Effect of Rare Earth Metals on the Microstructure of Al-Si Based Alloys
Abstract
:1. Introduction
2. Experimental Procedures
| Alloy | Elements (wt %) | |||||
|---|---|---|---|---|---|---|
| Si | Cu | Mg | Fe | Zn | Al | |
| A356 | 7 | <0.20 | 0.35 | <0.20 | <0.10 | Bal |
| Alloy | Mold Type | Preheating Mold Temperature (°C) | Alloy Code | Modifier Addition (wt %) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Aimed | Actual | ||||||||
| Sr | La | Ce | Sr | La | Ce | ||||
| A356 | Graphite | 600 | TB | 0 | 0 | 0 | 0 | 0 | 0 |
| TBS | 0.01 | 0 | 0 | 0.011 | 0 | 0 | |||
| T10 | 0 | 0.2 | 0 | 0 | 0.17 | 0 | |||
| T1 | 0 | 0.5 | 0 | 0 | 0.40 | 0 | |||
| T2 | 0 | 1 | 0 | 0 | 0.85 | 0 | |||
| T3 | 0 | 1.5 | 0 | 0 | 1.30 | 0 | |||
| T11 | 0 | 0 | 0.2 | 0 | 0 | 0.18 | |||
| T4 | 0 | 0 | 0.5 | 0 | 0 | 0.38 | |||
| T5 | 0 | 0 | 1 | 0 | 0 | 0.82 | |||
| T6 | 0 | 0 | 1.5 | 0 | 0 | 1.38 | |||
| T7 | 0 | 0.5 | 0.5 | 0 | 0.44 | 0.38 | |||
| T8 | 0 | 1 | 1 | 0 | 0.78 | 0.87 | |||
| T9 | 0 | 1.5 | 1.5 | 0 | 1.37 | 1.53 | |||
| T10S | 0.01 | 0.2 | 0 | 0.009 | 0.17 | 0 | |||
| T1S | 0.01 | 0.5 | 0 | 0.011 | 0.40 | 0 | |||
| T2S | 0.01 | 1 | 0 | 0.008 | 0.85 | 0 | |||
| T3S | 0.01 | 1.5 | 0 | 0.010 | 1.30 | 0 | |||
| T11S | 0.01 | 0 | 0.2 | 0.008 | 0 | 0.18 | |||
| T4S | 0.01 | 0 | 0.5 | 0.009 | 0 | 0.38 | |||
| T5S | 0.01 | 0 | 1 | 0.008 | 0 | 0.82 | |||
| T6S | 0.01 | 0 | 1.5 | 0.010 | 0 | 1.38 | |||
| T7S | 0.01 | 0.5 | 0.5 | 0.009 | 0.44 | 0.38 | |||
| T8S | 0.01 | 1 | 1 | 0.009 | 0.78 | 0.87 | |||
| T9S | 0.01 | 1.5 | 1.5 | 0.011 | 1.37 | 1.53 | |||

3. Results and Discussion
3.1. Thermal Analysis

- Introduction of La or Ce increased the start of solidification temperature of A356 alloy by about 11 °C at 1.5 wt % La or Ce with a marginal effect on the Al-Si eutectic temperature.
- Introduction of 100 ppm Sr to A356 alloy reduced the eutectic temperature by about 7 °C. Addition of La, Ce, or La + Ce up to 1.5 wt % each has no further effect on the eutectic temperature.
- No explicit peak corresponding to precipitation of rare earth (RE)—containing phases could be detected using the thermal analysis technique.

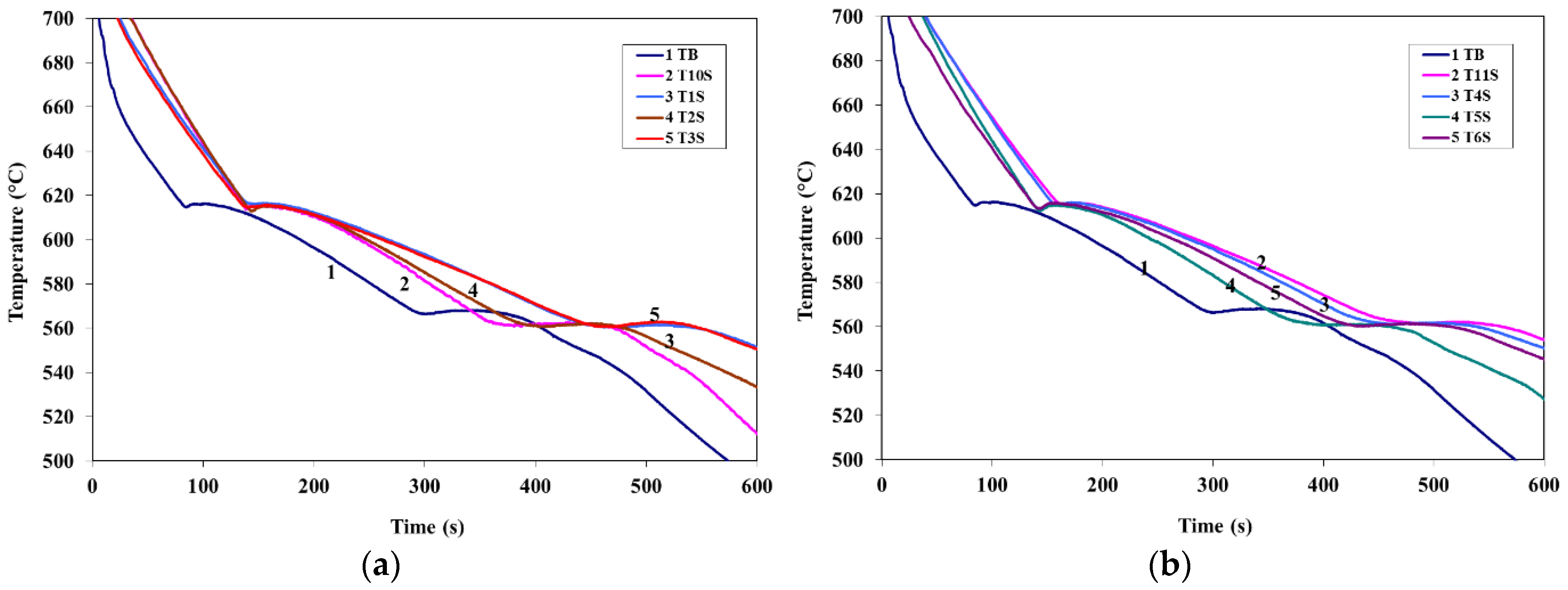
| Alloy Code | Recalescence of Eutectic Si | |||||
|---|---|---|---|---|---|---|
| Te1 * | Te2 | ΔTe | te1 ** | te2 | Δte | |
| T10 | 565.1 | 566.2 | 1.1 | 242.5 | 252.3 | 9.8 |
| T1 | 565.2 | 566.4 | 1.2 | 239.8 | 260.2 | 20.4 |
| T2 | 563.4 | 564.8 | 1.4 | 254.3 | 270.3 | 16.0 |
| T3 | 560.8 | 563.2 | 2.4 | 257.0 | 281.2 | 24.2 |
| Alloy Code | Recalescence of Eutectic Si | |||||
|---|---|---|---|---|---|---|
| Te1 | Te2 | ΔTe | te1 | te2 | Δte | |
| T10S | 560.7 | 562.6 | 1.9 | 264.6 | 308 | 43.4 |
| T1S | 560.4 | 561.8 | 1.4 | 339.6 | 388.8 | 49.2 |
| T2S | 560.6 | 562.2 | 1.6 | 279.6 | 322.2 | 42.6 |
| T3S | 560.7 | 563.0 | 2.3 | 347.8 | 389.4 | 41.6 |
3.2. Microstructural Characterization
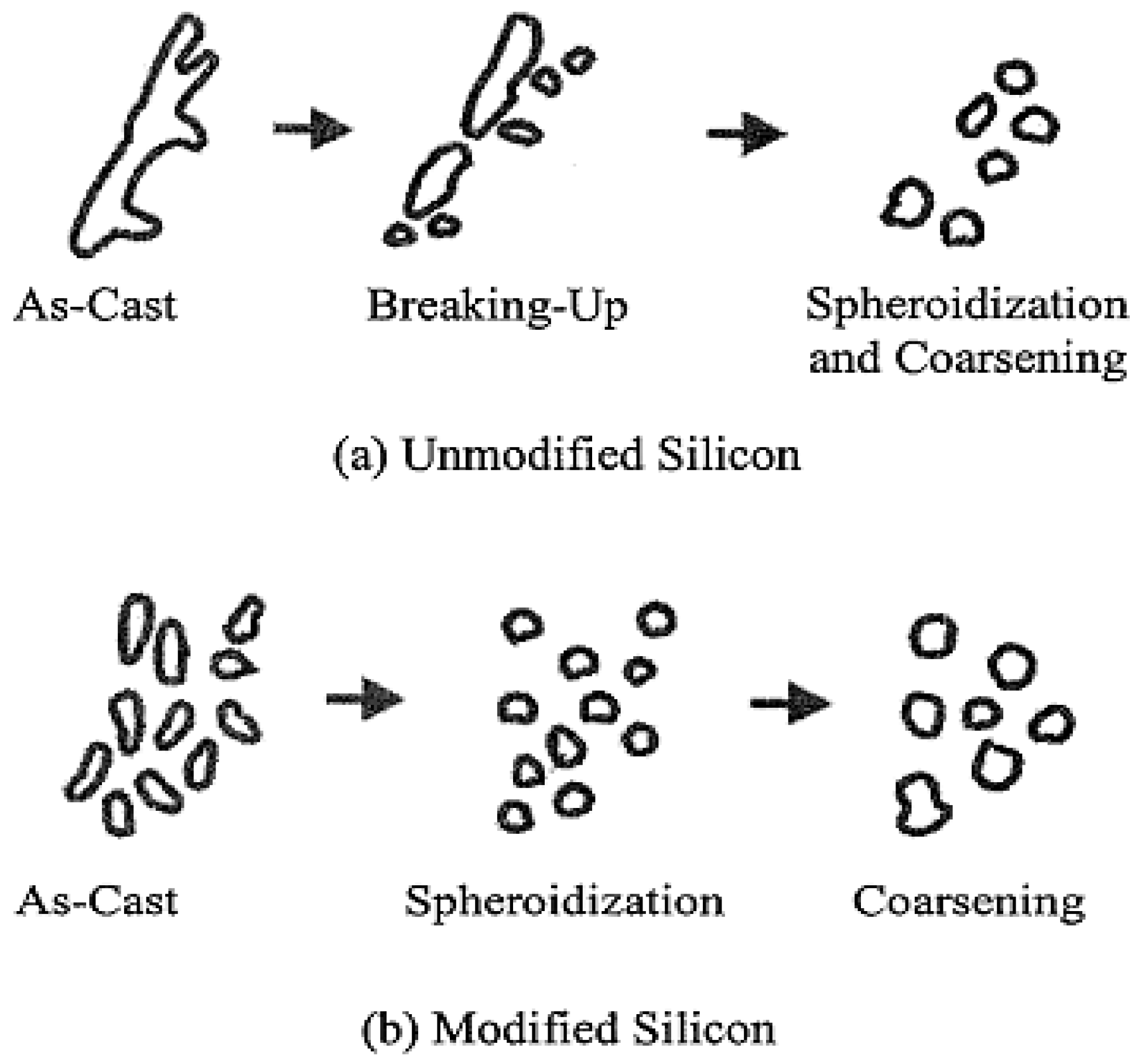
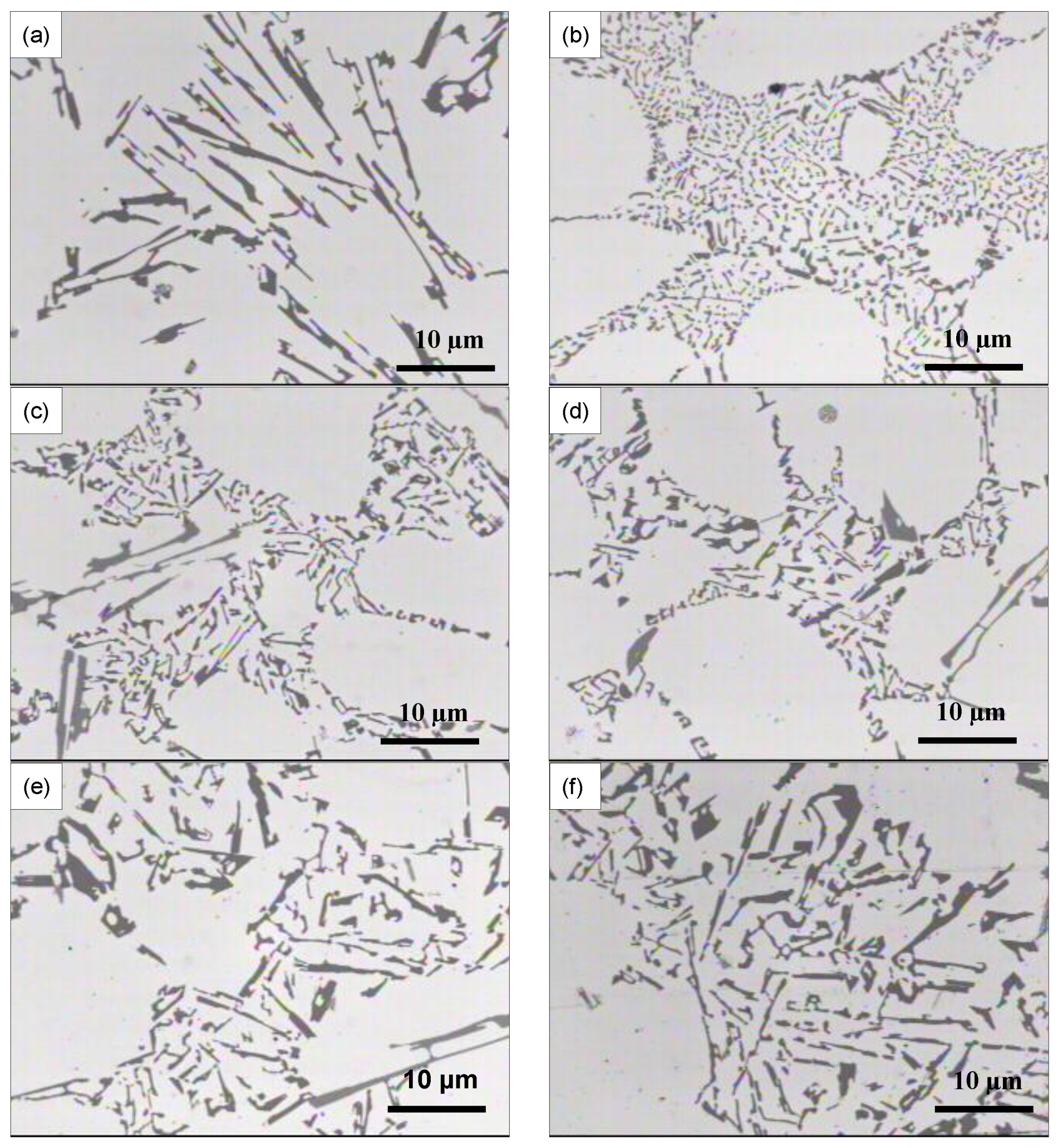
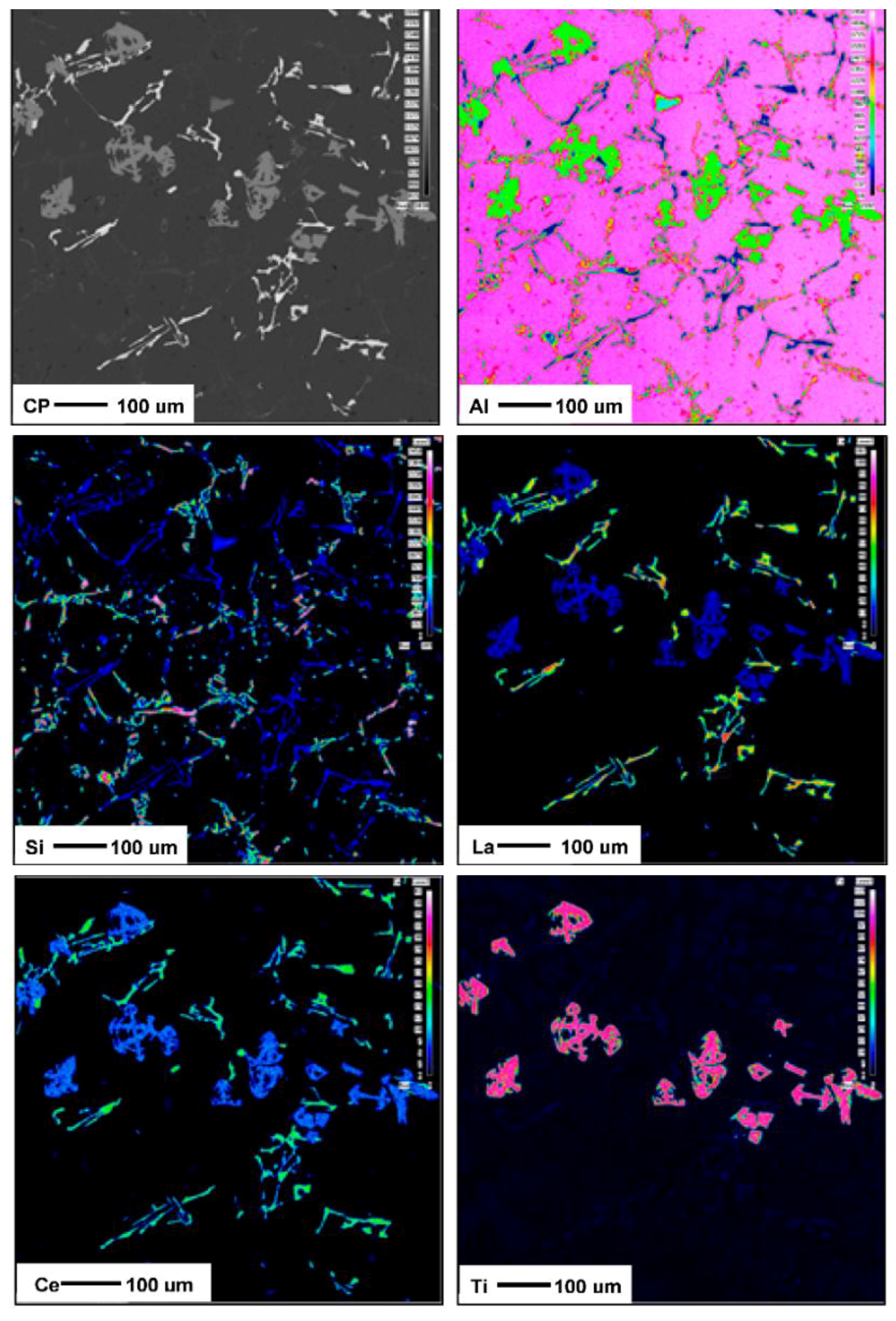
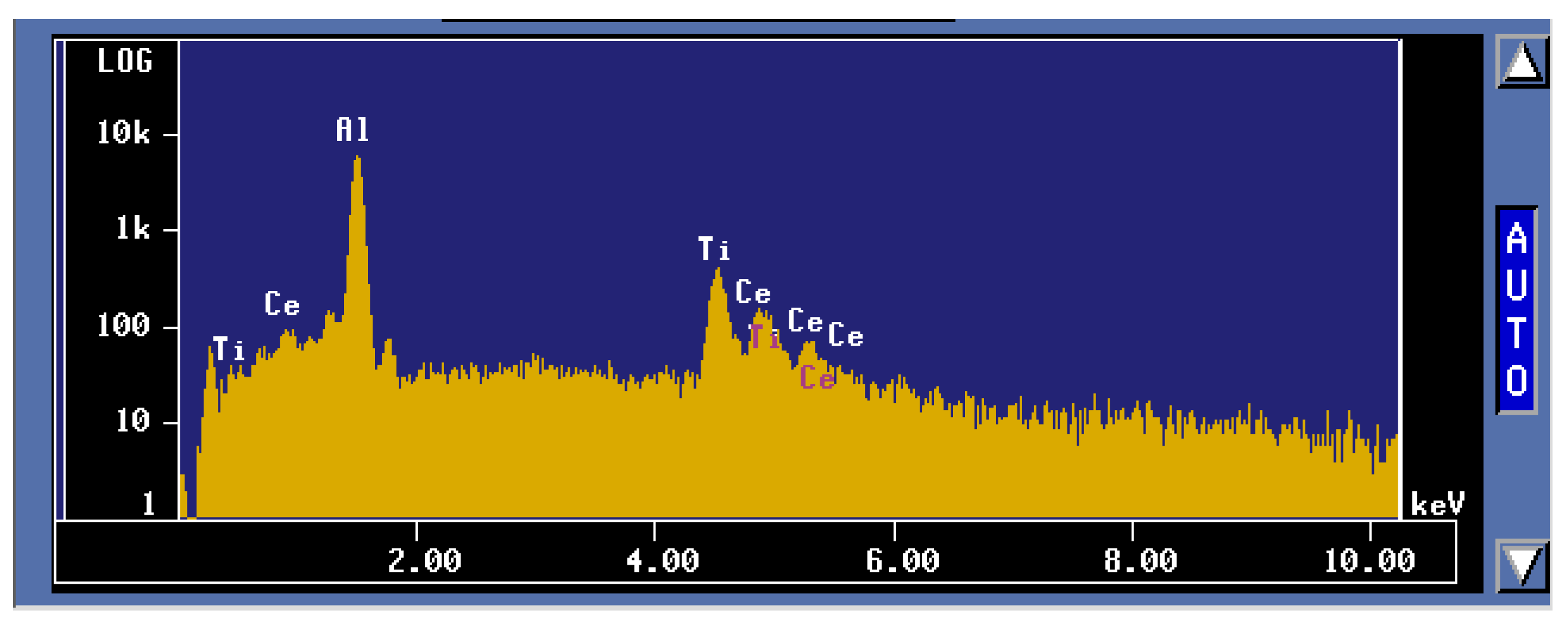
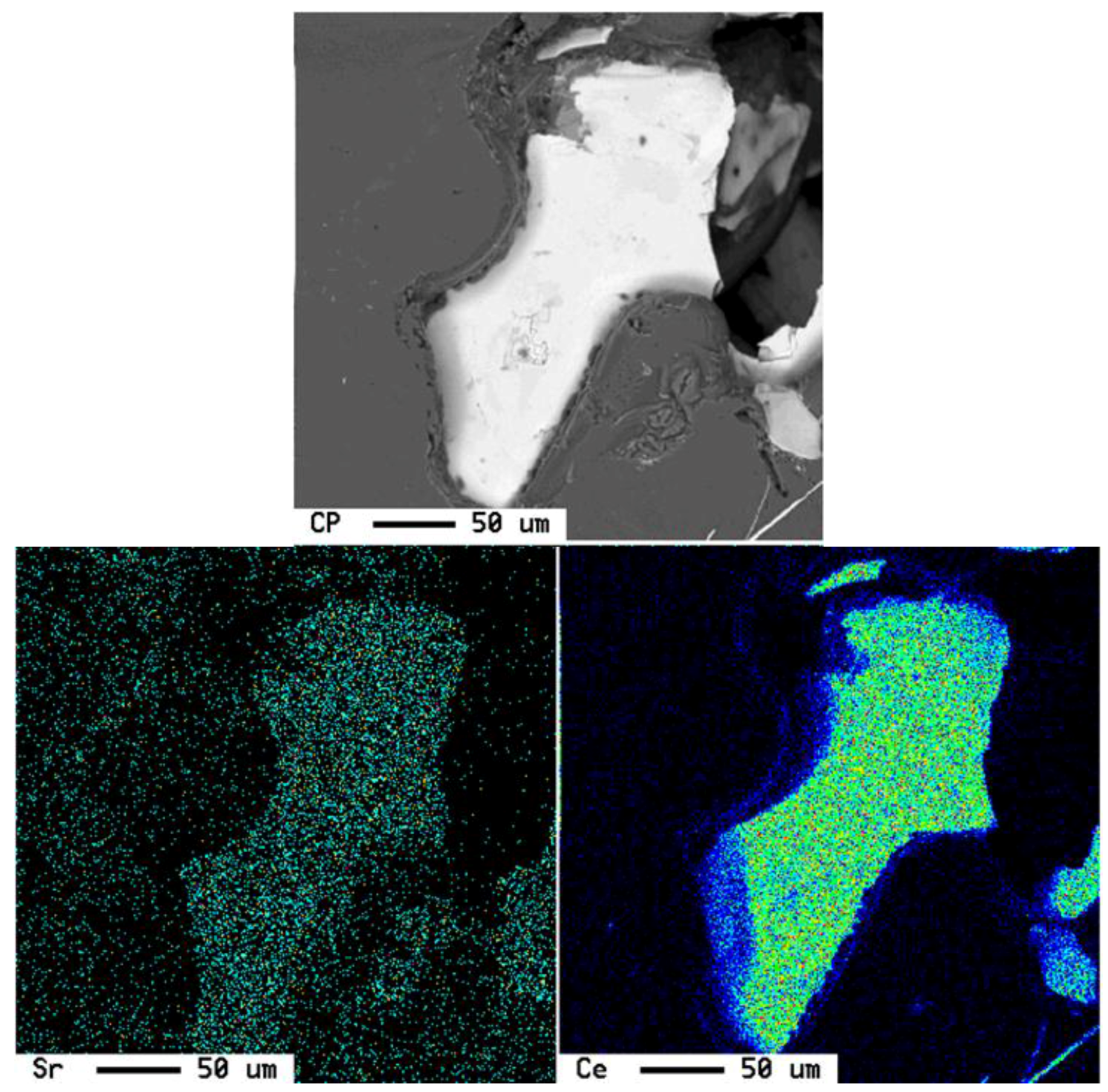
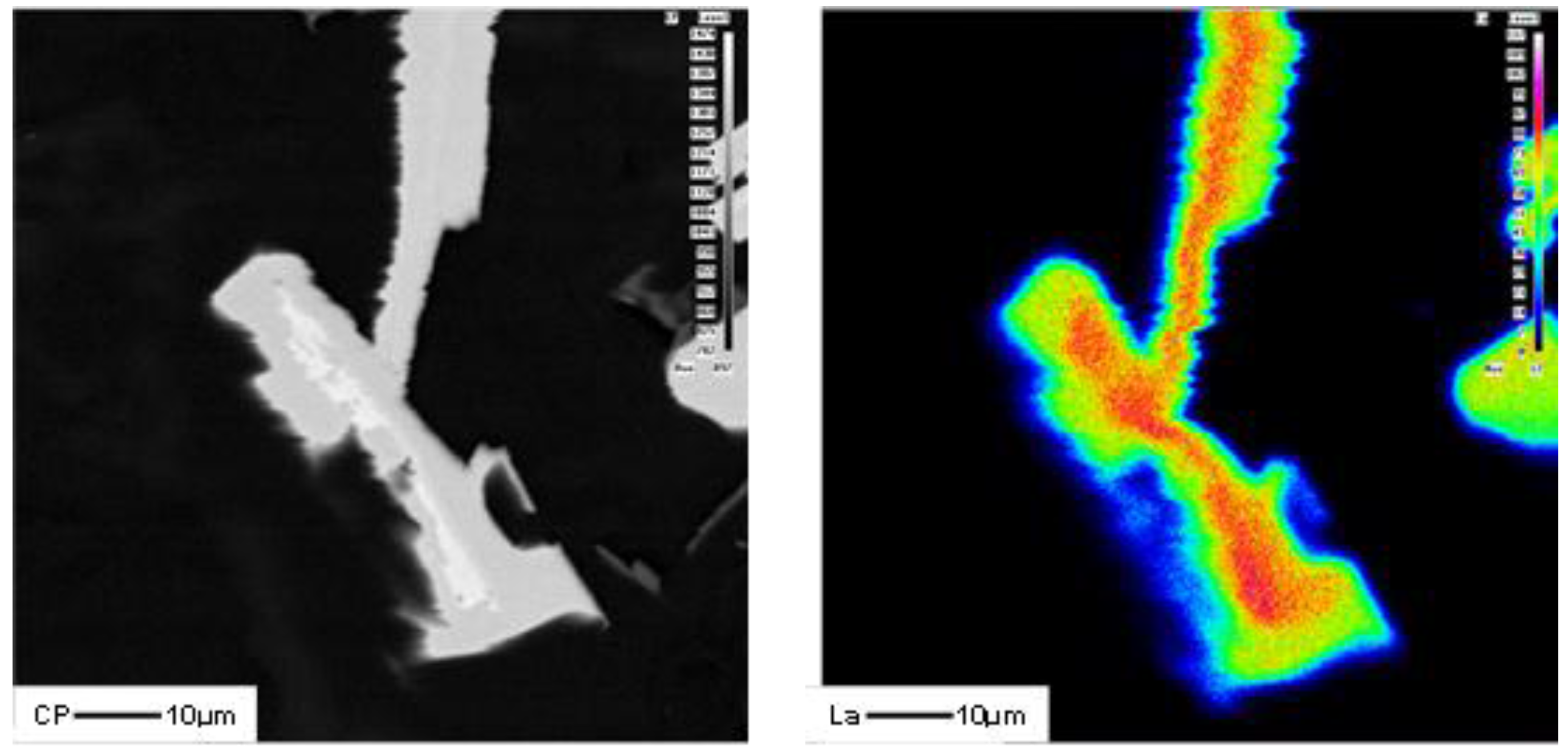
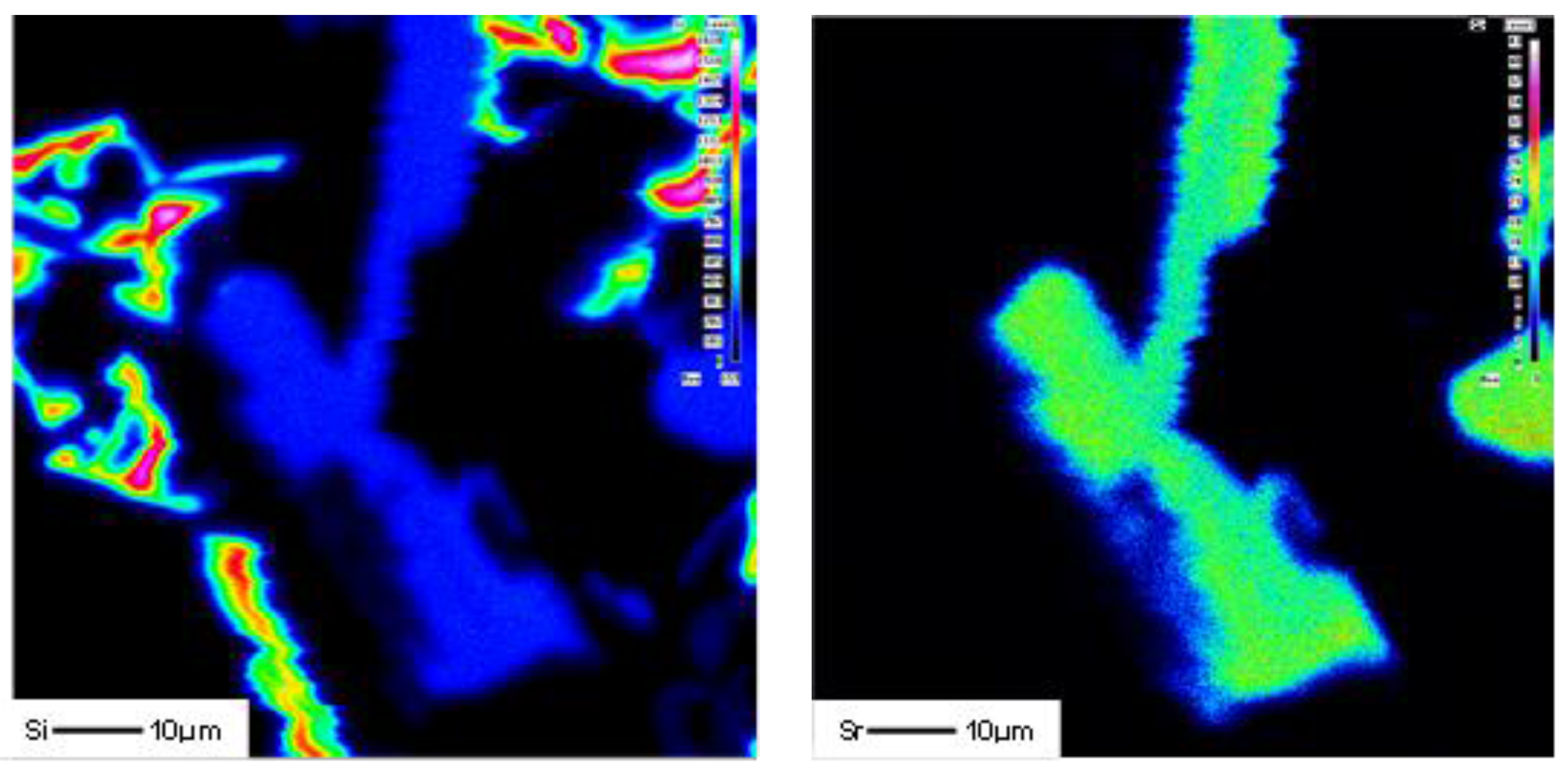


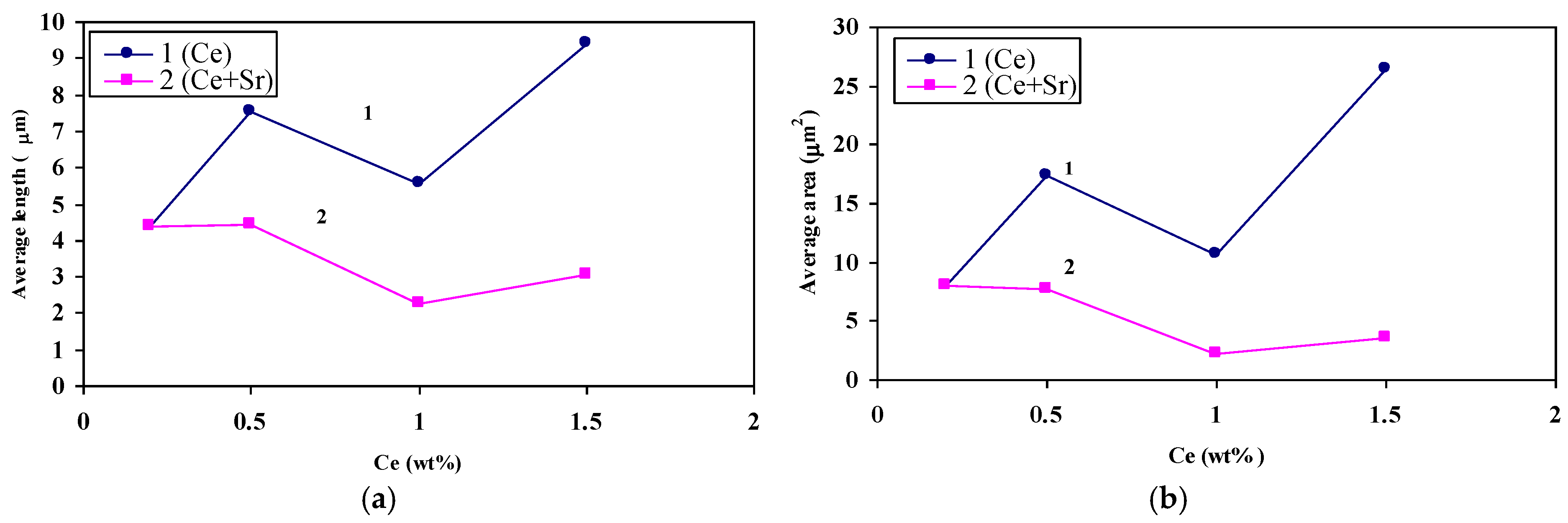
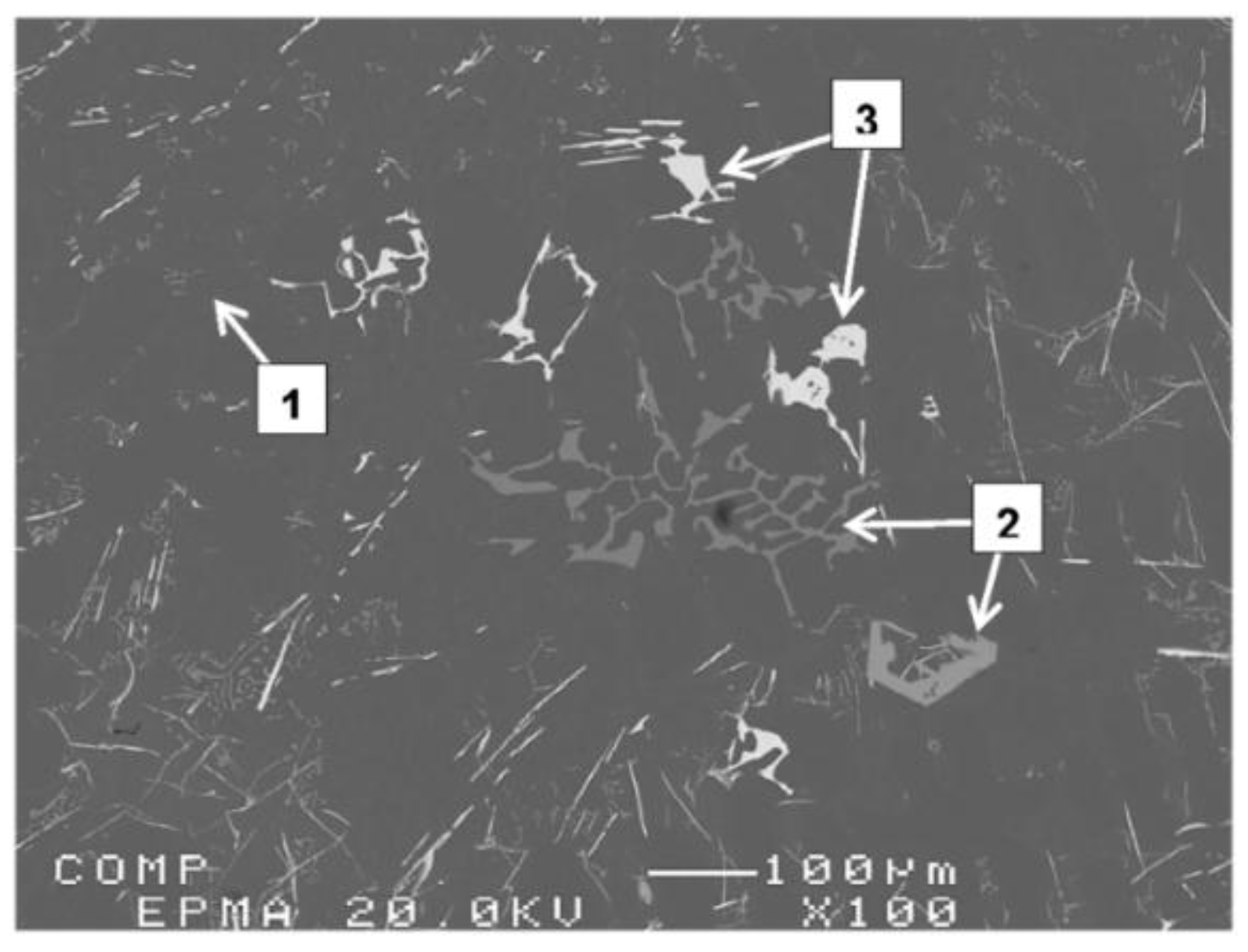
4. Conclusions
- Addition of La or Ce individually or combined up to 1.5 wt % increases the melting point of A356 alloy at approximately 1 °C/0.15 wt % RE metals.
- The addition of RE metals has no significant effect either on the Al-Si eutectic temperature or on the modification of the eutectic Si particles.
- Both La and Ce have high affinity to react with Sr resulting in marked reduction in the Sr modification effect.
- Modification of Si particles may take place when La concertation exceeds 1.5 wt %.
- Increasing the amount of La, however, leads to precipitation of insoluble intermetallics that could negatively affect the alloy performance.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aladar, P. Alloy. U.S. Patent 1,387,900, 16 August 1921. [Google Scholar]
- Closset, B.M.; Gruzleski, J.E. The Treatment Liquid Aluminum-Silicon Alloys; American Foundrymen’s Society: Des Plaines, IL, USA, 1990. [Google Scholar]
- Elsebaie, O.; Samuel, F.H.; Alkahtani, S.A. Intermetallic phases observed in non-modified and Sr modified Al-Si cast alloys containing mischmetal. Int. J. Cast Met. Res. 2013, 26, 1–15. [Google Scholar] [CrossRef]
- Elsebaie, O.; Samuel, A.M.; Samuel, F.H.; Doty, H.W. The effects of mischmetal, cooling rate and heat treatment on the eutectic Si particle characteristics of A319.1, A356.2 and A413.1 Al-Si casting alloys. Mater. Sci. Eng. A 2008, 480, 342–355. [Google Scholar]
- Samuel, A.M.; Garza-Elizondo, G.H.; Doty, H.W.; Samuel, F.H. Role of modification and melt thermal treatment processes on the microstructure and tensile properties of Al-Si alloys. Mater. Des. 2015, 80, 99–108. [Google Scholar] [CrossRef]
- Hegde, S.; Prabhu, K.N. Modification of eutectic silicon in Al-Si alloys. J. Mater. Sci. 2008, 43, 3009–3027. [Google Scholar] [CrossRef]
- Onyia, C.W.; Okorie, B.A.; Neife, S.I.; Obayi, C.S. Structural modification of sand cast eutectic Al-Si alloys with sulfur/sodium and its effect on mechanical properties. World J. Eng. Technol. 2013, 1, 9–16. [Google Scholar] [CrossRef]
- Sarada, B.N.; Srinivasamurthy, P.L. Swetha, microstructural characteristics of Sr and Na modified Al-Mg-Si alloy. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 3975–3983. [Google Scholar]
- Fat-Halla, N. Structural modification of Al-Si eutectic alloy by Sr and its effect on tensile and fracture characteristics. J. Mater. Sci. 1989, 24, 2488–2492. [Google Scholar] [CrossRef]
- Barrireroa, J.; Engstlera, M.; Ghafoorb, N.; Jongec, N.; Odénb, M.; Mücklicha, F. Comparison of segregations formed in unmodified and Sr-modified Al-Si alloys studied by atom probe tomography and transmission electron microscopy. J. Alloys Compd. 2014, 611, 410–421. [Google Scholar] [CrossRef]
- Srirangam, P.; Chattopadhyay, S.; Bhattacharya, A.; Nag, S.; Kaduke, J.; Shankar, S.; Banerjee, R.; Shibata, T. Probing the local atomic structure of Sr-modified Al-Si alloys. Acta Mater. 2014, 65, 185–193. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Yu, H.; Jin, Y. Study on dual modification of Al-17%Si alloys by structural heredity. Metals 2015, 5, 1112–1126. [Google Scholar] [CrossRef]
- Li, H.J.; Shivkumar, S.; Luos, X.J.; Apelian, D. Influence of modification on the solution heat treatment response of cast Al-Si-Mg Alloys. Cast Met. 1989, 1, 227–234. [Google Scholar]
- Nogita, K.; McDonald, S.D.; Dahle, A.K. Eutectic modification of Al-Si alloys with rare earth metals. Mater. Trans. JIM 2004, 45, 323–326. [Google Scholar] [CrossRef]
- Mousavi, G.S.; Emamyn, M.; Rassizadehghani, J. The effect of mischmetal and heat treatment on the microstructure and tensile properties of A357 Al-Si casting alloy. Mater. Sci. Eng. A 2012, 556, 573–581. [Google Scholar] [CrossRef]
- Ouyang, Z.-Y.; Mao, X.-M.; Hong, M. Multiplex modification with rare earth elements and P for hypereutectic Al-Si alloys. J. Shanghai Univ. Engl. Ed. 2007, 11, 400–402. [Google Scholar] [CrossRef]
- Zhang, H.; Duan, H.; Shao, G.; Xu, L.; Yin, J.; Yan, B. Modification mechanism of cerium on the Al-18Si alloy. Rare Met. 2006, 25, 11–15. [Google Scholar] [CrossRef]
- Dobrzański, L.A.; Maniara, R.; Sokolowski, J.H. The effect of cast Al-Si-Cu alloy solidification rate on alloy thermal characteristics. J. Achiev. Mater. Manuf. Eng. 2006, 17, 217–220. [Google Scholar]
- Kurz, W.; Fisher, D.J. Fundamentals of Solidification, 4th revised ed.; Trans Tech. Publications Ltd.: Uetikon-Zuerich, Switzerland, 1998. [Google Scholar]
- Paray, F.; Gruzleski, J.E. Microstructure-mechanical property relationship in A356 alloy. Part 1: Microstructure. Cast Met. 1994, 7, 29–40. [Google Scholar]
- Elsebaie, O. l’Effet de l’Addition du “Mischmetal”, du Taux de Refroidissement et du Traitement Thermique sur la Microstucture et la Dureté des Alliages Al-Si de Type 319, 356, et 413. Mater’s Thesis, Université du Québec à Chicoutimi (UQAC), Chicoutimi, QC, Canada, 2006. [Google Scholar]
- Hosseinifar, M.; Malakhov, D.V. The sequence of intermetallics formation during the solidification of an Al-Mg-Si alloy containing La. Metall. Mater. Trans. A 2011, 42, 825–833. [Google Scholar] [CrossRef]
- Yi, H.-K.; Zhang, D. Modification effect of pure rare earth metal La on the as cast Al-17%Si alloys. Trans. Nonferr. Met. Soc. China 2003, 13, 1–7. [Google Scholar]
- Hosseinifar, M. Aluminum Alloys Containing Cerium and Lanthanum. Ph.D. Thesis, McMaster University, Hamilton, ON, Canada, 2009. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkahtani, S.A.; Elgallad, E.M.; Tash, M.M.; Samuel, A.M.; Samuel, F.H. Effect of Rare Earth Metals on the Microstructure of Al-Si Based Alloys. Materials 2016, 9, 45. https://doi.org/10.3390/ma9010045
Alkahtani SA, Elgallad EM, Tash MM, Samuel AM, Samuel FH. Effect of Rare Earth Metals on the Microstructure of Al-Si Based Alloys. Materials. 2016; 9(1):45. https://doi.org/10.3390/ma9010045
Chicago/Turabian StyleAlkahtani, Saleh A., Emad M. Elgallad, Mahmoud M. Tash, Agnes M. Samuel, and Fawzy H. Samuel. 2016. "Effect of Rare Earth Metals on the Microstructure of Al-Si Based Alloys" Materials 9, no. 1: 45. https://doi.org/10.3390/ma9010045
APA StyleAlkahtani, S. A., Elgallad, E. M., Tash, M. M., Samuel, A. M., & Samuel, F. H. (2016). Effect of Rare Earth Metals on the Microstructure of Al-Si Based Alloys. Materials, 9(1), 45. https://doi.org/10.3390/ma9010045






