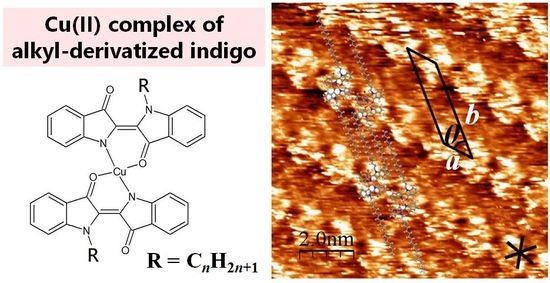
Surface Structures Formed by a Copper(II) Complex of Alkyl-Derivatized Indigo
Abstract
:1. Introduction
2. Results and Discussion
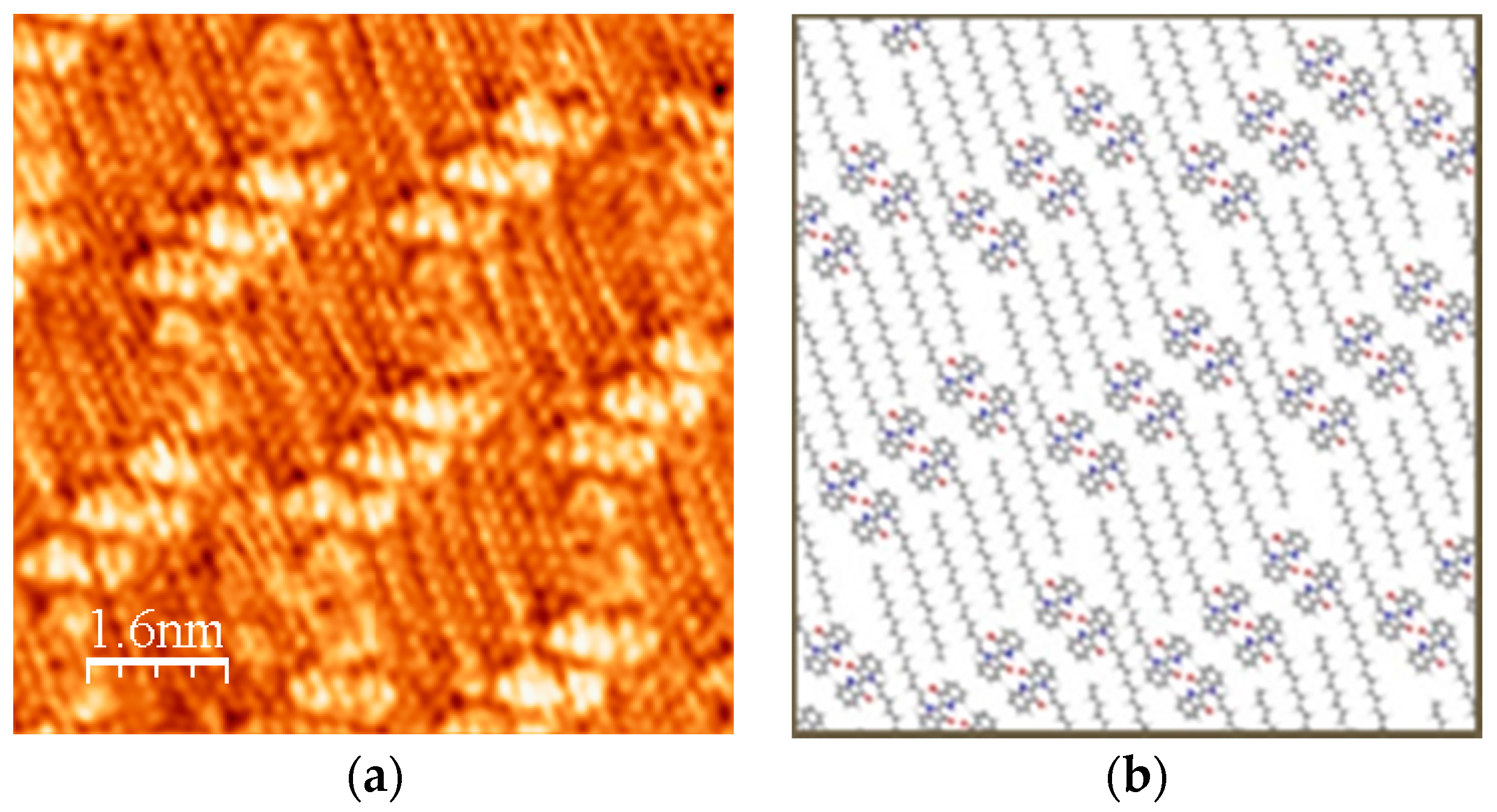
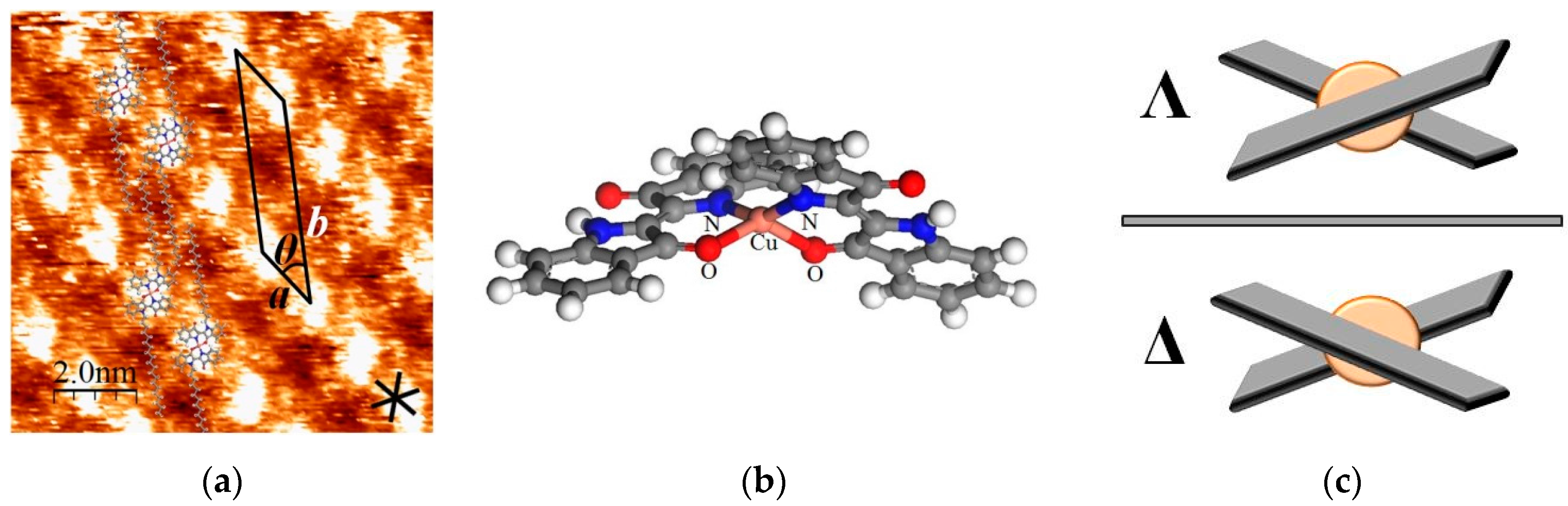
2.1. STM Images of [Cu(C16IND)2] on HOPG
2.2. Comparison between [Cu(C16IND)2] and C16IND
2.3. 2D Chirality of [Cu(C16IND)2]
2.4. STM Images of [Cu(C18IND)2] and [Cu(C20IND)2] on HOPG
2.5. The Effect of 3D Chirality of [Cu(C20IND)2] to 2D Self-Assembly
3. Materials and Methods
3.1. Materials and Identification
3.2. Synthesis of the Copper(II) Complex of Hexadecyl-Indigo ([Cu(C16IND)2])
3.3. Synthesis of the Copper(II) Complexes of Octadecyl-Indigo ([Cu(C18IND)2]) and Icosyl-Indigo ([Cu(C20IND)2])
3.4. Scanning Tunneling Microscope (STM) Measurements
4. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| STM | scanning tunneling microscope |
| HOPG | high orientated pyrolytic graphite |
| 2D | two-dimensional |
| 3D | three-dimensional |
Appendix A
References
- Kim, H.; Chang, J.Y. Reversible Thermochromic Polymer Film Embedded with Fluorescent Organogel Nanofibers. Langmuir 2014, 30, 13673–13679. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Kinuta, T.; Nagasaki, K.; Harada, T.; Sato, T.; Tajima, N.; Sasaki, Y.; Kuroda, R.; Matsubara, Y. Conformational and color polymorphism of achiral 2-methyl-3-(2-naphthalenylthio)-1,4-naphthalenedio-ne. Cryst. Eng. Comm. 2009, 11, 1223–1226. [Google Scholar] [CrossRef]
- Lee, J.H.; Naumov, P.; Chung, I.H.; Lee, S.C. Solid-State Thermochromism and Phase Transitions of Charge Transfer 1,3-Diamino-4,6-dinitrobenzene Dyes. J. Phys. Chem. A 2011, 115, 10087–10096. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.S.; Yao, H.; Matsuoka, O.; Kawabata, R.; Kitamura, N.; Yamamoto, S. Anisotropic Growth of J Aggregates of Pseudoisocyanine Dye at a Mica/Solution Interface Revealed by AFM and Polarization Absorption Measurements. J. Phys. Chem. B 1999, 103, 6909–6912. [Google Scholar] [CrossRef]
- Nguyen, N.D.; Zhang, G.; Lu, J.; Sherman, A.E.; Fraser, C.L. Alkyl chain length effects on solid-state difluoroboron β-diketonate mechanochromic luminescence. J. Mater. Chem. 2011, 21, 8409–8415. [Google Scholar] [CrossRef]
- Kuroda, S. J-aggregation and its characterization in Langmuir–Blodgett films of merocyanine dyes. Adv. Colloid Interface Sci. 2004, 111, 181–209. [Google Scholar] [CrossRef] [PubMed]
- Binnig, G.; Rohrer, H.; Gerber, C.; Weibel, E. Surface Studies by Scanning Tunneling Microscopy. Phys. Rev. Lett. 1982, 49, 57–61. [Google Scholar] [CrossRef]
- Thalacker, C.; Miura, A.; de Feyter, S.; de Schryver, F.C.; Würthner, F. Hydrogen bond directed self-assembly of core-substituted naphthalene bisimides with melamines in solution and at the graphite interface. Org. Biomol. Chem. 2005, 3, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wan, L.-J.; Wang, C.; Bai, C.-L. In Situ STM Evidence for Adsorption of Rhodamine B in Solution. J. Phys. Chem. B 2002, 106, 4223–4226. [Google Scholar] [CrossRef]
- Jaroch, T.; Maranda-Niedbala, A.; Kotwica, K.; Wamil, D.; Bujak, P.; Pron, A.; Nowakowski, R. Self-assembly of tetraalkoxydinaphthophenazines in monolayers on HOPG by scanning tunneling microscopy. Surface Sci. 2015, 641, 252–259. [Google Scholar] [CrossRef]
- Stawasz, M.E.; Sampson, D.L.; Parkinson, B.A. Scanning Tunneling Microscopy Investigation of the Ordered Structures of Dialkylamino Hydroxylated Squaraines Adsorbed on Highly Oriented Pyrolytic Graphite. Langmuir 2000, 16, 2326–2342. [Google Scholar] [CrossRef]
- Kawasaki, M.; Sato, T.; Yoshimoto, T. Controlled Layering of Two-Dimensional J-Aggregate of Anionic Cyanine Dye on Self-Assembled Cysteamine Monolayer on Au(111). Langmuir 2000, 16, 5409–5417. [Google Scholar] [CrossRef]
- Urano, K.; Ohno, T.; Tomono, K.; Miyamura, K. Observation of Dynamic Behavior of Self-Assembled N-Icosyl-Substituted Indigo by STM. Bull. Chem. Soc. Jpn. 2013, 86, 159–165. [Google Scholar] [CrossRef]
- Guzel, B.; Akgerman, A. Mordant dyeing of wool by supercritical processing. J. Supercrit. Fluids 2000, 18, 247–252. [Google Scholar] [CrossRef]
- Moiz, A.; Ahmed, M.A.; Kausar, N.; Ahmed, K.; Sohail, M. Study the effect of metal ion on wool fabric dyeing with tea as natural dye. J. Saudi Chem. Soc. 2010, 14, 69–76. [Google Scholar] [CrossRef]
- Mali, K.S.; Lava, K.; Binnemans, K.; de Feyter, S. Hydrogen Bonding Versus van der Waals Interactions: Competitive Influence of Noncovalent Interactions on 2D Self-Assembly at the Liquid–Solid Interface. Chem. Eur. J. 2010, 16, 14447–14458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamaki, Y.; Muto, K.; Miyamura, K. Odd-Even Effect in the Surface Structure of Alkyloxy-Substituted Anthraquinone on HOPG Observed by Scanning Tunneling Microscope. Bull. Chem. Soc. Jpn. 2013, 86, 354–362. [Google Scholar] [CrossRef]
- Vidal, F.; Delvigne, E.; Stepanow, S.; Lin, N.; Barth, J.V.; Kern, K. Chiral Phase Transition in Two-Dimensional Supramolecular Assemblies of Prochiral Molecules. J. Am. Chem. Soc. 2005, 127, 10101–10106. [Google Scholar] [CrossRef] [PubMed]
- Böhringer, M.; Schneider, W.-D.; Berndt, R. Real Space Observation of a Chiral Phase Transition in a Two-Dimensional Organic Layer. Angew. Chem. Int. Ed. 2000, 39, 792–795. [Google Scholar] [CrossRef]
- Honda, A.; Tamaki, Y.; Miyamura, K. The Effects of Noncovalent Interactions on Surface Structures Formed by Diketopyrrolopyrrole Pigment and Its Alkyl-Derivatives on HOPG Substrate. Bull. Chem. Soc. Jpn. 2015, 88, 969–975. [Google Scholar] [CrossRef]
- Miyake, K.; Hori, Y.; Ikeda, T.; Asakawa, M.; Shimizu, T.; Sasaki, S. Alkyl Chain Length Dependence of the Self-Organized Structure of Alkyl-Substituted Phthalocyanines. Langmuir 2008, 24, 4708–4714. [Google Scholar] [CrossRef] [PubMed]
- De Feyter, S.; Larsson, M.; Schuurmans, N.; Verkuijl, B.; Zoriniants, G.; Gesquière, A.; Abdel-Mottaleb, M.M.; van Esch, J.; Feringa, B.L.; van Stam, J.; et al. Supramolecular Control of Two-Dimensional Phase Behavior. Chem. Eur. J. 2003, 9, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yan, H.-J.; Yan, C.-J.; Pan, G.-B.; Wan, L.-J.; Wen, G.-Y.; Zhang, D.-Q. STM investigation of the dependence of alkane and alkane (C18H38, C19H40) derivatives self-assembly on molecular chemical structure on HOPG surface. Surface Sci. 2008, 602, 1256–1266. [Google Scholar] [CrossRef]
- Mamdouh, W.; Uji-i, H.; Ladislaw, J.S.; Dulcey, A.E.; Percec, V.; de Schryver, F.C.; de Feyter, S. Solvent Controlled Self-Assembly at the Liquid-Solid Interface Revealed by STM. J. Am. Chem. Soc. 2006, 128, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Hibino, M.; Sumi, A.; Tsuchiya, H.; Hatta, I. Microscopic Origin of the Odd-Even Effect in Monolayer of Fatty Acids Formed on a Graphite Surface by Scanning Tunneling Microscopy. J. Phys. Chem. B 1998, 102, 4544–4547. [Google Scholar] [CrossRef]
- Tahara, K.; Furukawa, S.; Uji-I, H.; Uchino, T.; Ichikawa, T.; Zhang, J.; Mamdouh, W.; Sonoda, M.; de Schryver, F.C.; de Feyter, S.; Tobe, Y. Two-Dimensional Porous Molecular Networks of Dehydrobenzo[12]annulene Derivatives via Alkyl Chain Interdigitation. J. Am. Chem. Soc. 2006, 128, 16613–16625. [Google Scholar] [CrossRef] [PubMed]
- Accelrys Inc. Materials Studio, version 7.0; Accelrys Inc.: San Diego, CA, USA, 2013. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honda, A.; Noda, K.; Tamaki, Y.; Miyamura, K. Surface Structures Formed by a Copper(II) Complex of Alkyl-Derivatized Indigo. Materials 2016, 9, 837. https://doi.org/10.3390/ma9100837
Honda A, Noda K, Tamaki Y, Miyamura K. Surface Structures Formed by a Copper(II) Complex of Alkyl-Derivatized Indigo. Materials. 2016; 9(10):837. https://doi.org/10.3390/ma9100837
Chicago/Turabian StyleHonda, Akinori, Keisuke Noda, Yoshinori Tamaki, and Kazuo Miyamura. 2016. "Surface Structures Formed by a Copper(II) Complex of Alkyl-Derivatized Indigo" Materials 9, no. 10: 837. https://doi.org/10.3390/ma9100837