Synthesis, Characterization, Antimicrobial Studies and Corrosion Inhibition Potential of 1,8-dimethyl-1,3,6,8,10,13-hexaazacyclotetradecane: Experimental and Quantum Chemical Studies
Abstract
:1. Introduction
2. Results and Discussion
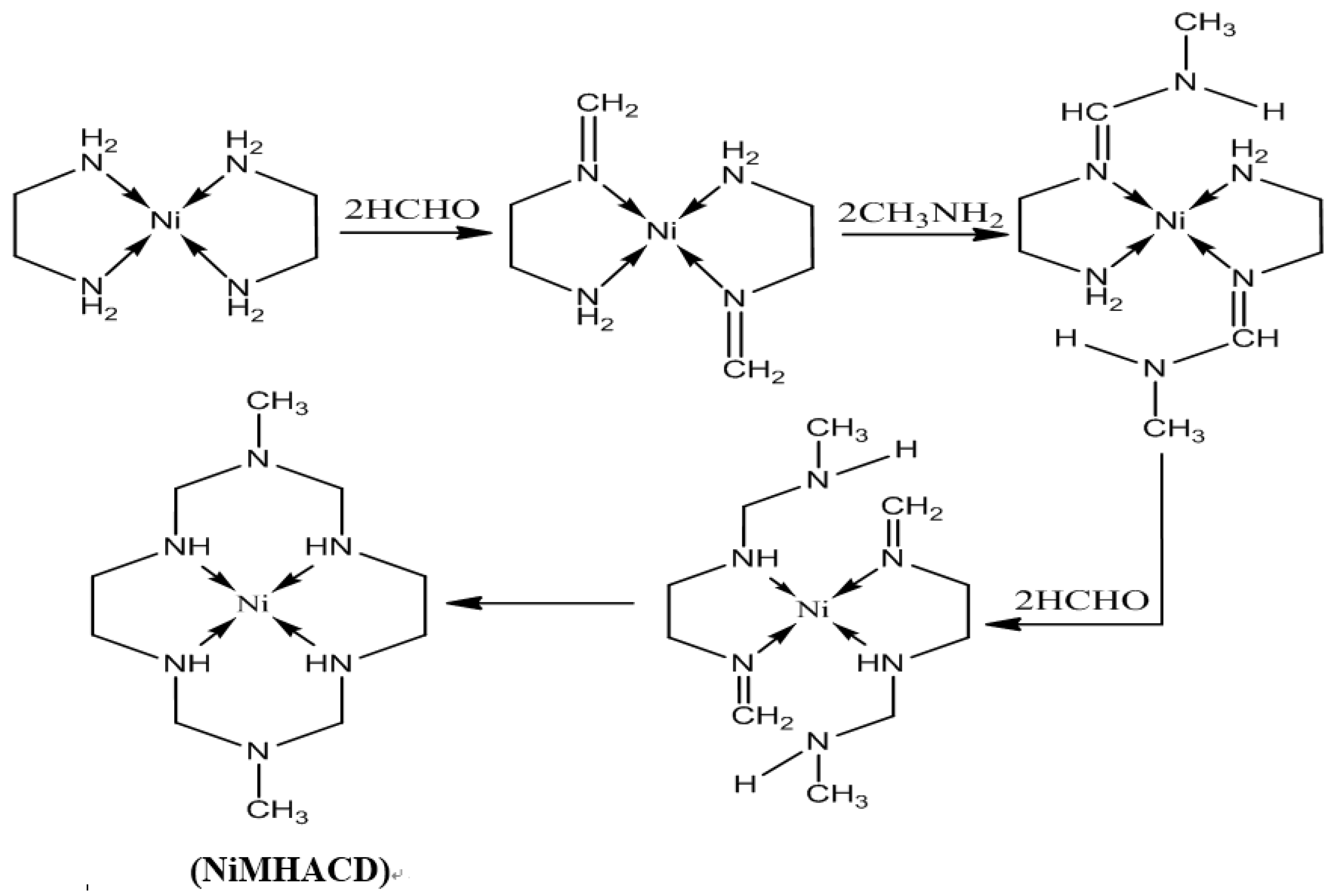
2.1. Synthesis and Characterization of NiMHACD and MHACD
2.2. Antimicrobial Study
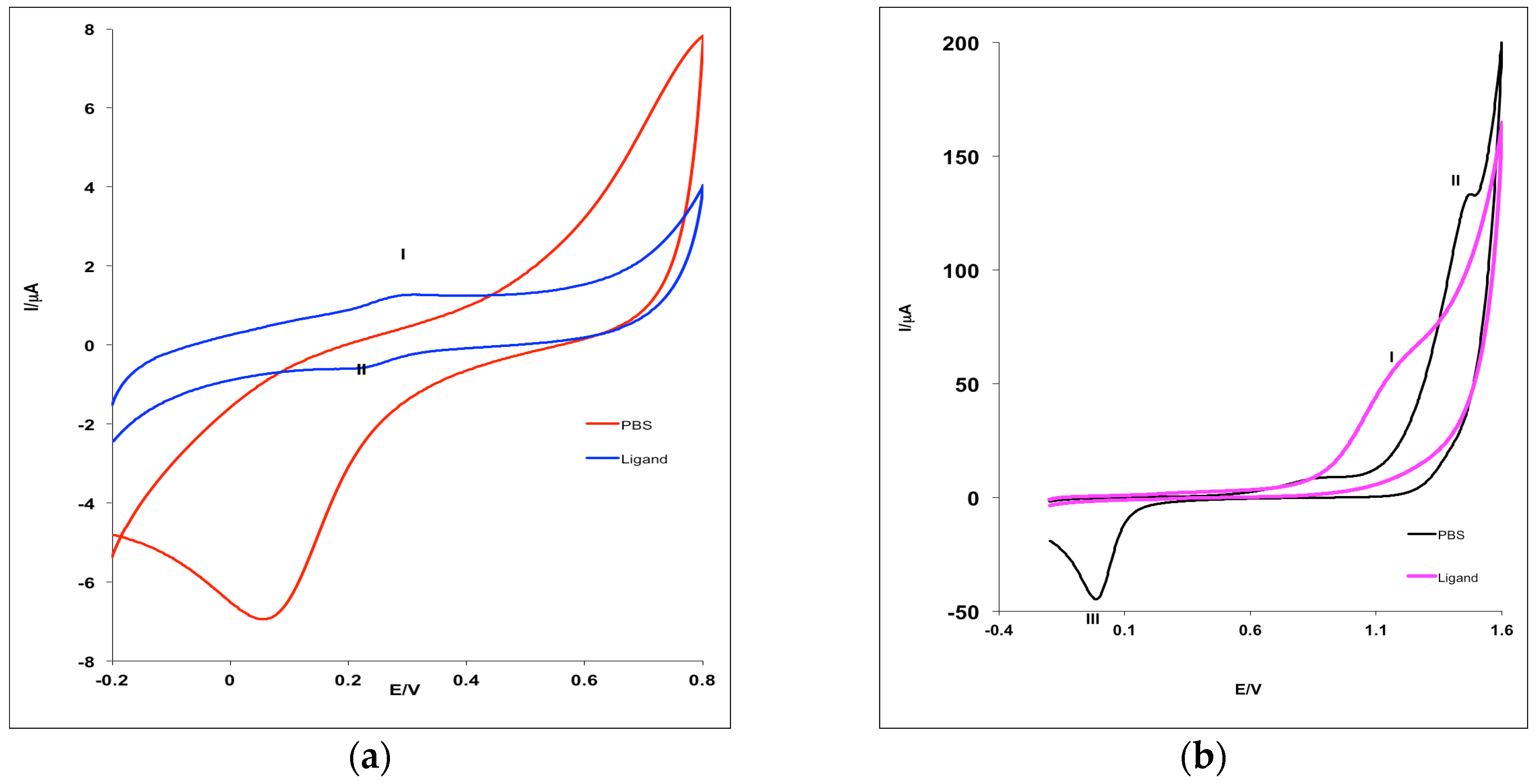
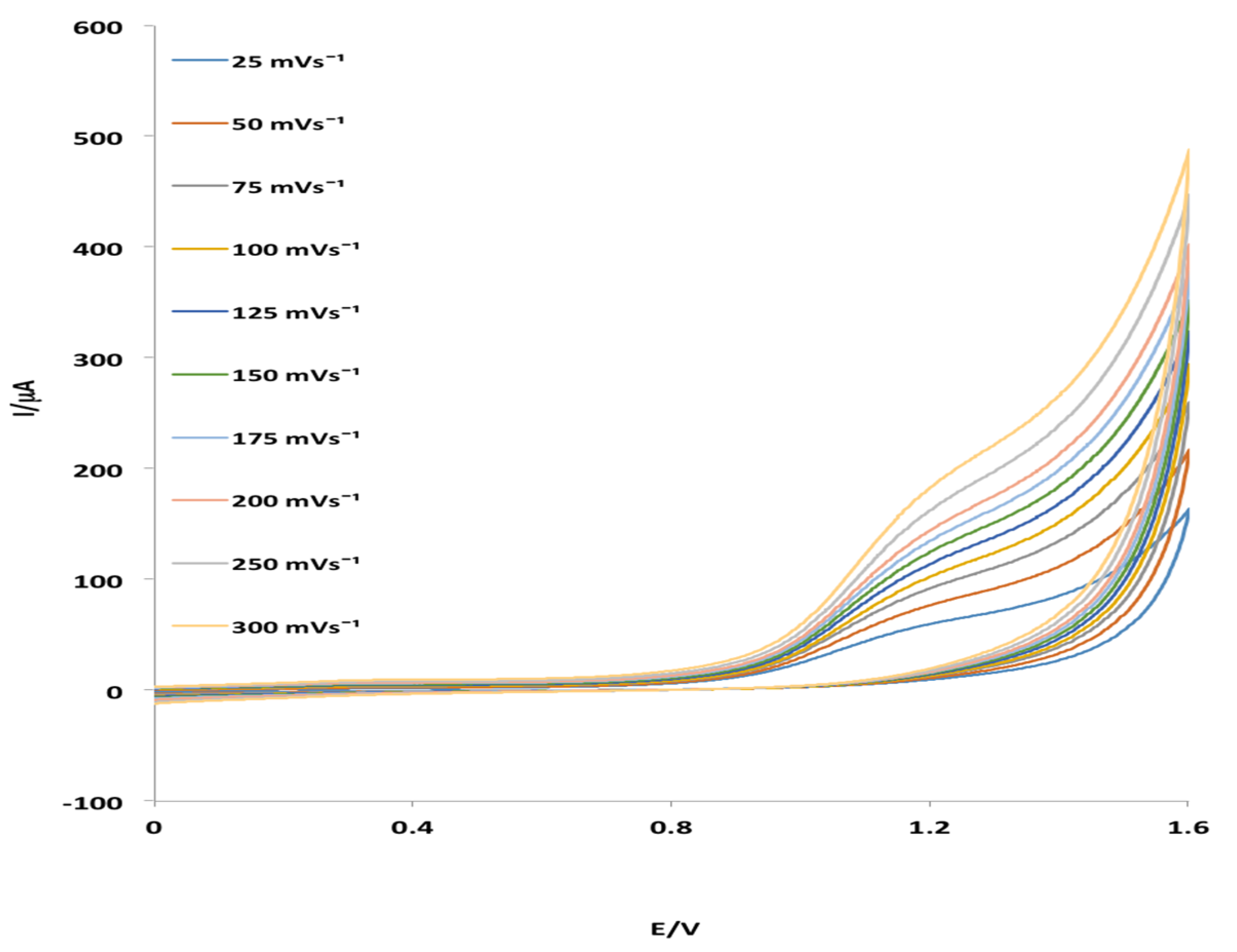
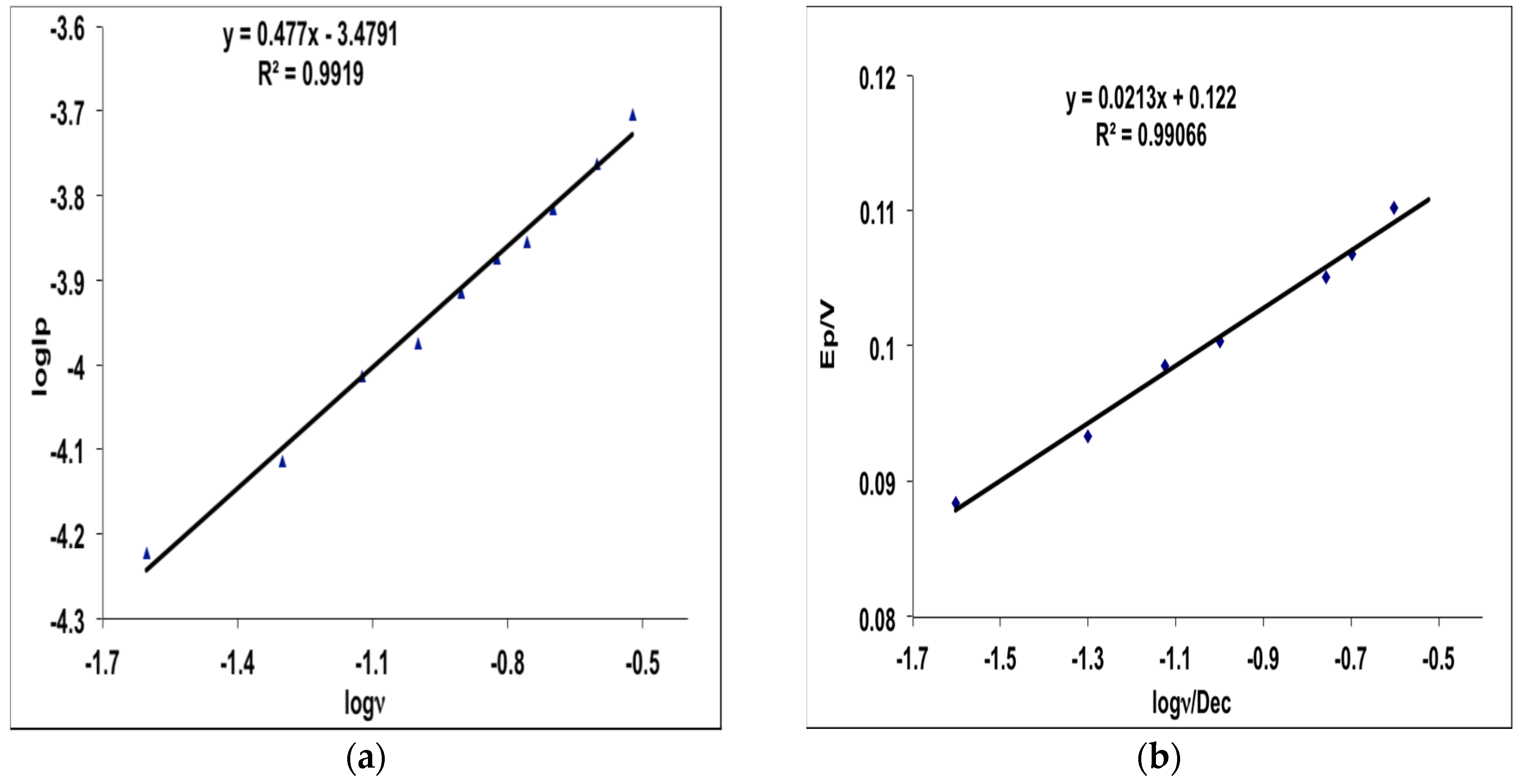
2.3. Electrochemical Behavior of MHACD: Cyclic Voltammetry Study
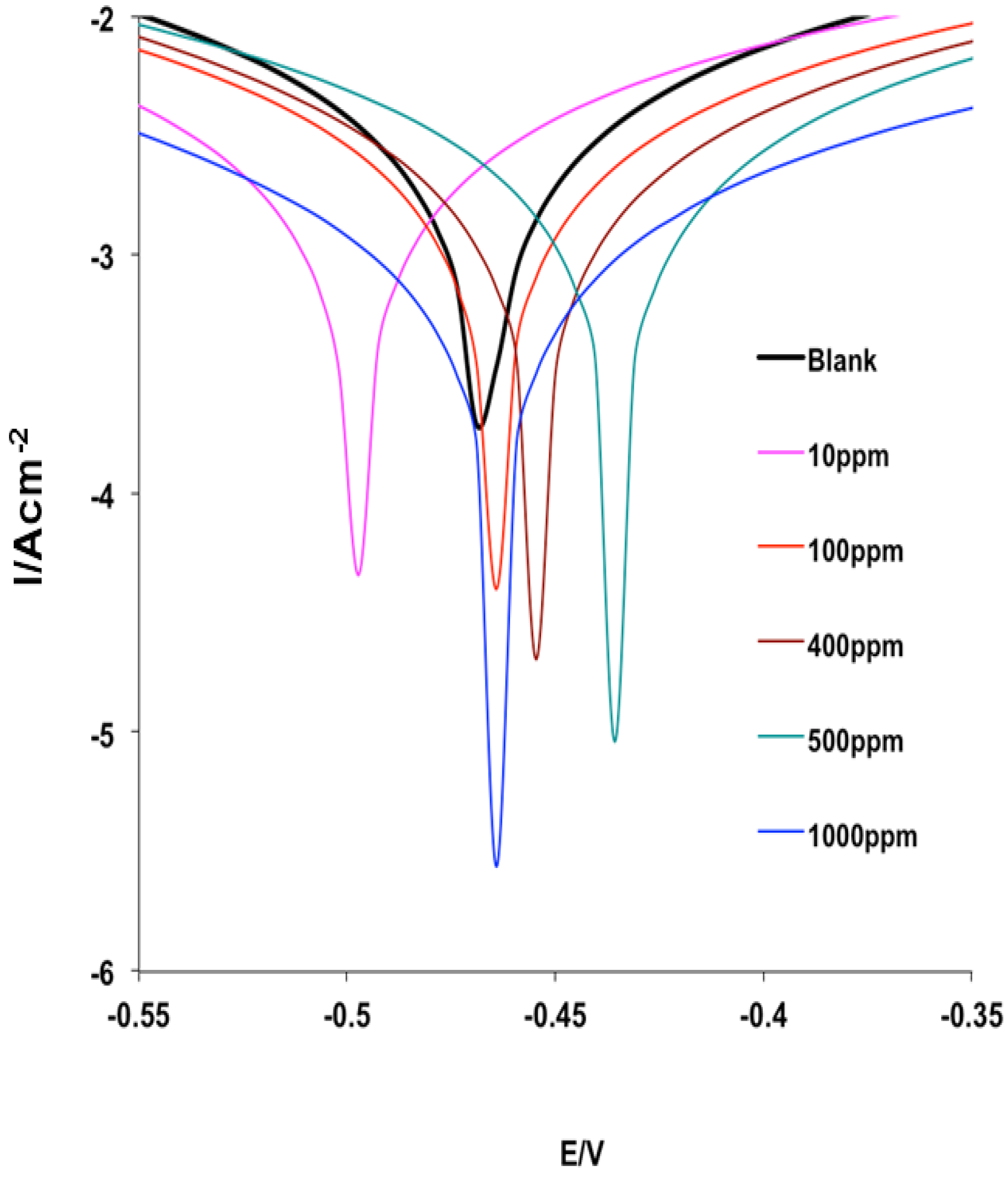
2.4. Corrosion Inhibition Study
2.4.1. Potentiodynamic Polarization Measurements
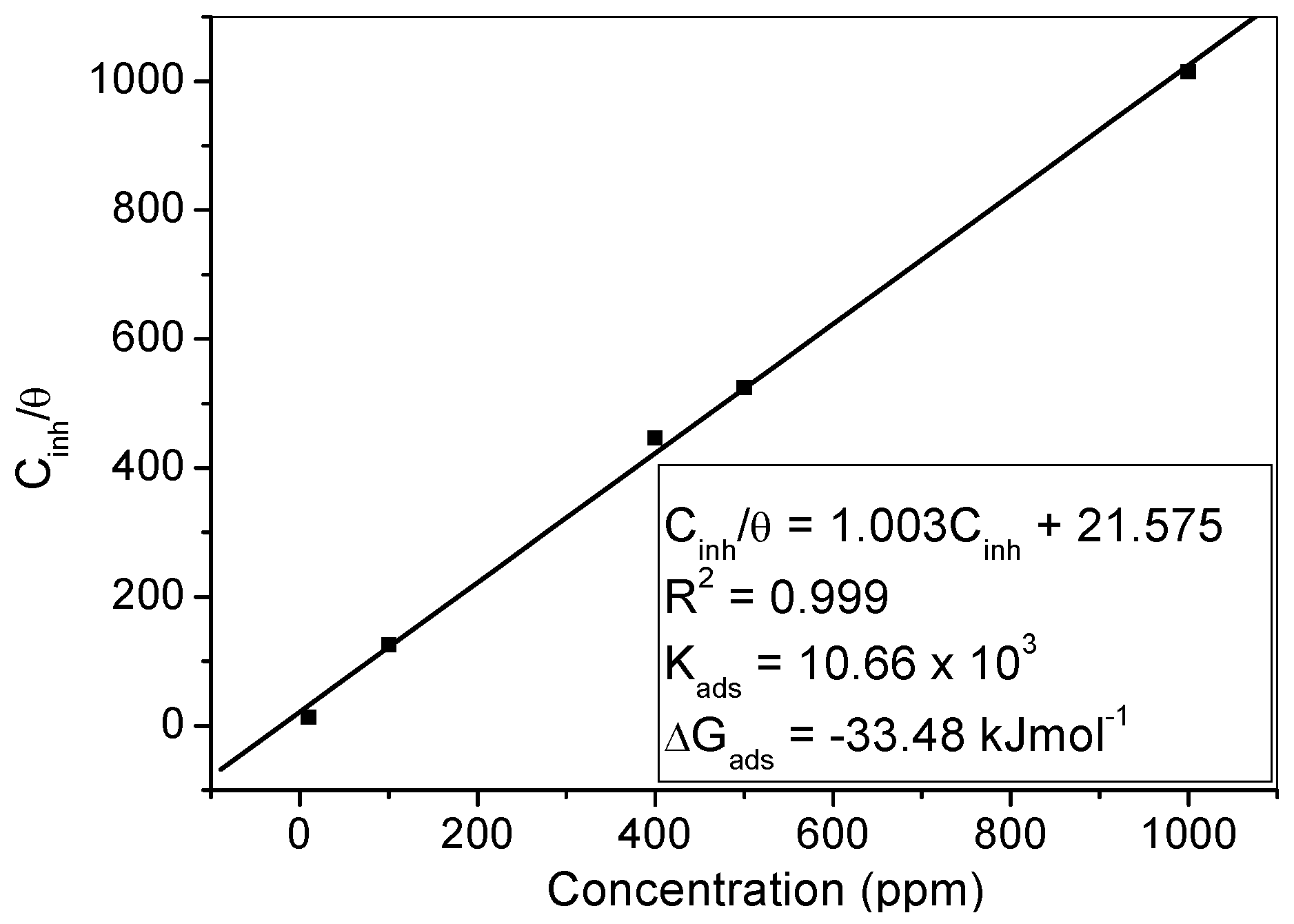
2.4.2. Adsorption Isotherm
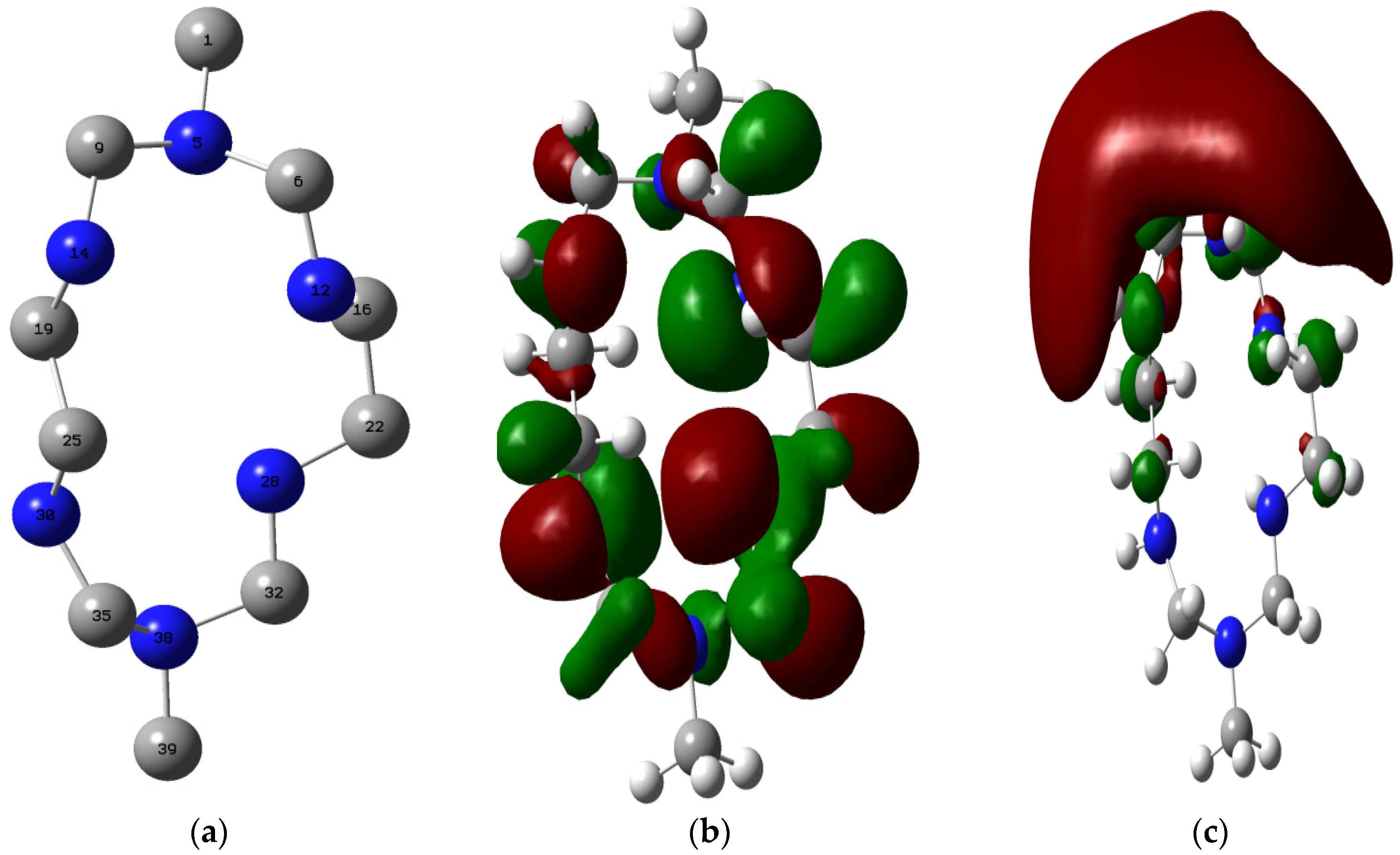
2.4.3. Quantum Chemical Study
3. Experimental Section
3.1. Materials, Reagents and Strains
3.2. Synthesis
3.2.1. Synthesis of 1,8-dimethyl-1,3,6,8,10,13-hexaazacyclotetradecanenickel(II) Complex (NiMHACD)
- FTIR (v/cm−1): 1026s (C–N); 1503m (N–H, bend); 3222m (C–H, stretch), 3550b (N–H, stretch) 13CNMR (CDCl3, δ, ppm ): three distinct peaks: 39.90 (–N–CH3), 71.20 (–N–C–N–), 172.60 (–N–CH2–CH2–N)
- 1HNMR (CDCl3, δ, ppm ): 0.9 (s, 6H, –N–CH3), 1.2 (m, 8H, –N–CH2–CH2–N–), 1.5 (d, 8H, –N–CH2–N–), 7.2 (m, 4H, –C–NH–C–)
- UV-vis (nm): 214 (1A1g→1A2g), 324 nm (1A1g→1B1g)
- EDX: 22.5 % Ni
3.2.2. Synthesis of 1,8-dimethyl-1,3,6,8,10,13-hexaazacyclotetradecane (MHACD) by Demetallation of NiMHACD
- FTIR (v/cm−1): 1421s (C–N); 1584m (N–H, bend); 3300b (N–H, stretch)
- 13CNMR (CDCl3, δ, ppm): three distinct peaks: 28.56 (–N–CH3), 78.43 (–N–C–N–), 174.60 ()
- 1HNMR (CDCl3, δ, ppm): 0.9 (s, 6H, –N–CH3), 1.3 (m, 8H, –N–CH2–CH2–N–), 1.6 (d, 8H, –N-CH2–N–), 7.3 (m, 4H, –C–NH–C–)
- UV-vis (nm): 286 (n→σ*)
- EDX: 0 % Ni
3.3. Spectroscopic Analyses
3.4. Electrochemical Characterization of MHACD: Cyclic Voltammetry (CV) Study
3.5. Biological Activity
Antibacterial Study
3.6. Corrosion Inhibition Study
3.6.1. Metal Specimen and Inhibitor Solution
3.6.2. Potentiodynamic Polarization Measurements
3.6.3. Quantum Chemical Study
4. Conclusions
- 1)
- The results FT-IR, 1HNMR, 13CNMR, UV-Vis, and EDX characterization revealed successful synthesis of NiMHACD and dematallation to MHACD.
- 2)
- MHACD was found to exhibit anti-bacteria activities against Staphylococcus aureus and Enterococcus species, being more active against the latter and the zone of inhibition increases with increasing concentration of MHACD.
- 3)
- MHACD displayed electrochemical redox properties that suggest its possible catalytic properties in electrochemical applications.
- 4)
- Potentiodynamic polarization study suggested that MHACD is a mixed-type corrosion inhibitor for mild steel in 1 M HCl.
- 5)
- The adsorption of MHACD was found to be spontaneous, obey the Langmuir adsorption isotherm, and involve competitive physisorption and chemisorption mechanisms.
- 6)
- Quantum chemical study showed that the HOMO of MHACD is high enough to favor forward donation of charges to the metal, while various orbitals in the MHACD that are capable of donating or accepting electrons were identified using the NBO analysis.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bowman-James, K. Macrocyclic Ligands. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Gerbelue, N.V.; Arion, V.B.; Burges, J. Template Synthesis of Macrocyclic Compounds, 2nd ed.; Wiley-VCH: Weinleim, Germany, 1999; pp. 1–80. [Google Scholar]
- Sujatha, S.; Balasubramanian, S.; Varghese, B. Synthesis, structural, spectral, electrochemical and spin equilibrium studies of hexaaza macrotricyclic complexes. Polyhedron 2009, 28, 3723–3730. [Google Scholar] [CrossRef]
- Krishna, E.R.; Reddy, P.M.; Sarangapi, M.; Hanmanthu, G.; Geeta, B.; Rani, K.S.; Ravinder, V. Synthesis of N4 donor macrocyclic Schiff base ligands and their Ru(II), Pd(II), Pt(II) metal complexes for biological studies and catalytic oxidation of didanosine in pharmaceuticals. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 97, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Dey, K. A series of transition and non-transition metal complexes from a N4O2 hexadentate Schiff base ligand: Synthesis, spectroscopic characterization and efficient antimicrobial activities. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2010, 77, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, C.S.; Broderick, W.E.; Sabat, M.A.; Barrett, G.M.; Hoffman, B.M. Metal-encapsulated porphyrazines: Synthesis, X-ray crystal structure and spectroscopy of a tetratin-star-nickel(porphyrazine) S8 complex. J. Am. Chem. Soc. 1990, 112, 7408–7410. [Google Scholar] [CrossRef]
- Singh, K.; Kumar, Y.; Puri, P.; Kumar, M.; Sharma, C. Cobalt, nickel, copper and zinc complexes with 1,3-diphenyl-1H-pyrazole-4-carboxaldehyde Schiff bases: Antimicrobial, spectroscopic, thermal and fluorescence studies. Eur. J. Med. Chem. 2012, 52, 313–321. [Google Scholar] [CrossRef] [PubMed]
- El-Bayouki, K.A.H.M.; Basyouni, W.M.; Mohamed, Y.A.F.; Aly, M.M.; Abbas, S.Y. Novel 4 (3H)-quinazolinones containing biologically active thiazole, pyridinone and chromene of expected antitumor and antifungal activities. Eur. J. Chem. 2011, 2, 455–462. [Google Scholar] [CrossRef]
- Samee, W.; Vajragupta, O. Antifungal, cytotoxic activities and docking studies of 2,5-dimercapto-1,3,4-thiaduazole derivatives. Afr. J. Pharm. Pharmacol. 2011, 5, 477–485. [Google Scholar] [CrossRef]
- Patel, N.B.; Patel, J.C.; Patel, S.D.; Barat, G.G. Synthesis, antibacterial and antifungal activity of pyrazolyl-quinazolin-4(3H)-one derivatives. Orbital Elec. J. Chem. 2010, 2, 248–262. [Google Scholar]
- Shujah, S.; Zia-ur-Rehman; Muhammad, N.; Ali, S.; Khalid, N.; Tahir, M.N. New dimeric and supramolecular organotin(IV) complexes with tridentate Schiff base as potential biocidal agents. J. Organomet. Chem. 2011, 696, 2772–2781. [Google Scholar] [CrossRef]
- Kurogi, Y.; Inoue, Y.; Tsutsuni, K.; Nakamura, S.; Nagao, K.; Yoshisugu, H.; Tsuda, Y. Synthesis and hypolipidemic activities of novel 2-[4-(diethoxyphosphoryl)methyl]phenyl]quinazolines and 4-(3H)-quinazolinones. J. Med. Chem. 1996, 39, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Helali, A.Y.H.; Sarg, M.T.M.; Koraa, M.M.S.; El-Zoghbi, M.S.F. Utility of 2-methyl-quinazolin-4(3H)-one in the synthesis of heterocyclic compounds with anticancer activity. Open J. Med. Chem. 2014, 4, 12–37. [Google Scholar] [CrossRef]
- Alagarsamy, V.; Solomon, V.R.; Murugan, M. Synthesis and pharmacological investigation of novel 4-benzyl-1-substituted-4H-[1,2,4]triazolo[4,3-a]quinazolin-5-ones as new class of H1-antihistaminic agents. Bioorg. Med. Chem. 2007, 15, 4009–4015. [Google Scholar] [CrossRef] [PubMed]
- Alagarsamy, V.; Meena, S.; Ramseshu, K.V.; Solomon, V.R.; Thirumurugan, K.; Dhanabal, K.; Murugan, M. Synthesis, analgesic, anti-inflammatory, ulcerogenic index and antibacterial activities of novel 2-methythio-3-substituted-5,6,7,8-tetrahydrobenzo (b)thieno[2,3-d]pyrmidin-4(3H)-ones. Eur. J. Med. Chem. 2006, 41, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Alagarsamy, V.; Solomon, V.R.; Dhanabal, K. Synthesis and pharmacological evaluation of some 3-phenyl-2-substituted-3H-quinazolin-4-one as analgesic, anti-inflammatory agents. Bioorg. Med. Chem. 2007, 15, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Mosaad, S.M.; Mohammed, K.I.; Ahmed, M.A.; Abdel-Hamide, S.G. Synthesis of certain new 6-iodoquinazolines as potential antitubercular agents. J. Appl. Sci. 2004, 4, 302–307. [Google Scholar]
- George, S.; Kochupappy, R.T. Design, synthesis and antitubercular screening of certain novel thiadiazolyl pyrrolidine carboxamides as enoyl ACP reductase inhibitors. Int. J. Pharm. Pharm. Sci. 2011, 3, 280–284. [Google Scholar]
- Georgey, H.; Abdel-Gawad, N.; Abbas, S. Synthesis and anticonvulsant activity of some quinazolin-4-(3H)-one derivatives. Molecules 2008, 13, 2557–2569. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.R.; Raju, S.; Goud, P.S.; Sailaja, M.; Sarma, M.R.; Reddy, G.O.; Kumar, M.P.; Reddy, V.V.R.M.K.; Suresh, T.; Hegde, P. Synthesis and biological evaluation of thiophene [3,2-b] pyrrol derivatives as potential anti-inflammatory agents. Bioorg. Med. Chem. 2004, 12, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Rawal, R.K.; Solomon, V.R.; Prabhakar, Y.S.; Katti, S.B.; De Clercq, E. Synthesis and QSAR studies on thiazolidinones as anti-HIV agents. Comb. Chem. High T. Scr. 2005, 8, 439–443. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Ansari, K.R.; Ebenso, E.E. A new and effective macrocyclic compound as corrosion inhibitor for mild steel in hydrochloric acid solution. Int. J. Electrochem. Sci. 2012, 7, 13106–13120. [Google Scholar]
- Babić-Samardžija, K.; Khaled, K.F.; Hackerman, N. Investigation of the inhibiting action of O-, S- and N-dithiocarbamato(1,4,8,11-tetraazacyclotetradecane)cobalt(III) complexes on the corrosion of iron in HClO4 acid. Appl. Surf. Sci. 2005, 240, 327–340. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Rawat, J. Influence of iodide ions on inhibitive performance of tetraphenyl-dithia-octaaza-cyclotetradeca-hexaene (PTAT) during pickling of mild steel in hot sulphuric acid. Mater. Chem. Phys. 2001, 70, 95–99. [Google Scholar] [CrossRef]
- Singh, A.; Lin, Y.; Obot, I.B.; Ebenso, E.E.; Ansari, K.R.; Quraishi, M.A. Corrosion mitigation of J55 steel in 3.5% NaCl solution by a macrocyclic inhibitor. Appl. Surf. Sci. 2015, 356, 341–347. [Google Scholar] [CrossRef]
- Singh, A.; Lin, Y.; Quraishi, M.A.; Olasunkanmi, L.O.; Fayemi, O.E.; Sasikumar, Y.; Ramaganthan, B.; Bahadur, I.; Obot, I.B.; Adekunle, A.S.; et al. Porphyrins as corrosion inhibitors for N80 Steel in 3.5% NaCl solution: Electrochemical, quantum chemical, QSAR and Monte Carlo simulations studies. Molecules 2015, 20, 15122–15146. [Google Scholar] [CrossRef] [PubMed]
- Dibetsoe, M.; Olasunkanmi, L.O.; Fayemi, O.E.; Yesudass, S.; Ramaganthan, B.; Bahadur, I.; Adekunle, A.S.; Kabanda, M.M.; Ebenso, E.E. Some phthalocyanine and naphthalocyanine derivatives as corrosion inhibitors for aluminium in acidic medium: Experimental, quantum chemical calculations, QSAR studies and synergistic effect of iodide ions. Molecules 2015, 20, 15701–15734. [Google Scholar] [CrossRef] [PubMed]
- Niasari, M.S. Synthesis and properties of 16-membered hexaaza macrocycles molecules of copper(II) produced by one-pot template. Inorg. Chem. Comm. 2004, 7, 698–700. [Google Scholar] [CrossRef]
- Suh, M.P.; Kang, S.G. Synthesis and properties of nickel (II) and copper (II) complexes of 14-membered hexaaza macrocycles, 1,8-dimethyl- and 1,8-diethyl-1,3.6,8,10,13-hexaazacyclotetradecane. Inorg. Chem. 1988, 27, 2544–2546. [Google Scholar] [CrossRef]
- Melson, G.A. Coordination Chemistry of Macrocyclic Compounds; Plenum Press: New York, NY, USA, 1979; pp. 46–186. [Google Scholar]
- Siegfried, L.; Kaden, T.A. Kinetics and mechanism of the demetallation of macrocyclic Nickel(II) complexes by cyanide. Helv. Chim. Acta 2005, 88, 380–390. [Google Scholar] [CrossRef]
- Raman, N.; Raja, J.D.; Sakthivel, A. Template synthesis of novel 14-membered tetraazamacrocyclic transition metal complexes: DNA cleavage and antimicrobial studies. J. Chil. Chem. Soc. 2008, 53, 1568–1571. [Google Scholar] [CrossRef]
- Postlethwaite, T.A.; Hutchison, J.E.; Hathcock, K.W.; Murray, R.W. Optical, electrochemical, and electrocatalytic properties of self-assembled thio-derivatized porphyrins on transparent gold films. Langmuir 1995, 11, 4109–4116. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Cai, R.; Rao, W.; Long, F. Molecularly imprinted electrochemical sensor based on nickel nanoparticles-graphene nanocomposites modified electrode for determination of tetrabromobisphenol A. Electrochim. Acta 2014, 117, 385–395. [Google Scholar] [CrossRef]
- Moghaddam, H.M.; Malakootian, M.; Beitollah, H.; Biparva, P. Nanostructured base electrochemical sensor for determination of sulphite. Int. J. Electrochem. Sci. 2014, 9, 327–341. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: NJ, NJ, USA, 2001; pp. 44–156. [Google Scholar]
- Adekunle, A.S.; Pillay, J.; Ozoemena, K.I. Probing the electrochemical behaviour of SWCNT-cobalt nanoparticles and their electrocatalytic activities towards the detection of nitrite at acidic and physiological pH conditions. Electrochim. Acta 2010, 55, 4319–4327. [Google Scholar] [CrossRef]
- Soderberg, J.N.; Co, A.C.; Sirk, A.H.C.; Birss, V.I. Impact of porous electrode properties on the electrochemical transfer coefficient. J. Phys. Chem. B 2006, 110, 10401–10410. [Google Scholar] [CrossRef] [PubMed]
- Olasunkanmi, L.O.; Obot, I.B.; Kabanda, M.M.; Ebenso, E.E. Some quinoxalin-6-yl derivatives as corrosion inhibitors for mild steel in hydrochloric acid: Experimental and theoretical studies. J. Phys. Chem. C 2015, 119, 16004–16019. [Google Scholar] [CrossRef]
- Ferreira, E.S.; Giancomelli, C.; Giacomelli, F.C.; Spinelli, A. Evaluation of the inhibitor effect of L-ascorbic acid on the corrosion of mild steel. Mater. Chem. Phys. 2004, 83, 129–134. [Google Scholar] [CrossRef]
- Li, W.H.; He, Q.; Zhang, S.T.; Pei, C.L.; Hou, B.R. Some new triazole derivatives as inhibitors for mild steel corrosion in acidic medium. J. Appl. Electrochem. 2008, 38, 289–295. [Google Scholar] [CrossRef]
- Mahdavian, M.; Attar, M.M. Electrochemical behaviour of some transition metal acetylacetonate complexes as corrosion inhibitors for mild steel. Corr. Sci. 2009, 51, 409–414. [Google Scholar] [CrossRef]
- Abdel-Gaber, A.M.; Masoud, M.S.; Khalil, E.A.; Shehata, E.E. Electrochemical study on the effect of Schiff base and its cobalt complex on the acid corrosion of steel. Corr. Sci. 2009, 51, 3021–3024. [Google Scholar] [CrossRef]
- Sasikumar, Y.; Adekunle, A.S.; Olasunkanmi, L.O.; Bahadur, I.; Baskar, R.; Kabanda, M.M.; Obot, I.B.; Ebenso, E.E. Experimental, quantum chemical and Monte Carlo simulation studies on the corrosion inhibition of some alkyl imidazolium ionic liquids containing tetrafluoroborate anion on mild steel in acidic medium. J. Mol. Liq. 2015, 211, 105–118. [Google Scholar] [CrossRef]
- Mashuga, M.E.; Olasunkanmi, L.O.; Adekunle, A.S.; Yesudass, S.; Kabanda, M.M.; Ebenso, E.E. Adsorption, thermodynamics and quantum chemical studies of 1-hexyl-3-methylimidazolium based ionic liquids as corrosion inhibitors for mild steel in HCl. Materials 2015, 8, 3607–3632. [Google Scholar] [CrossRef]
- Asegbeloyin, J.N.; Ejikeme, P.M.; Olasunkanmi, L.O.; Adekunle, A.S.; Ebenso, E.E. A Novel Schiff base of 3-acetyl-4-hydroxy-6-methyl-(2H)pyran-2-one and 2,2′-(ethylenedioxy)diethylamine as potential corrosion inhibitor for mild steel in acidic medium. Materials 2015, 8, 2918–2934. [Google Scholar] [CrossRef]
- Torres, V.V.; Rayol, V.A.; Magalhães, M.; Viana, G.M.; Aguiar, L.C.S.; Machado, S.P.; Orofino, H.; D’Elia, E. Study of thioureas derivatives synthesized from a green route as corrosion inhibitors for mild steel in HCl solution. Corr. Sci. 2014, 79, 108–118. [Google Scholar] [CrossRef]
- Emregül, K.C.; Hayyal, M. Studies on the effect of a newly synthesized Schiff base compound from phenazone and vanillin on the corrosion of steel in 2 M HCl. Corr. Sci. 2006, 48, 796–812. [Google Scholar] [CrossRef]
- Mohammed, K.Z.; Hamdy, A.; Abdel-Wahab, A.; Farid, N.A. Temperature effect on corrosion inhibition of carbon steel in formation water by non-ionic inhibitor and synergistic influence of halide ions. Life Sci. J. 2012, 9, 424–434. [Google Scholar]
- Ahamad, I.; Prasa, R.; Quaraishi, M.A. Experimental and theoretical investigation of adsorption of fexofenadine at mild steel/hydrochloric acid interface as corrosion. J. Solid State Electr. 2010, 14, 2095–2105. [Google Scholar] [CrossRef]
- Prabhu, R.A.; Venkatesha, T.V.; Shanbhag, A.V.; Kulkarni, G.M.; Kalkhambkar, R.G. Inhibition effects of some Schiff bases as the corrosion mild steel hydochloric acid solution. Corr. Sci. 2008, 50, 3356–3362. [Google Scholar] [CrossRef]
- Behpour, M.; Ghoreishi, S.M.; Soltani, N.; Salavati-Niasari, M.; Hamadanian, M.; Gandomi, A. Electrochemical and theoretical investigation on the corrosion inhibition of mild steel by thiosalicylaldehyde derivatives in hydrochloric acid solution. Corr. Sci. 2008, 50, 2172–2181. [Google Scholar] [CrossRef]
- Bentiss, F.; Mernari, B.; Traisnel, M.; Vezin, H.; Lagrenée, M. On the relationship between corrosion inhibiting effect and molecular structure of 2,5-bis(n-pyridyl)-1,3,4-thiadiazole derivatives in acidic media: AC impedance and DFT studies. Corr. Sci. 2011, 53, 487–495. [Google Scholar] [CrossRef]
- Lukovits, I.; Kálmán, E.; Zucchi, F. Corrosion inhibitors-correlation between electronic structure and efficiency. Corrosion 2001, 57, 3–8. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Martinez, S. Inhibitory mechanism of Mimosa Tannin using molecular modeling and substitutional adsorption isotherms. Mater. Chem. Phys. 2002, 77, 97–102. [Google Scholar] [CrossRef]
- Pearson, R.G. Absolute Electronegativity and Hardness: Application to inorganic chemistry. Inorg. Chem. 1988, 27, 734–740. [Google Scholar] [CrossRef]



| NiMHACD | |
|---|---|
| FT-IR (v/cm−1) | 1026s (C–N); 1503m (N–H, bend); 3222m (C–H, stretch), 3550b (N–H, stretch) |
| 13C-NMR (CDCl3, δ, ppm) | 39.90 (–N–CH3), 71.20 (–N–C–N–), 172.60 (–N–CH2–CH2–N) |
| 1H-NMR (CDCl3, δ, ppm) | 0.9 (s, 6H, –N–CH3), 1.2 (m, 8H, –N-CH2-CH2–N–), 1.5 (d, 8H, –N–CH2–N–), 7.2 (m, 4H, –C–NH–C–) |
| UV-vis (nm) | 214 (1A1g → 1A2g), 324 nm (1A1g → 1B1g) |
| EDX | 22.5% Ni |
| MHACD | |
| FT-IR (v/cm−1) | 1421s (C–N); 1584m (N–H, bend); 3300b (N–H, stretch) |
| 13C-NMR (CDCl3, δ, ppm ) | 28.56 (–N–CH3), 78.43 (–N–C–N–), 174.60 (–N–CH2–CH2–N) |
| 1H-NMR (CDCl3, δ, ppm) | 0.9 (s, 6H, –N–CH3), 1.3 (m, 8H, –N–CH2–CH2–N–), 1.6 (d, 8H, –N–CH2–N–), 7.3 (m, 4H, –C–NH–C–) |
| UV-vis (nm) | 286 (n → σ*) |
| EDX | 0% Ni |
| Inhibitor Conc. (ppm) | −Ecorr (mV) | icorr (mA·cm−2) | ba (mV·dec−1) | bc (mV·dec−1) | %IEPDP |
|---|---|---|---|---|---|
| – | – | Blank (1 M HCl) | – | – | – |
| – | 467 | 2.838 | 134 | 185 | – |
| – | – | Inhibitor (MHACD) | – | – | – |
| 10 | 498 | 2.288 | 153 | 215 | 76.54 |
| 100 | 465 | 1.947 | 128 | 176 | 79.53 |
| 400 | 445 | 1.296 | 90 | 120 | 89.61 |
| 500 | 436 | 1.251 | 90 | 118 | 95.28 |
| 1000 | 464 | 0.581 | 86 | 129 | 98.58 |
| Geometry Parameter | Gas Phase |
|---|---|
| C1–N5 | 1.458 |
| C6–N5 | 1.466 |
| C6–N12 | 1.455 |
| C9–N5 | 1.465 |
| C9–N14 | 1.449 |
| C16–N12 | 1.456 |
| C19–N14 | 1.465 |
| C16–C22 | 1.533 |
| C19–C25 | 1.530 |
| C22–N28 | 1.452 |
| C25–N30 | 1.462 |
| C32–N28 | 1.446 |
| C35–N30 | 1.455 |
| C32–N38 | 1.460 |
| C35–N38 | 1.476 |
| C39–N38 | 1.458 |
| Quantum Chemical Parameters | ||||||
|---|---|---|---|---|---|---|
| EHOMO (eV) | ELUMO (eV) | ∆E (eV) | χ (eV) | η (eV) | ∆N | Dipole Moment (Debye) |
| −4.17 | 0.11 | 4.27 | 2.03 | 2.14 | 1.16 | 1.74 |
| Atom | Orbital Type | Occupancy | Energy (a.u) |
|---|---|---|---|
| N5 | 2s | 1.283 | −0.517 |
| 2px | 1.576 | −0.203 | |
| 2py | 1.26 | −0.187 | |
| 2pz | 1.435 | −0.195 | |
| N12 | 2s | 1.339 | −0.523 |
| 2px | 1.365 | −0.179 | |
| 2py | 1.639 | −0.193 | |
| 2pz | 1.35 | −0.177 | |
| N14 | 2s | 1.334 | −0.525 |
| 2px | 1.249 | −0.171 | |
| 2py | 1.543 | −0.189 | |
| 2pz | 1.575 | −0.197 | |
| N28 | 2s | 1.333 | −0.521 |
| 2px | 1.285 | −0.174 | |
| 2py | 1.502 | −0.188 | |
| 2pz | 1.59 | −0.193 | |
| N30 | 2s | 1.32 | −0.514 |
| 2px | 1.265 | −0.168 | |
| 2py | 1.563 | −0.184 | |
| 2pz | 1.561 | −0.188 | |
| N38 | 2s | 1.291 | −0.513 |
| 2px | 1.263 | −0.182 | |
| 2py | 1.256 | −0.181 | |
| 2pz | 1.728 | −0.204 |
| Atom | Orbital Type | Energy (a.u) |
|---|---|---|
| C1 | 3dxy | 2.164 |
| C6 | 3dxz | 2.206 |
| C9 | 3dxy | 2.180 |
| C16 | 3dxz | 2.133 |
| C19 | 3dz2 | 2.077 |
| C25 | 3dz2 | 2.092 |
| C35 | 3dx2 − y2 | 2.209 |
| C39 | 3dxz | 2.239 |
| N5 | 3dxy | 2.122 |
| N12 | 3dxy | 2.190 |
| N14 | 3dz2 | 2.103 |
| N28 | 3dxz | 2.179 |
| N30 | 3dz2 | 2.098 |
| N38 | 3dxz | 2.094 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nwankwo, H.U.; Ateba, C.N.; Olasunkanmi, L.O.; Adekunle, A.S.; Isabirye, D.A.; Onwudiwe, D.C.; Ebenso, E.E. Synthesis, Characterization, Antimicrobial Studies and Corrosion Inhibition Potential of 1,8-dimethyl-1,3,6,8,10,13-hexaazacyclotetradecane: Experimental and Quantum Chemical Studies. Materials 2016, 9, 107. https://doi.org/10.3390/ma9020107
Nwankwo HU, Ateba CN, Olasunkanmi LO, Adekunle AS, Isabirye DA, Onwudiwe DC, Ebenso EE. Synthesis, Characterization, Antimicrobial Studies and Corrosion Inhibition Potential of 1,8-dimethyl-1,3,6,8,10,13-hexaazacyclotetradecane: Experimental and Quantum Chemical Studies. Materials. 2016; 9(2):107. https://doi.org/10.3390/ma9020107
Chicago/Turabian StyleNwankwo, Henry U., Collins N. Ateba, Lukman O. Olasunkanmi, Abolanle S. Adekunle, David A. Isabirye, Damian C. Onwudiwe, and Eno E. Ebenso. 2016. "Synthesis, Characterization, Antimicrobial Studies and Corrosion Inhibition Potential of 1,8-dimethyl-1,3,6,8,10,13-hexaazacyclotetradecane: Experimental and Quantum Chemical Studies" Materials 9, no. 2: 107. https://doi.org/10.3390/ma9020107




