, I.; Kang, S.M.; Lee, S.; Cho, Y.O.; Kim, J.B.; Lee, S.B.; Nam, Y.S.; Lee, H. Polydopamine microfluidic system toward a two-dimensional, gravity-driven mixing device. Angew. Chem. Int. Ed. 2012, 51, 6126–6130. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.P.; Mano, J.F. Superhydrophobic paper in the development of disposable labware and lab-on-paper devices. ACS Appl. Mater. Interfaces 2013, 5, 3731–3737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsharkawy, M.; Schutzius, T.M.; Megaridis, C.M. Inkjet patterned superhydrophobic paper for open-air surface microfluidic devices. Lab Chip 2014, 14, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, Z.; Niu, H.; Wang, X.; Lin, T. Magnetic liquid marbles: Toward “lab in a droplet”. Adv. Funct. Mater. 2015, 25, 437–444. [Google Scholar] [CrossRef]
- Kim, D.; Seo, J.; Shin, S.; Lee, S.; Lee, K.; Cho, H.; Shim, W.; Lee, H.-B.-R.; Lee, T. Reversible liquid adhesion switching of superamphiphobic Pd-decorated Ag dendrites via gas-induced structural changes. Chem. Mater. 2015, 27, 4964–4971. [Google Scholar] [CrossRef]
- Jonsson-Niedziolka, M.; Lapierre, F.; Coffinier, Y.; Parry, S.J.; Zoueshtiagh, F.; Foat, T.; Thomy, V.; Boukherroub, R. EWOD driven cleaning of bioparticles on hydrophobic and superhydrophobic surfaces. Lab Chip 2011, 11, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, F.; Piret, G.; Drobecq, H.; Melnyk, O.; Coffinier, Y.; Thomy, V.; Boukherroub, R. High sensitive matrix-free mass spectrometry analysis of peptides using silicon nanowires-based digital microfluidic device. Lab Chip 2011, 11, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, F.; Harnois, M.; Coffinier, Y.; Boukherroub, R.; Thomy, V. Split and flow: Reconfigurable capillary connection for digital microfluidic devices. Lab Chip 2014, 14, 3589–3593. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Breedveld, V.; Hess, D.W. Hysteresis controlled water droplet splitting on superhydrophobic paper. Colloid Polym. Sci. 2013, 291, 417–426. [Google Scholar] [CrossRef]
- You, I.; Lee, T.G.; Nam, Y.S.; Lee, H. Fabrication of a micro-omnifluidic device by omniphilic/omniphobic patterning on nanostructured surfaces. ACS Nano 2014, 8, 9016–9024. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Lee, S.-K.; Lee, J.; Seung, L.J.; Kwon, H.; Cho, S.-W.; Ahn, J.-H.; Lee, T. Path-programmable water droplet manipulations on an adhesion controlled superhydrophobic surface. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.G.; Levenberg, S.; Langer, R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem Cells. Nat. Biotechnol. 2004, 22, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Gerecht, S.; Taylor, M.; Urquhart, A.J.; Bogatyrev, S.R.; Cho, S.-W.; Davies, M.C.; Alexander, M.R.; Langer, R.S.; Anderson, D.G. Mapping the interactions among biomaterials, adsorbed proteins, and human embryonic stem cells. Adv. Mater. 2009, 21, 2781–2786. [Google Scholar] [CrossRef]
- Khan, F.; Tare, R.S.; Kanczler, J.M.; Oreffo, R.O.C.; Bradley, M. Strategies for cell manipulation and skeletal tissue engineering using high-throughput polymer blend formulation and microarray techniques. Biomaterials 2010, 31, 2216–2228. [Google Scholar] [CrossRef] [PubMed]
- Hook, A.L.; Chang, C.-Y.; Yang, J.; Luckett, J.; Cockayne, A.; Atkinson, S.; Mei, Y.; Bayston, R.; Irvine, D.J.; Langer, R.; et al. Combinatorial discovery of polymers resistant to bacterial attachment. Nat. Biotechnol. 2012, 30, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Zonca, M.R.; Yune, P.S.; Heldt, C.L.; Belfort, G.; Xie, Y. High-throughput screening of substrate chemistry for embryonic stem cell attachment, expansion, and maintaining pluripotency. Macromol. Biosci. 2013, 13, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.B.; Mano, J.F. On-chip assessment of the protein-release profile from 3D hydrogel arrays. Anal. Chem. 2013, 85, 2391–2396. [Google Scholar] [CrossRef] [PubMed]
- Ueda, E.; Geyer, F.L.; Nedashkivska, V.; Levkin, P.A. Dropletmicroarray: Facile formation of arrays of Microdroplets and hydrogel micropads for cell screening applications. Lab Chip 2012, 12, 5218–5224. [Google Scholar] [CrossRef] [PubMed]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.; Seo, J.; Han, H.; Kang, S.; Kim, H.; Lee, T. Bio-Inspired Extreme Wetting Surfaces for Biomedical Applications. Materials 2016, 9, 116. https://doi.org/10.3390/ma9020116
Shin S, Seo J, Han H, Kang S, Kim H, Lee T. Bio-Inspired Extreme Wetting Surfaces for Biomedical Applications. Materials. 2016; 9(2):116. https://doi.org/10.3390/ma9020116
Chicago/Turabian StyleShin, Sera, Jungmok Seo, Heetak Han, Subin Kang, Hyunchul Kim, and Taeyoon Lee. 2016. "Bio-Inspired Extreme Wetting Surfaces for Biomedical Applications" Materials 9, no. 2: 116. https://doi.org/10.3390/ma9020116