Improved Electrochemical Detection of Zinc Ions Using Electrode Modified with Electrochemically Reduced Graphene Oxide
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of GCE/ERGO
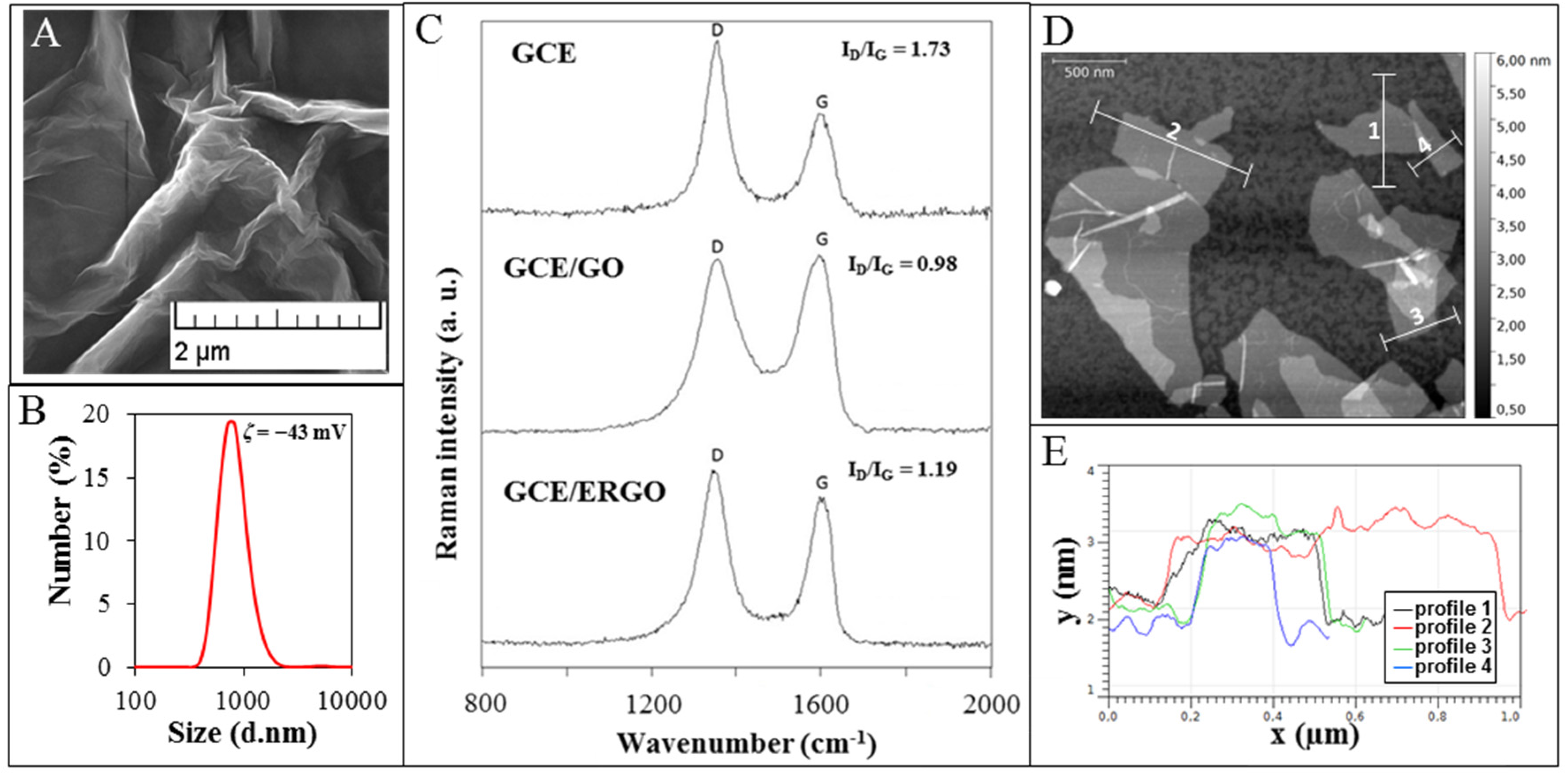

2.2. Characterization of GCE/ERGO
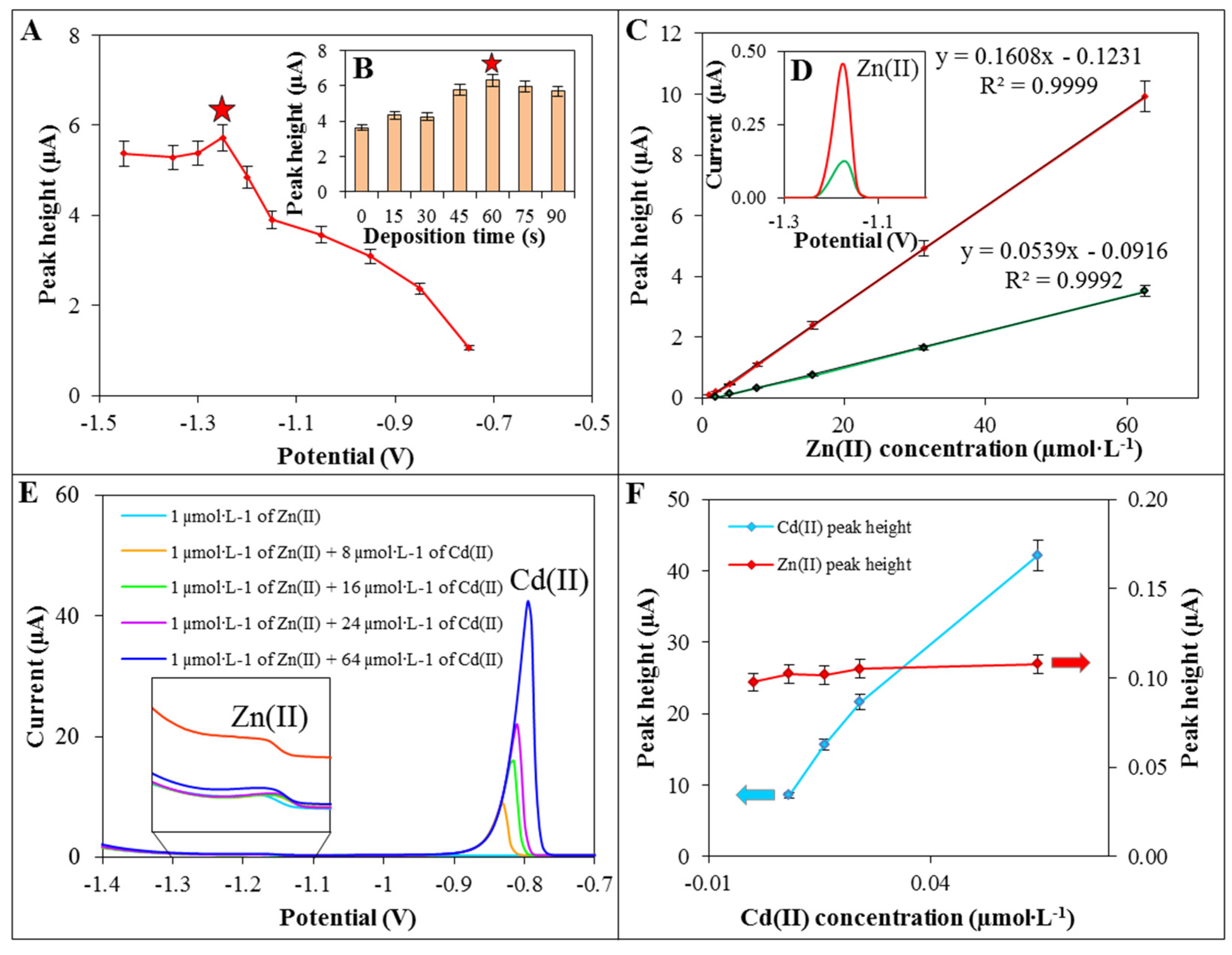
2.3. Detection of Zn(II)
| Substance | Working Electrode | Regression Equation | Linear Dynamic Range (µmol·L−1) | R2 a | LOD b (µmol·L−1) | LOQ c (µmol·L−1) | RSD (%) |
|---|---|---|---|---|---|---|---|
| Zn(II) | GCE/ERGO | y = 0.1608x − 0.1231 | 62.5 – 1.0 | 0.9999 | 0.1 | 0.4 | 4.8 |
| Zn(II) | GCE | y = 0.0539x − 0.0916 | 500.0 – 2.0 | 0.9992 | 0.5 | 2.0 | 5.2 |
3. Experimental Section
3.1. Chemicals and Material
3.2. Preparation of GO
3.3. Glassy Carbon Electrode Modification with Graphene
3.4. Instrumentation
3.5. The Electroactive Surface Determination
3.6. Scanning Electron Microscopy (SEM)
3.7. Dynamic Light Scattering (DLS)
3.8. Raman Spectroscopy
3.9. Interference Measurement
3.10. Atomic Force Microscopy Measurement
3.10.1. GO Immobilization
3.10.2. Visualization of GO
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haider, S.; Anis, L.; Batool, Z.; Sajid, I.; Naqvi, F.; Khaliq, S.; Ahmed, S. Short term cadmium administration dose dependently elicits immediate biochemical, neurochemical and neurobehavioral dysfunction in male rats. Metab. Brain Dis. 2015, 30, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, V.; Longo, G.; Brundo, M.V.; Sinatra, F.; Copat, C.; Conti, G.O.; Ferrante, M. Bioaccumulation of cadmium and lead and its effects on hepatopancreas morphology in three terrestrial isopod crustacean species. Ecotoxical. Environ. Saf. 2014, 110, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Blazovics, A.; Szentmihalyi, K.; Vinkler, P.; Kovacs, A. Zn overdose may cause disturbance in iron metabolism in inactive inflammatory bowel diseases. Trace Elem. Electrolytes 2004, 21, 240–247. [Google Scholar] [CrossRef]
- Murakami, M.; Hirano, T. Intracellular zinc homeostasis and zinc signaling. Cancer Sci. 2008, 99, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jing, X.P.; Zhang, S.P.; Gu, R.X.; Tang, F.X.; Wang, X.L.; Xiong, Y.; Qiu, M.; Sun, X.Y.; Ke, D.; et al. High dose zinc supplementation induces hippocampal zinc deficiency and memory impairment with inhibition of BDNF signaling. PLoS ONE 2013, 8, e55384. [Google Scholar] [CrossRef] [PubMed]
- Krizkova, S.; Ryvolova, M.; Hynek, D.; Eckschlager, T.; Hodek, P.; Masarik, M.; Adam, V.; Kizek, R. Immunoextraction of zinc proteins from human plasma using chicken yolk antibodies immobilized onto paramagnetic particles and their electrophoretic analysis. Electrophoresis 2012, 33, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Ryvolova, M.; Hynek, D.; Skutkova, H.; Adam, V.; Provaznik, I.; Kizek, R. Structural changes in metallothionein isoforms revealed by capillary electrophoresis and Brdicka reaction. Electrophoresis 2012, 33, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Frederickson, C.J.; Koh, J.Y.; Bush, A.I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005, 6, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Masarik, M.; Gumulec, J.; Sztalmachova, M.; Hlavna, M.; Babula, P.; Krizkova, S.; Ryvolova, M.; Jurajda, M.; Sochor, J.; Adam, V.; et al. Isolation of metallothionein from cells derived from aggressive form of high-grade prostate carcinoma using paramagnetic antibody-modified microbeads off-line coupled with electrochemical and electrophoretic analysis. Electrophoresis 2011, 32, 3576–3588. [Google Scholar] [CrossRef] [PubMed]
- Krizkova, S.; Ryvolova, M.; Gumulec, J.; Masarik, M.; Adam, V.; Majzlik, P.; Hubalek, J.; Provaznik, I.; Kizek, R. Electrophoretic fingerprint metallothionein analysis as a potential prostate cancer biomarker. Electrophoresis 2011, 32, 1952–1961. [Google Scholar] [CrossRef] [PubMed]
- Krizkova, S.; Ryvolova, M.; Hrabeta, J.; Adam, V.; Stiborova, M.; Eckschlager, T.; Kizek, R. Metallothioneins and zinc in cancer diagnosis and therapy. Drug Metab. Rev. 2012, 44, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Gumulec, J.; Masarik, M.; Krizkova, S.; Adam, V.; Hubalek, J.; Hrabeta, J.; Eckschlager, T.; Stiborova, M.; Kizek, R. Insight to physiology and pathology of zinc(II) ions and their actions in breast and prostate carcinoma. Curr. Med. Chem. 2011, 18, 5041–5051. [Google Scholar] [CrossRef] [PubMed]
- Adam, V.; Petrlova, J.; Wang, J.; Eckschlager, T.; Trnkova, L.; Kizek, R. Zeptomole electrochemical detection of metallothioneins. PLoS ONE 2010, 5, e11441. [Google Scholar] [CrossRef] [PubMed]
- Babula, P.; Masarik, M.; Adam, V.; Eckschlager, T.; Stiborova, M.; Trnkova, L.; Skutkova, H.; Provaznik, I.; Hubalek, J.; Kizek, R. Mammalians’ metallothioneins and their properties and functions. Metallomics 2012, 4, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Sobrova, P.; Vyslouzilova, L.; Stepankova, O.; Ryvolova, M.; Anyz, J.; Trnkova, L.; Adam, V.; Hubalek, J.; Kizek, R. Tissue specific electrochemical fingerprinting. PLoS ONE 2012, 7, e49654. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, P.; Benedetti, G.; Albarede, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.; Rink, L. Zinc signals and immune function. Biofactors 2014, 40, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, M.; Trefon, J.; Milnerowicz, H. The role of metallothionein interactions with other proteins. Proteomics 2014, 14, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Nejdl, L.; Kudr, J.; Cihalova, K.; Chudobova, D.; Zurek, M.; Zalud, L.; Kopecny, L.; Burian, F.; Ruttkay-Nedecky, B.; Krizkova, S.; et al. Remote-controlled robotic platform ORPHEUS as a new tool for detection of bacteria in the environment. Electrophoresis 2014, 35, 2333–2345. [Google Scholar] [CrossRef] [PubMed]
- Prasek, J.; Adamek, M.; Hubalek, J.; Adam, V.; Trnkova, L.; Kizek, R. New hydrodynamic electrochemical arrangement for cadmium ions detection using thick-film chemical sensor electrodes. Sensors 2006, 6, 1498–1512. [Google Scholar] [CrossRef]
- Krystofova, O.; Trnkova, L.; Adam, V.; Zehnalek, J.; Hubalek, J.; Babula, P.; Kizek, R. Electrochemical microsensors for the detection of cadmium(II) and lead(II) ions in plants. Sensors 2010, 10, 5308–5328. [Google Scholar] [CrossRef] [PubMed]
- Krizkova, S.; Krystofova, O.; Trnkova, L.; Hubalek, J.; Adam, V.; Beklova, M.; Horna, A.; Havel, L.; Kizek, R. Silver(I) ions ultrasensitive detection at carbon electrodes—Analysis of waters, tobacco cells and fish tissues. Sensors 2009, 9, 6934–6950. [Google Scholar] [CrossRef] [PubMed]
- Nejdl, L.; Ruttkay-Nedecky, B.; Kudr, J.; Kremplova, M.; Cernei, N.; Prasek, J.; Konecna, M.; Hubalek, J.; Zitka, O.; Kynicky, J.; et al. Behaviour of zinc complexes and zinc sulphide nanoparticles revealed by using screen printed electrodes and spectrometry. Sensors 2013, 13, 14417–14437. [Google Scholar] [CrossRef] [PubMed]
- Adam, V.; Baloun, J.; Fabrik, I.; Trnkova, L.; Kizek, R. An electrochemical detection of metallothioneins at the zeptomole level in nanolitre volumes. Sensors 2008, 8, 2293–2305. [Google Scholar] [CrossRef]
- Zhang, W.; Ou, J.Z.; Tang, S.Y.; Sivan, V.; Yao, D.D.; Latham, K.; Khoshmanesh, K.; Mitchell, A.; O’Mullane, A.P.; Kalantar-zadeh, K. Liquid metal/metal oxide frameworks. Adv. Funct. Mater. 2014, 24, 3799–3807. [Google Scholar] [CrossRef]
- Cincotto, F.H.; Martinez-Garcia, G.; Yanez-Sedeno, P.; Canevari, T.C.; Machado, S.A.S.; Pingarron, J.M. Electrochemical immunosensor for ethinylestradiol using diazonium salt grafting onto silver nanoparticles-silica-graphene oxide hybrids. Talanta 2016, 147, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Hui, N.; Wang, S.Y.; Xie, H.B.; Xu, S.H.; Niu, S.Y.; Luo, X.L. Nickel nanoparticles modified conducting polymer composite of reduced graphene oxide doped poly(3,4-ethylenedioxythiophene) for enhanced nonenzymatic glucose sensing. Sens. Actuators B Chem. 2015, 221, 606–613. [Google Scholar] [CrossRef]
- Campbell, J.L.; Breedon, M.; Latham, K.; Kalantar-Zadeh, K. Electrowetting of superhydrophobic ZnO nanorods. Langmuir 2008, 24, 5091–5098. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Kaner, R.B. Materials science—Graphene-based materials. Science 2008, 320, 1170–1171. [Google Scholar] [CrossRef] [PubMed]
- Le, T.X.H.; Bechelany, M.; Lacour, S.; Oturan, N.; Oturan, M.A.; Cretin, M. High removal efficiency of dye pollutants by electron-Fenton process using a graphene based cathode. Carbon 2015, 94, 1003–1011. [Google Scholar] [CrossRef]
- Li, B.; Pan, G.H.; Avent, N.D.; Lowry, R.B.; Madgett, T.E.; Waines, P.L. Graphene electrode modified with electrochemically reduced graphene oxide for label-free DNA detection. Biosens. Bioelectron. 2015, 72, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Le, T.X.H.; Bechelany, M.; Champavert, J.; Cretin, M. A highly active based graphene cathode for the electro-Fenton reaction. RSC Adv. 2015, 5, 42536–42539. [Google Scholar] [CrossRef]
- Kim, Y.R.; Bong, S.; Kang, Y.J.; Yang, Y.; Mahajan, R.K.; Kim, J.S.; Kim, H. Electrochemical detection of dopamine in the presence of ascorbic acid using graphene modified electrodes. Biosens. Bioelectron. 2010, 25, 2366–2369. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Tang, Y.H.; Wang, K.; Liu, C.B.; Luo, S.L. Direct electrodeposition of reduced graphene oxide on glassy carbon electrode and its electrochemical application. Electrochem. Commun. 2011, 13, 133–137. [Google Scholar] [CrossRef]
- Cheemalapati, S.; Palanisamy, S.; Chen, S.M. Electrochemical determination of isoniazid at electrochemically reduced graphene oxide modified electrode. Int. J. Electrochem. Sci. 2013, 8, 3953–3962. [Google Scholar]
- Sehat, A.A.; Khodadadi, A.A.; Shemirani, F.; Mortazavi, Y. Fast immobilization of glucose oxidase on graphene oxide for highly sensitive glucose biosensor fabrication. Int. J. Electrochem. Sci. 2015, 10, 272–286. [Google Scholar]
- Ping, J.F.; Wang, Y.X.; Fan, K.; Wu, J.; Ying, Y.B. Direct electrochemical reduction of graphene oxide on ionic liquid doped screen-printed electrode and its electrochemical biosensing application. Biosens. Bioelectron. 2011, 28, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Zhang, X.L. A method based on electrodeposition of reduced graphene oxide on glassy carbon electrode for sensitive detection of theophylline. J. Solid State Electrochem. 2013, 17, 167–173. [Google Scholar] [CrossRef]
- Li, G.N.; Li, T.T.; Deng, Y.; Cheng, Y.; Shi, F.; Sun, W.; Sun, Z.F. Electrodeposited nanogold decorated graphene modified carbon ionic liquid electrode for the electrochemical myoglobin biosensor. J. Solid State Electrochem. 2013, 17, 2333–2340. [Google Scholar] [CrossRef]
- Wang, F.; Wu, Y.J.; Lu, K.; Ye, B.X. A sensitive voltammetric sensor for taxifolin based on graphene nanosheets with certain orientation modified glassy carbon electrode. Sens. Actuator B Chem. 2015, 208, 188–194. [Google Scholar] [CrossRef]
- Adam, V.; Petrlova, J.; Potesil, D.; Zehnalek, J.; Sures, B.; Trnkova, L.; Jelen, F.; Kizek, R. Study of metallothionein modified electrode surface behavior in the presence of heavy metal ions-biosensor. Electroanalysis 2005, 17, 1649–1657. [Google Scholar] [CrossRef]
- Kudr, J.; Nguyen, V.H.; Gumulec, J.; Nejdl, L.; Blazkova, I.; Ruttkay-Nedecky, B.; Hynek, D.; Kynicky, J.; Adam, V.; Kizek, R. Simultaneous automatic electrochemical detection of zinc, cadmium, copper and lead ions in environmental samples using a thin-film mercury electrode and an artificial neural network. Sensors 2015, 15, 592–610. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.Y.; Ma, X.Y.; Li, X. Graphene-modified electrode for the selective determination of uric acid under coexistence of dopamine and ascorbic acid. Int. J. Electrochem. Sci. 2012, 7, 2201–2213. [Google Scholar]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.J.; Hyde, M.E.; Compton, R.G. Nanotrench arrays reveal insight into graphite electrochemistry. Angew. Chem. Int. Ed. 2005, 44, 5121–5126. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.P.; Pei, S.F.; Ren, W.C.; Gao, L.B.; Cheng, H.M. Efficient preparation of large-area graphene oxide sheets for transparent conductive films. ACS Nano 2010, 4, 5245–5252. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Feng, H.B.; Li, J.H. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Castro, S.S.L.; de Oliveira, M.F.; Stradiotto, N.R. Study of the electrochemical behavior of histamine using a Nafion (R)-Copper(II) hexacyanoferrate film-modified electrode. Int. J. Electrochem. Sci. 2010, 5, 1447–1456. [Google Scholar]
- Gilje, S.; Han, S.; Wang, M.; Wang, K.L.; Kaner, R.B. A chemical route to graphene for device applications. Nano Lett. 2007, 7, 3394–3398. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.C.; Zhang, X.J.; Chen, Y.; Du, Y.L.; Zhou, F.; Wang, C.M. Pulsed electrodeposition of reduced graphene oxide on glass carbon electrode as an effective support of electrodeposited Pt microspherical particles: Nucleation studies and the application for methanol electro-oxidation. Int. J. Electrochem. Sci. 2013, 8, 2122–2139. [Google Scholar]
- Zhang, Z.P.; Yan, J.; Jin, H.Z.; Yin, J.G. Tuning the reduction extent of electrochemically reduced graphene oxide electrode film to enhance its detection limit for voltammetric analysis. Electrochim. Acta 2014, 139, 232–237. [Google Scholar] [CrossRef]
- Guo, H.L.; Wang, X.F.; Qian, Q.Y.; Wang, F.B.; Xia, X.H. A green approach to the synthesis of graphene nanosheets. ACS Nano 2009, 3, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Mohan, R.; Kim, S.J. Investigation of Raman and photoluminescence studies of reduced graphene oxide sheets. Appl. Phys. A Mater. Sci. Process. 2012, 106, 501–506. [Google Scholar] [CrossRef]
- Eigler, S.; Dotzer, C.; Hirsch, A. Visualization of defect densities in reduced graphene oxide. Carbon 2012, 50, 3666–3673. [Google Scholar] [CrossRef]
- Bi, S.; Zhao, T.T.; Jia, X.Q.; He, P. Magnetic graphene oxide-supported hemin as peroxidase probe for sensitive detection of thiols in extracts of cancer cells. Biosens. Bioelectron. 2014, 57, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Xu, C.; Lin, J.; Wang, C.; Wang, X.S.; Zhang, C.L.; Zhou, X.J.; Guo, S.W.; Cui, D.X. Folic acid-conjugated graphene oxide loaded with photosensitizers for targeting photodynamic therapy. Theranostics 2011, 1, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Casero, E.; Parra-Alfambra, A.M.; Petit-Dominguez, M.D.; Pariente, F.; Lorenzo, E.; Alonso, C. Differentiation between graphene oxide and reduced graphene by electrochemical impedance spectroscopy (EIS). Electrochem. Commun. 2012, 20, 63–66. [Google Scholar] [CrossRef]
- Nejdl, L.; Kudr, J.; Ruttkay-Nedecky, B.; Heger, Z.; Zima, L.; Zalud, L.; Krizkova, S.; Adam, V.; Vaculovicova, M.; Kizek, R. Remote-controlled robotic platform for electrochemical determination of water contaminated by heavy metal ions. Int. J. Electrochem. Sci. 2015, 10, 3635–3643. [Google Scholar]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339–1339. [Google Scholar] [CrossRef]
- Prathish, K.P.; Barsan, M.M.; Geng, D.S.; Sun, X.L.; Brett, C.M.A. Chemically modified graphene and nitrogen-doped graphene: Electrochemical characterisation and sensing applications. Electrochim. Acta 2013, 114, 533–542. [Google Scholar] [CrossRef]
- Necas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudr, J.; Richtera, L.; Nejdl, L.; Xhaxhiu, K.; Vitek, P.; Rutkay-Nedecky, B.; Hynek, D.; Kopel, P.; Adam, V.; Kizek, R. Improved Electrochemical Detection of Zinc Ions Using Electrode Modified with Electrochemically Reduced Graphene Oxide. Materials 2016, 9, 31. https://doi.org/10.3390/ma9010031
Kudr J, Richtera L, Nejdl L, Xhaxhiu K, Vitek P, Rutkay-Nedecky B, Hynek D, Kopel P, Adam V, Kizek R. Improved Electrochemical Detection of Zinc Ions Using Electrode Modified with Electrochemically Reduced Graphene Oxide. Materials. 2016; 9(1):31. https://doi.org/10.3390/ma9010031
Chicago/Turabian StyleKudr, Jiri, Lukas Richtera, Lukas Nejdl, Kledi Xhaxhiu, Petr Vitek, Branislav Rutkay-Nedecky, David Hynek, Pavel Kopel, Vojtech Adam, and Rene Kizek. 2016. "Improved Electrochemical Detection of Zinc Ions Using Electrode Modified with Electrochemically Reduced Graphene Oxide" Materials 9, no. 1: 31. https://doi.org/10.3390/ma9010031
APA StyleKudr, J., Richtera, L., Nejdl, L., Xhaxhiu, K., Vitek, P., Rutkay-Nedecky, B., Hynek, D., Kopel, P., Adam, V., & Kizek, R. (2016). Improved Electrochemical Detection of Zinc Ions Using Electrode Modified with Electrochemically Reduced Graphene Oxide. Materials, 9(1), 31. https://doi.org/10.3390/ma9010031










