Synthesis, Characterization, Tautomeric Structure and Solvatochromic Behavior of Novel 4-(5-Arylazo-2-Hydroxystyryl)-1-Methylpyridinium Iodide as Potential Molecular Photoprobe
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterizations
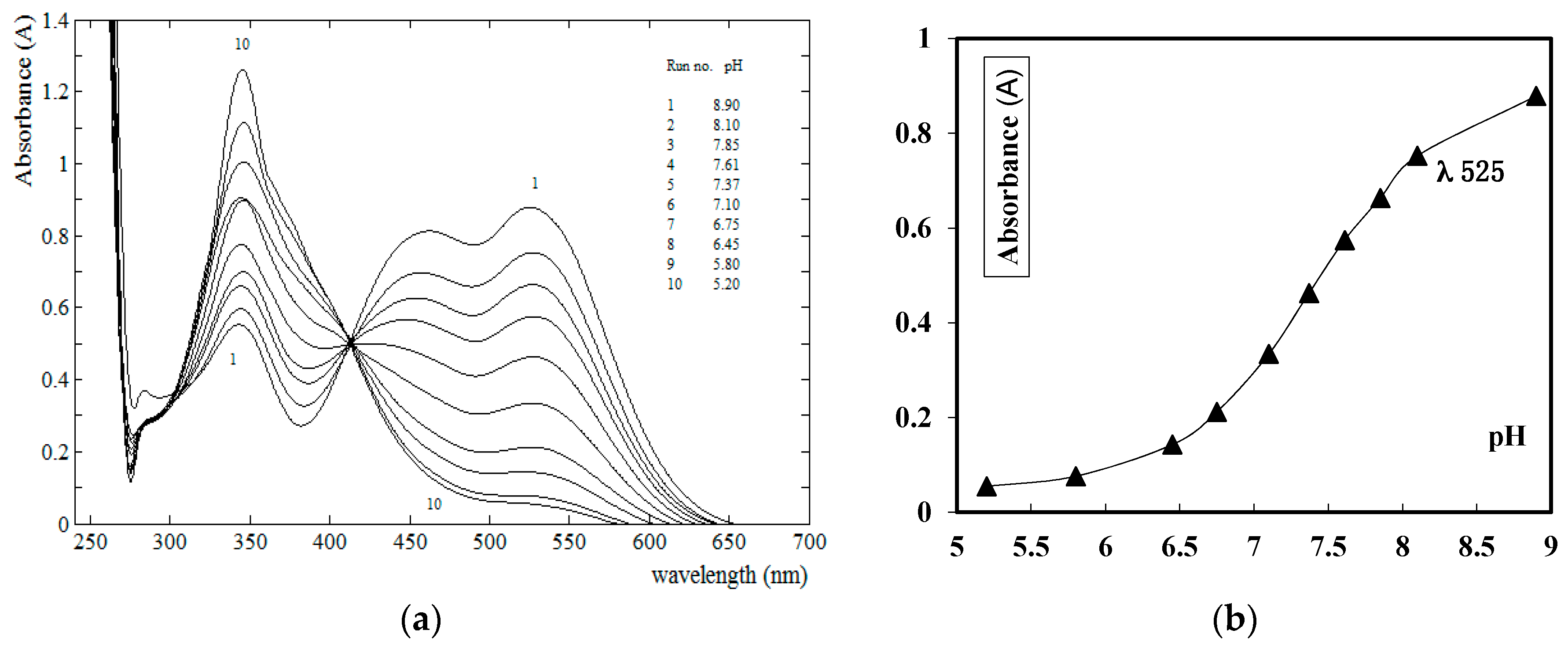
2.2. Determination of Acid Dissociation Constants and Tautomeric Structure for the Prepared Azo Disperse Dyes 6a–i
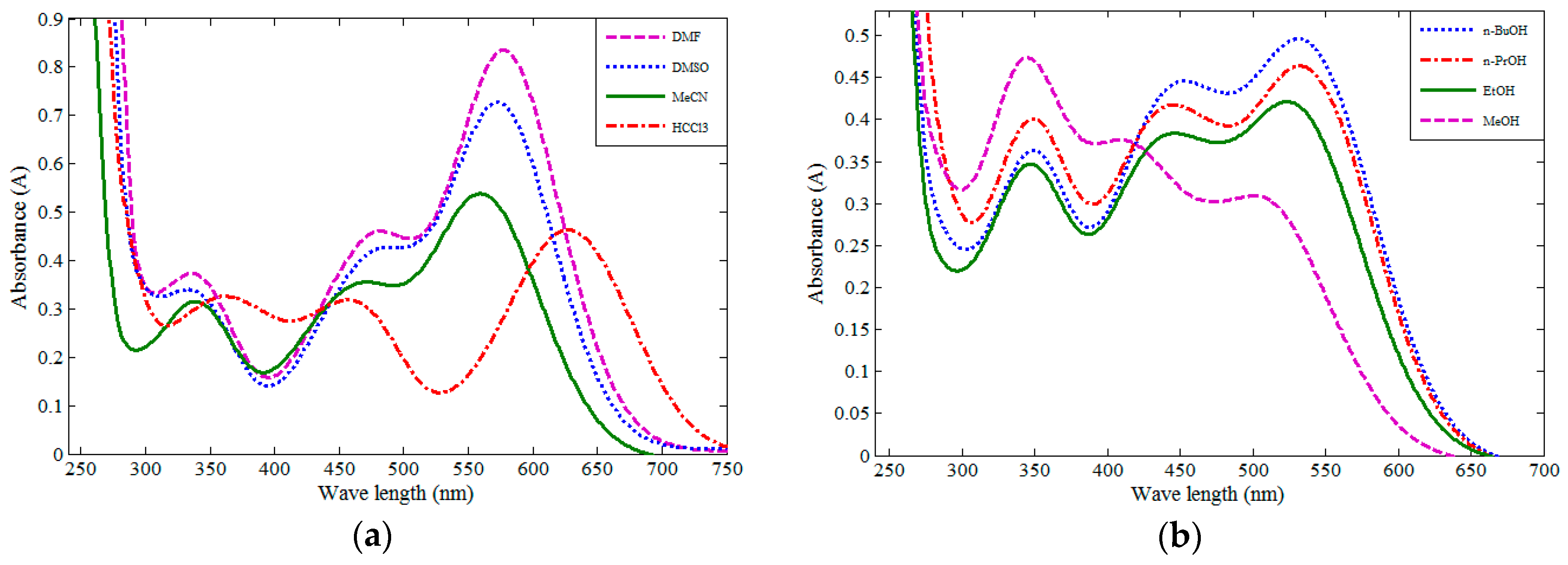
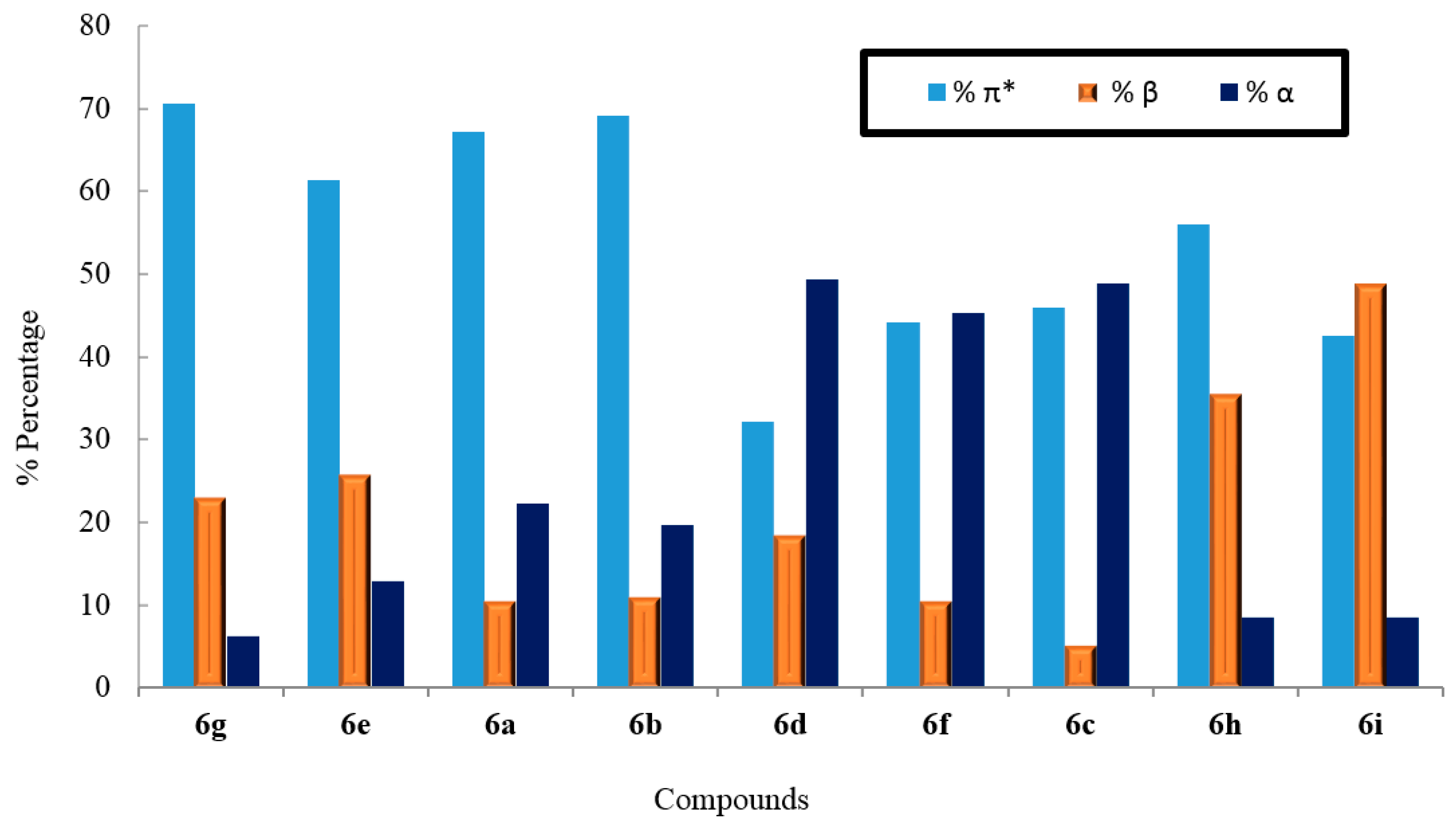
2.3. Solvatochromic Behavior
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Synthetic Procedures
3.2.1. General Procedure for the Synthesis of Arylazosalicylaldehyde 4a–j
3.2.2. 5-(3-Chlorophenylazo)salicylaldehyde (4d)
3.2.3. 5-(4-Bromophenylazo)salicylaldehyde (4e)
3.2.4. 5-(3-Nitrophenylazo)salicylaldehyde (4f)
3.2.5. 5-(4-Acetylphenylazo)salicylaldehyde (4g)
3.2.6. 5-(4-Ethoxycarbonylphenylazo)salicylaldehyde (4h)
3.2.7. General Procedure for the Synthesis of 4-(5-Arylazo-2-hydroxystyryl)-1-methylpyridinium Iodide (6a–i)
3.2.8. 4-(2-Hydroxy-5-(4-methoxyphenylazo)styryl)-1-methylpyridinium Iodide (6a)
3.2.9. 4-(2-Hydroxy-5-(4-methylphenylazo)styryl)-1-methylpyridinium Iodide (6b)
3.2.10. 4-(2-Hydroxy-5-phenylazostyryl)-1-methylpyridinium Iodide (6c)
3.2.11. 4-(2-Hydroxy-5-(3-chlorophenylazo)styryl)-1-methylpyridinium Iodide (6d)
3.2.12. 4-(2-Hydroxy-5-(4-bromophenylazo)styryl)-1-methylpyridinium Iodide (6e)
3.2.13. 4-(2-Hydroxy-5-(3-nitrophenylazo)styryl)-1-methylpyridinium Iodide (6f)
3.2.14. 4-(2-Hydroxy-5-(4-acetylphenylazo)styryl)-1-methylpyridinium Iodide (6g)
3.2.15. 4-(2-Hydroxy-5-(4-Ethoxycarbonylphenylazo)styryl)-1-methylpyridinium Iodide (6h)
3.2.16. 4-(2-Hydroxy-5-(4-nitrophenylazo)styryl)-1-methylpyridinium Iodide (6i)
3.3. pK′s and pK*′s Determination of the Prepared Azo Merocyanine Dyes 6a–i
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interests
References
- Shawali, A.S.; Zeid, I.F.; Abdelkader, M.H.; Elsherbini, A.A.; Altalbawy, F.M.A. Synthesis, acidity constants and tautomeric structure of 7-arylhydrazono[1,2,4]triazole[3,4-b][1,3,4]thiadiazines in ground and excited states. J. Chin. Chem. Soc. 2001, 48, 65–72. [Google Scholar] [CrossRef]
- Shawali, A.S.; Abdelkader, M.H.; Altalbawy, F.M.A. Synthesis and tautomeric structure of novel 3,7-bis(arylazo)-2,6-diphenyl-1H-imidazo-[1,2-b]pyrazoles in ground and excited states. Tetrahedron 2002, 58, 2875–2880. [Google Scholar] [CrossRef]
- Shawali, A.S.; Mosselhi, A.M.; Altalbawy, F.M.A.; Farghaly, T.A.; Tawfik, N.M. Synthesis and tautomeric structure of 3,7-bis(arylazo)-6-methyl-2-phenyl-1H-imidazo-[1,2-b]pyrazoles in ground and excited states. Tetrahedron 2008, 64, 5524–5530. [Google Scholar] [CrossRef]
- Pawar, G.G.; Bineesh, P.; Kumar, P.S.R.; Rangnekar, D.W.; Kanetkar, V.R. The synthesis and application of 3-arylazo-4-phenylthieno-2,3-c-isothiazole and ethyl 3-arylazo-4-phenylthieno-2,3-c-isothiazole-5-carboxylate. J. Serb. Chem. Soc. 2005, 70, 799–805. [Google Scholar] [CrossRef]
- Waring, D.R.; Hallas, G. The Chemistry and Application of Dyes; Plenum Press: New York, NY, USA, 1990; p. 381. [Google Scholar]
- Matsuoka, M. Infrared Absorbing Dyes; Plenum Press: New York, NY, USA, 1990; p. 95. [Google Scholar]
- Garg, H.G.; Prakash, C. Potential antidiabetics. 7. N1-(beta-hydroxybenzylmethyl)-3-methyl-4-arylhydrazono-2-pyrazolin-5-ones and N1-(beta-hydroxybenzylmethyl)-3-methyl-4-arylazo-5-methyl- or -phenylpyrazoles. J. Med. Chem. 1971, 14, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Shawali, A.S.; Darwish, S.S.; Altalbawy, F.M.A. Synthesis of (4-amino-5-phenyl-1,2,4-triazol-3-yl)-thiohydrazonates and spectrophotometric study of their cyclization products in ground and excited states. Asian J. Spectrosc. 2007, 11, 115–125. [Google Scholar]
- Habibi, M.H.; Hassanzadeh, A.; Zeini-Isfahani, A. Spectroscopic studies of solophenyl red 3BL polyazo dye tautomerism in different solvents using UV-visible, 1H NMR and steady-state fluorescence techniques. Dyes Pigm. 2006, 69, 93–101. [Google Scholar] [CrossRef]
- Otutu, J.O.; Osabohien, E.; Efurhievwe, E.M. Synthesis and spectral properties of hetarylmonoazo dyes derived from 2-amino-5-nitrothiazole. Orient. J. Chem. 2011, 27, 1389–1396. [Google Scholar]
- Li, Q.; Lin, G.L.; Peng, B.X.; Li, Z.X. Synthesis, characterization and photographic properties of some new styryl cyanine dyes. Dyes Pigm. 1998, 38, 211–218. [Google Scholar]
- Yoshida, T.; Zhang, J.; Komatsu, D.; Seiichi, S.; Hideki, M.; Thierry, P.; Daniel, L.; Torsten, O.; Derck, S.; Hirokazu, T.; et al. Electrodeposition of Inorganic/Organic Hybrid Thin Films. Adv. Funct. Mater. 2009, 19, 17–43. [Google Scholar] [CrossRef]
- Campagnola, P.J.; Low, L.M. Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. Nat. Biotechnol. 2003, 21, 1356–1360. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.O.; Morley, R.M.; Docherty, R.; Charlton, M.H. Fundamental Studies on Brooker’s Merocyanine. J. Am. Chem. Soc. 1997, 119, 10192–10202. [Google Scholar] [CrossRef]
- Safavi, A.; Abdollahi, H. Thermodynamic characterization of weak association equilibria accompanied with spectral overlapping by a SVD-based chemometric method. Talanta 2001, 53, 1001–1007. [Google Scholar] [CrossRef]
- Hisamoto, H.; Tohma, H.; Yamada, T.; Yamauchi, K.-I.; Siswanta, D.; Yoshioka, N.; Suzuki, K. Molecular design, characterization, and application of multi-information dyes for multi-dimensional optical chemical sensing. Molecular design concepts of the dyes and their fundamental spectral characteristics. Anal. Chim. Acta 1998, 373, 271–289. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.; Campo, J.; Lee, S.; Jeon, W.; Wenseleers, W.; Jazbinsek, M.; Yun, H.; Kwon, O. N-methylquinolinium derivatives for photonic applications: Enhancement of electron-withdrawing character beyond that of the widely-used N-methylpyridinium. Dyes Pigm. 2015, 113, 8–17. [Google Scholar] [CrossRef]
- Castro, D.J.; Saxton, R.E.; Haghighat, S.; Reisler, E.; Plant, D.; Soudant, J. The synergistic effects of rhodamine-123 and merocyanine-540 laser dyes on human tumor cell lines: A new approach to laser phototherapy. Otolaryngol. Head Neck Surg. 1993, 108, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Lv, A.; Stolte, M.; Würthner, F. Head-to-tail zig-zag packing of dipolar merocyanine dyes affords high-performance organic thin-film transistors. Angew. Chem. Int. Ed. 2015, 54, 10512–10515. [Google Scholar] [CrossRef] [PubMed]
- Poronik, Y.M.; Hugues, V.; Blanchard-Desce, M.; Gryko, D.T. Octupolarmerocyanine dyes: A new class of nonlinear optical chromophores. Chem. Eur. J. 2012, 18, 9258–9266. [Google Scholar] [CrossRef] [PubMed]
- Yamin, P.; Piechowski, A.P.; Bird, G.R.; Morel, D. Comparison of the action of some merocyanine dyes as solar photovoltaic elements and as photographic sensitizers. J. Phys. Chem. 1982, 86, 3796–3802. [Google Scholar] [CrossRef]
- Würthner, F.; Wortmann, R.; Matschiner, R.; Lukaszuk, K.; Meerholz, K.; DeNardin, Y.; Bittner, R.; Bräuchle, C.; Sens, R. Merocyanine dyes in the cyanine limit: A new class of chromophores for photorefractive materials. Angew. Chem. Int. Ed. 1997, 36, 2765–2768. [Google Scholar] [CrossRef]
- Altalbawy, F.M.A.; Al-Sherbini, E.A.M. Absorption, fluorescence, photochemical and thermal cis/transisomerization reactivity of 1-methyl-4-(4′-aminostyryl)pyridinium iodide. Chem. Sci. Trans. 2015, 4, 1018–1030. [Google Scholar]
- Altalbawy, F.M.A.; Al-Sherbini, E.A.M. Spectrophotometric determination of acidity constant of 1-methyl-4-[4′-aminostyryl]quinolinium iodide in aqueous buffer and micellar solutions in the ground and excited states. Asian J. Chem. 2013, 25, 6181–6185. [Google Scholar]
- Darwish, E.S.; Mosselhi, M.A.; Altalbawy, F.M.; Saad, H.A. Synthesis, acidity constants and tautomeric structure of the diazonium coupling products of 2-(benzylsulfanyl)-7H-purin-6-one in its ground and excited. Molecules 2011, 16, 8788–8802. [Google Scholar] [CrossRef]
- Shawali, A.S.; Darwish, S.S.; Altalbawy, F.M.A. Site selectivity in diazonium coupling of Ethyl (3-phenyl-7H-[1,2,4]-triazolo[3,4-b][1,3,4] thiadiazin-6-yl)acetate and tautomeric structure of the coupling products in ground and excited states. Asian J. Spectrosc. 2008, 12, 113–120. [Google Scholar]
- Altalbawy, F.M.A.; Darwish, E.S.S. Synthesis and tautomeric structure of 7-arylhydrazono-3,5-diphenyl-5H-pyrazolo[5,1-c][1,2,4]triazol-6(7H)-ones in its ground and excited states. Asian J. Spectrosc. 2012, 16, 45–54. [Google Scholar]
- Altalbawy, F.M.A.; Darwish, E.S.S.; Abdelkader, M.H.; Elnagdi, M.H. Synthesis, electronic absorption, fluorescence and life time spectroscopic study of some new coumarin dyes. Asian J. Chem. 2016, 28, 2303–2310. [Google Scholar] [CrossRef]
- Padmini, V. Synthesis and characterization of new sciff’s bases containing an azo group. Arch. Apll. Sci. Res. 2010, 2, 356–363. [Google Scholar]
- Yahyazadeh, A.; Azimi, V. Synthesis of some unsymmetrical new schiff bases from azo dyes. Eur. Chem. Bull. 2013, 2, 453–455. [Google Scholar]
- Eldaly, S.A.; Abdelkader, M.H.; Issa, R.M.; Elsherbini, E.A. Influence of solvent polarity and medium acidity on the UV-Vis spectral behavior of 1-methyl-4-[4-amino-styryl] pyridinum iodide. Spectrochim. Acta A 2003, 59, 405–411. [Google Scholar] [CrossRef]
- Hanna, M.A.; Girges, M.M.; El-Hossini, M.S.; Sofan, M.A. Spectrophotometric studies on some 4-hydroxy-5-arylazopyrazoles-3-(N-pyrid-2-yl)carboxamide in organic solvents of varying polarities and in buffer solutions. Bull. Chem. Soc. Fr. 1989, 1, 68–72. [Google Scholar]
- John, C.D. The Hammett Equation; Press Syndicate of Cambridge University: New York, NY, USA, 1973. [Google Scholar]
- Tsuno, Y.; Ibata, T.; Yukawa, Y. Resonance effect in hammett relationship. I. The substituent effect in the acid catalyzed decompositions of ω-diazoacetophenones in acetic acid. Bull. Chem. Soc. Jpn. 1595, 32, 960–965. [Google Scholar] [CrossRef]
- Shawali, A.S.; Harb, N.M.; Badahdah, K.O. A study of tautomerism in diazonium coupling products of 4-hydroxycoumarin. J. Heterocycl. Chem. 1985, 22, 1397–1403. [Google Scholar] [CrossRef]
- Förster, T. Elektrolytische dissoziation angeregter moleküle. Z. Elektrochem. Angew. Phys. Chem. 1950, 54, 42–46. [Google Scholar]
- Jaffe, H.H.; Jones, H.L. Excited state pK values: III. The application of the Hammett equation. J. Org. Chem. 1965, 30, 964–969. [Google Scholar] [CrossRef]
- Jaffe, H.H.; Jones, H.L.; Isaks, M. Excited State pK′s. II. Δv-σ Relations. J. Am. Chem. Soc. 1964, 86, 2934–2935. [Google Scholar] [CrossRef]
- Jaffe, H.H.; Beveridge, D.L.; Jones, H.L. Excited state pK′s: I. Azobenzene and azoxybenzene. J. Am. Chem. Soc. 1964, 86, 2932–2934. [Google Scholar] [CrossRef]
- Reichardt, C. Solvents and Solvent Effects in Organic Chemistry, 3rd ed.; Wiley–VCH: Weinheim, Germany, 2004. [Google Scholar]
- Rageh, N.M. Electronic spectra, solvatochromic behavior and acid-base properties of some azo cinnoline compounds. Spectrochim. Acta A 2004, 60, 103–109. [Google Scholar] [CrossRef]
- Mahmoud, M.R.; Abde-El-Gaber, A.A.; Roudi, A.M.; Soliman, E.M. More light on the low electronic transition energy bands of dithizone. Spectrochim. Acta A 1987, 43, 1281–1285. [Google Scholar] [CrossRef]
- Taft, R.W.; Kamlet, M.J.; Abboud, J.L.M. An examination of linear solvation energy relationships. Prog. Phys. Org. Chem. 1981, 13, 485–630. [Google Scholar]
- Kamlet, M.J.; Abboud, J.L.M.; Abraham, M.H.; Taft, R.W. Linear solvation energy relationships. 23. A comprehensive collection of the solvatochromic parameters, pi*, alpha, and beta, and some methods for simplifying the generalized solvatochromic equation. J. Org. Chem. 1983, 48, 2877–2887. [Google Scholar] [CrossRef]
- Zakerhamidi, M.S.; Keshavarz, M.; Tajalli, H.; Ghanadzadeh, A.; Ahmadi, S.; Moghadam, M.; Hosseini, S.H.; Hooshangi, V. Isotropic and anisotropic environment effects on the UV/vis absorption spectra of three disperse azo dyes. J. Mol. Liq. 2010, 154, 94–101. [Google Scholar] [CrossRef]
- Yazdanbakhsh, M.R.; Mohammad, A. Synthesis, substituent effects and solvatochromic properties of some disperse azo dyes derived from N-phenyl-2,2′-iminodiethanol. J. Mol. Liq. 2009, 148, 35–39. [Google Scholar] [CrossRef]



| Compound No. | λmax nm (EtOH) (log ε) | Compound No. | λmax nm (EtOH) (log ε) |
|---|---|---|---|
| 6a | 542 (4.01), 421 (4.16), 361 (4.25) | 6f | 525 (4.17), 443 (4.11), 347 (4.11) |
| 6b | 538 (3.95), 411 (4.06), 355 (4.12) | 6g | 535 (4.48), 459 (4.42), 350 (4.45) |
| 6c | 530 (3.81), 412 (4.00), 353 (4.15) | 6h | 528 (4.48), 452 (4.43), 345 (4.32) |
| 6d | 530 (4.18), 428 (4.17), 353 (4.16) | 6i | 543 (3.45), 484 (3.38), 347 (3.14) |
| 6e | 534 (4.51), 433 (4.50), 352 (4.43) |
| Compd. No. | σX | pK | λmax a | λmax b | Δν cm−1 | pK* | |
|---|---|---|---|---|---|---|---|
| 6a | −0.27 | −0.27 | 7.92 | 353 | 537 | 9706 | −12.46 |
| 6b | −0.17 | −0.17 | 7.70 | 352 | 529 | 9505 | −12.26 |
| 6c | 0 | 0 | 7.57 | 350 | 524 | 9487 | −12.35 |
| 6d | 0.37 | 0.37 | 7.18 | 344 | 520 | 9838 | −13.48 |
| 6e | 0.23 | 0.23 | 7.38 | 346 | 525 | 9854 | −13.31 |
| 6f | 0.71 | 0.71 | 6.86 | 346 | 527 | 9926 | −13.98 |
| 6g | 0.5 | 0.87 | 6.94 | 347 | 525 | 9771 | −13.58 |
| 6h | 0.45 | 0.68 | 7.09 | 349 | 533 | 9891 | −13.68 |
| 6i | 0.78 | 1.28 | 6.75 | 363 | 575 | 10157 | −14.58 |
| Compd. No. | λmax nm (log ε) | |||||||
|---|---|---|---|---|---|---|---|---|
| MeCN | n-BuOH | HCCl3 | DMF | DMSO | MeOH | EtOH | n-PrOH | |
| 6a | 578 (3.84), | 547 (4.08), | 665 (3.66), | 560 (3.11), 357 (4.36) | 588 (4.17), | 526 (3.34), 354 (4.33) | 542(4.01), | 549 (3.85), |
| 434 (3.96), | 426 (4.15), | 439 (3.99), | 459 (4.07), | 421(4.16), | 425 (4.08), | |||
| 354 (4.22) | 567 (4.18) | 361 (4.21) | 350 (4.09) | 361(4.25) | 363 (4.23) | |||
| 6b | 572 (3.99), | 543 (4.15), | 653 (3.9), | 749 (2.67), | 582 (4.34), | 511(3.82), | 538(3.95), | 547 (3.98), |
| 435 (3.96), | 420 (4.14), | 432 (4.02), | 556 (3.57), | 460 (4.19), | 411 (4.12), | 411(4.06), | 416 (4.09), | |
| 346 (4.16) | 362 (4.13) | 353 (4.13) | 347(4.36) | 342 (4.15) | 347 (4.30) | 355(4.12) | 357(4.18); | |
| 6c | 575 (3.04), | 538 (3.94), | 587 (2.60), | 587 (4.29), | 578 (3.94), | 513 (3.58), | 530(3.81), | 538 (3.77), |
| 519 (3.15), | 423 (3.97), | 418 (3.85), | 460 (4.15), | 457 (3.86), | 402 (3.97), | 412(4.00), | 410 (4.08), | |
| 344 (4.48) | 359 (4.02) | 348 (4.16) | 347 (4.08) | 344 (4.07) | 348 (4.17) | 353(4.15) | 350 (4.26) | |
| 6d | 564 (4.21), | 536 (3.94), | 643 (3.82), | 581 (4.35), | 574 (4.38), | 508 (3.85), | 530(4.18), | 538 (3.98), |
| 455 (4.06), | 432 (3.89), | 429 (3.98), | 475 (4.09), | 475 (4.13), | 404 (4.17), | 428(4.17), | 430 (4.02), | |
| 343 (4.24) | 356 (3.83) | 356 (4.11) | 338 (3.97) | 339 (4.02) | 347 (4.29) | 353(4.16) | 353 (4.06) | |
| 6e | 564 (4.69), | 538 (4.55), | 647 (4.67), | 551 (4.18), 347 (4.62) | 567 (4.76), | 509 (4.27), | 534(4.51), | 543 (4.53), |
| 458 (4.54), | 438 (4.52), | 455 (4.48), | 474 (4.55), | 405 (4.49), | 433(4.50), | 432 (4.53), | ||
| 340 (4.39) | 353 (4.41) | 392 (4.35) | 338 (4.37) | 348 (4.60) | 352(4.43) | 353 (4.47) | ||
| 6f | 561(4.28), | 532 (4.23), | 628 (4.21) | 577 (4.47), | 573 (4.41), | 507 (4.02), | 525(4.17), | 533 (4.19), |
| 469 (4.09), | 450 (4.18), | 459 (4.04), | 483 (4.21), | 483 (4.17), | 414 (4.11), | 443(4.11), | 444 (4.15), | |
| 340 (4.08) | 349 (4.14) | 362 (4.04) | 337 (4.14) | 336 (4.09) | 345 (4.25) | 347(4.11) | 350 (4.18) | |
| 6g | 571 (4.43), | 544 (4.55), | 637 (4.27), | 564 (4.59), | 586 (4.78), | 507 (4.02), | 535(4.48), | 545 (4.44), |
| 478 (4.25), | 466 (4.48), | 461 (4.27), | 477 (4.40), | 492 (4.49), | 414 (4.11), | 459 (4.42), | 456 (4.40), | |
| 348 (4.48) | 351 (4.38) | 359 (4.51) | 343 (4.61) | 340 (4.41) | 345 (4.25) | 350(4.45) | 351 (4.45) | |
| 6h | 569 (4.52), | 539 (4.66), | 639 (4.6), | 533 (4.52), | 584 (4.75), | 516 (4.25), | 528(4.48), | 542 (4.48), |
| 476 (4.47), | 459 (4.46), | 470 (4.41), | 453 (4.46), | 489 (4.45), | 413 (4.39), | 452(4.43), | 454 (4.43), | |
| 344 (4.32) | 346 (4.32) | 371 (4.25) | 344 (4.33) | 333 (4.28) | 349 (4.55) | 345(4.32) | 346 (4.36) | |
| 6i | 591 (3.67), | 548 (3.45), | 648 (3.17), 506 (3.44) | 619 (4.67), | 618 (4.48), | 521 (3.94), | 543(3.45), | 550 (3.49), |
| 503 (3.47), | 478 (3.38), | 508 (4.30), | 504 (4.12), | 420 (4.04), | 484(3.38), | 488 (3.41), | ||
| 352 (3.47) | 346 (3.36) | 342 (4.26) | 336 (4.07) | 350 (4.24) | 347(3.14) | 344 (3.44) | ||
| Solvents | π* | Β | Α |
|---|---|---|---|
| Acetonitrile | 0.75 | 0.31 | 0.19 |
| 1-Butanol | 0.47 | 0.88 | 0.79 |
| Chloroform | 0.58 | 0 | 0.44 |
| DMF | 0.88 | 0.69 | 0 |
| DMSO | 1 | 0.76 | 0 |
| Ethanol | 0.54 | 0.77 | 0.83 |
| Methanol | 0.6 | 0.62 | 0.93 |
| 1-Propanol | 0.52 | 0.83 | 0.78 |
| Compd. No. | υo (103·cm−1) | s | b | a | R 1 | ±S 2 |
|---|---|---|---|---|---|---|
| 6a | 24.38 | 4.778 | −0.752 | 1.588 | 0.987 | 0.092 |
| 6b | 24.819 | 5.158 | −0.825 | 1.471 | 0.982 | 0.136 |
| 6c | 13.738 | 3.410 | 0.390 | 3.630 | 0.987 | 0.197 |
| 6d | 19.389 | 3.103 | −1.782 | 4.769 | 0.993 | 0.202 |
| 6e | 22.80 | 5.448 | 2.292 | 1.136 | 0.810 | 0.974 |
| 6f | 16.62 | 5.23 | −1.25 | 5.36 | 0.991 | 0.208 |
| 6g | 26.462 | 2.428 | 0.792 | 0.216 | 0.983 | 0.114 |
| 6h | 24.58 | 3.217 | 2.047 | 0.483 | 0.921 | 0.438 |
| 6i | 16.544 | 7.699 | 8.838 | 1.542 | 0.896 | 1.925 |
| Compod. No. | π* (%) | β (%) | α (%) |
|---|---|---|---|
| 6a | 67.12 | 10.56 | 22.31 |
| 6b | 69.19 | 11.07 | 19.73 |
| 6c | 45.89 | 5.24 | 48.86 |
| 6d | 32.14 | 18.45 | 49.39 |
| 6e | 61.38 | 25.82 | 12.79 |
| 6f | 44.17 | 10.55 | 45.27 |
| 6g | 70.66 | 23.05 | 6.28 |
| 6h | 55.97 | 35.61 | 8.40 |
| 6i | 42.58 | 48.88 | 8.53 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altalbawy, F.; Darwish, E.; Medhat, M.; El-Zaiat, S.; Saleh, H. Synthesis, Characterization, Tautomeric Structure and Solvatochromic Behavior of Novel 4-(5-Arylazo-2-Hydroxystyryl)-1-Methylpyridinium Iodide as Potential Molecular Photoprobe. Materials 2016, 9, 1022. https://doi.org/10.3390/ma9121022
Altalbawy F, Darwish E, Medhat M, El-Zaiat S, Saleh H. Synthesis, Characterization, Tautomeric Structure and Solvatochromic Behavior of Novel 4-(5-Arylazo-2-Hydroxystyryl)-1-Methylpyridinium Iodide as Potential Molecular Photoprobe. Materials. 2016; 9(12):1022. https://doi.org/10.3390/ma9121022
Chicago/Turabian StyleAltalbawy, Farag, Elham Darwish, Mohamed Medhat, Sayed El-Zaiat, and Hagar Saleh. 2016. "Synthesis, Characterization, Tautomeric Structure and Solvatochromic Behavior of Novel 4-(5-Arylazo-2-Hydroxystyryl)-1-Methylpyridinium Iodide as Potential Molecular Photoprobe" Materials 9, no. 12: 1022. https://doi.org/10.3390/ma9121022






