Energetic Study of Helium Cluster Nucleation and Growth in 14YWT through First Principles
Abstract
:1. Introduction
2. Methods
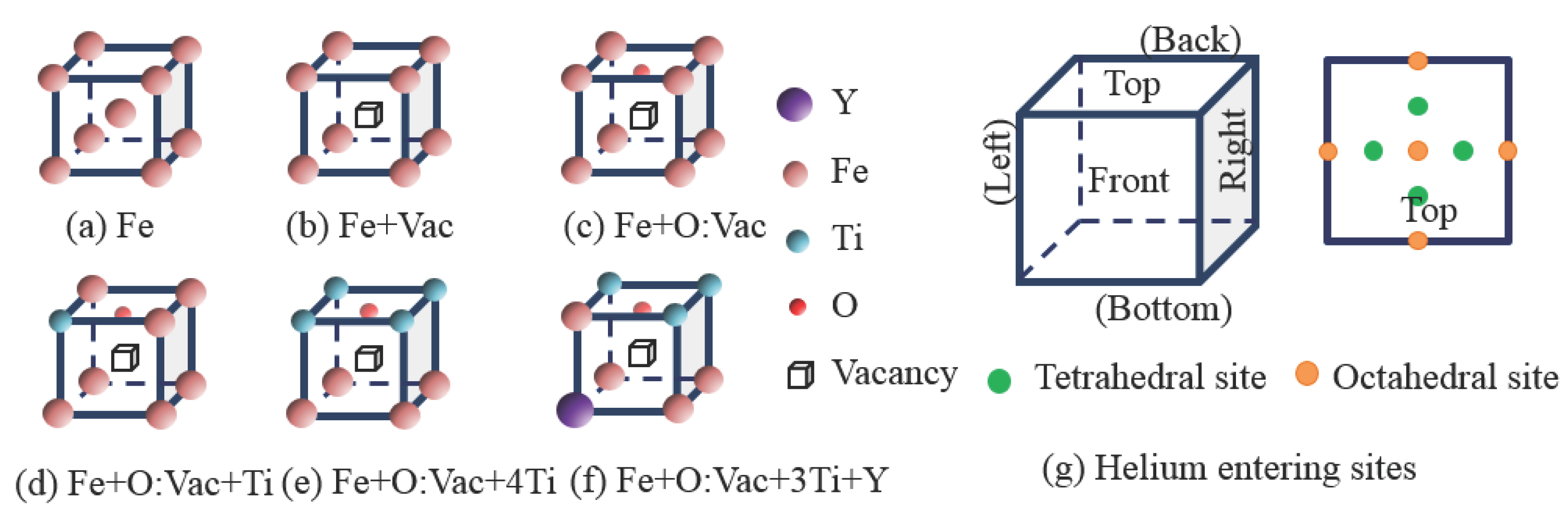
3. Results and Discussion
3.1. He Interaction with a Vacancy and the O:Vac Pair
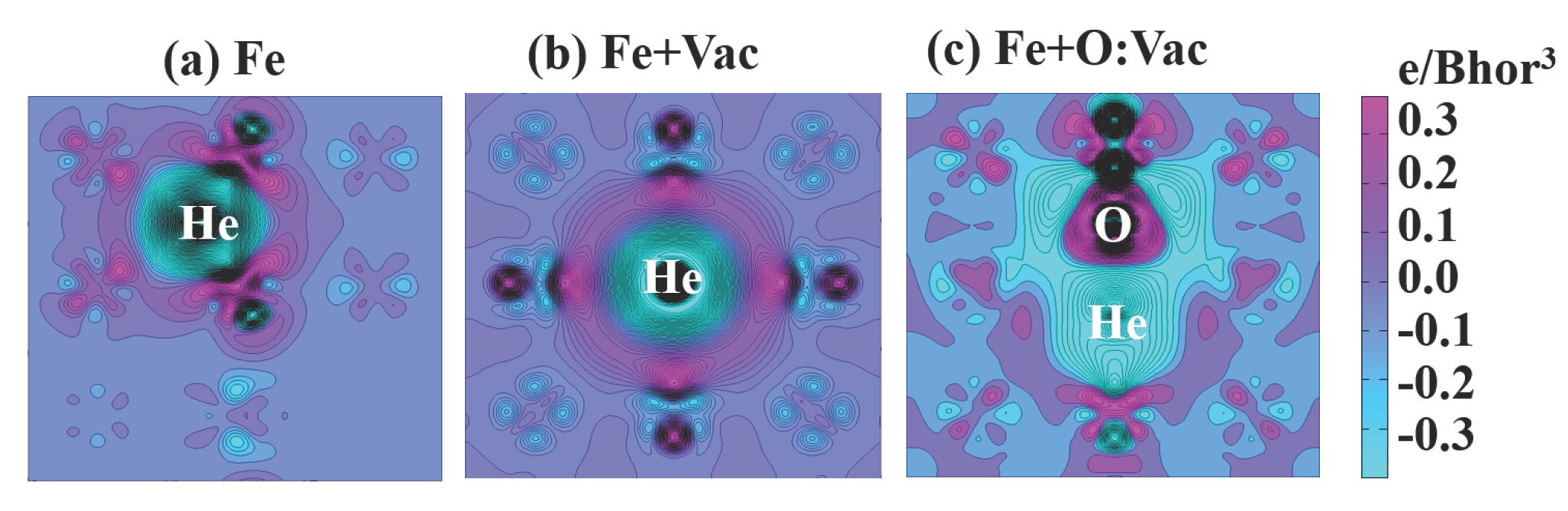
3.2. Energetics of He Clusters with a Vacancy and the O:Vac Pair
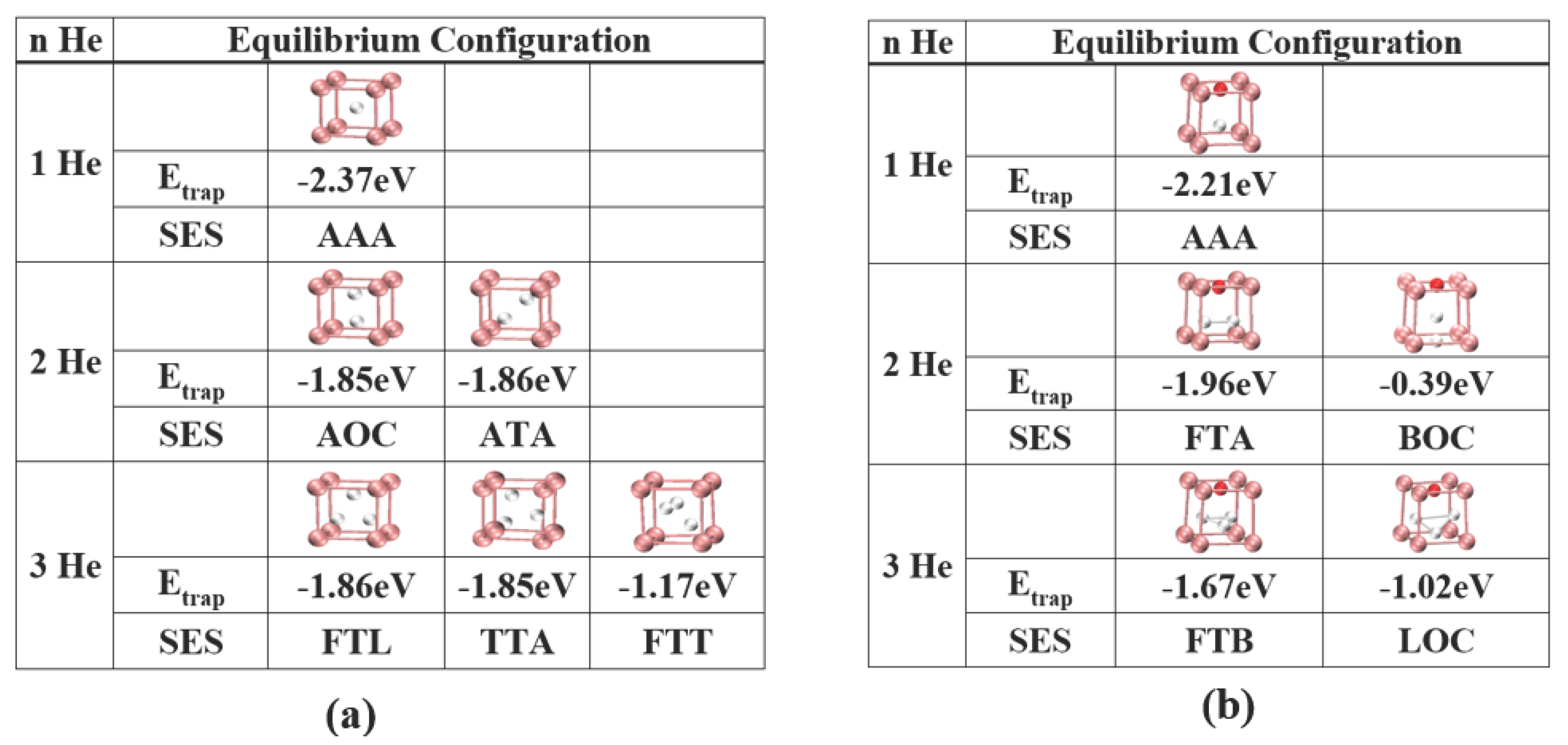

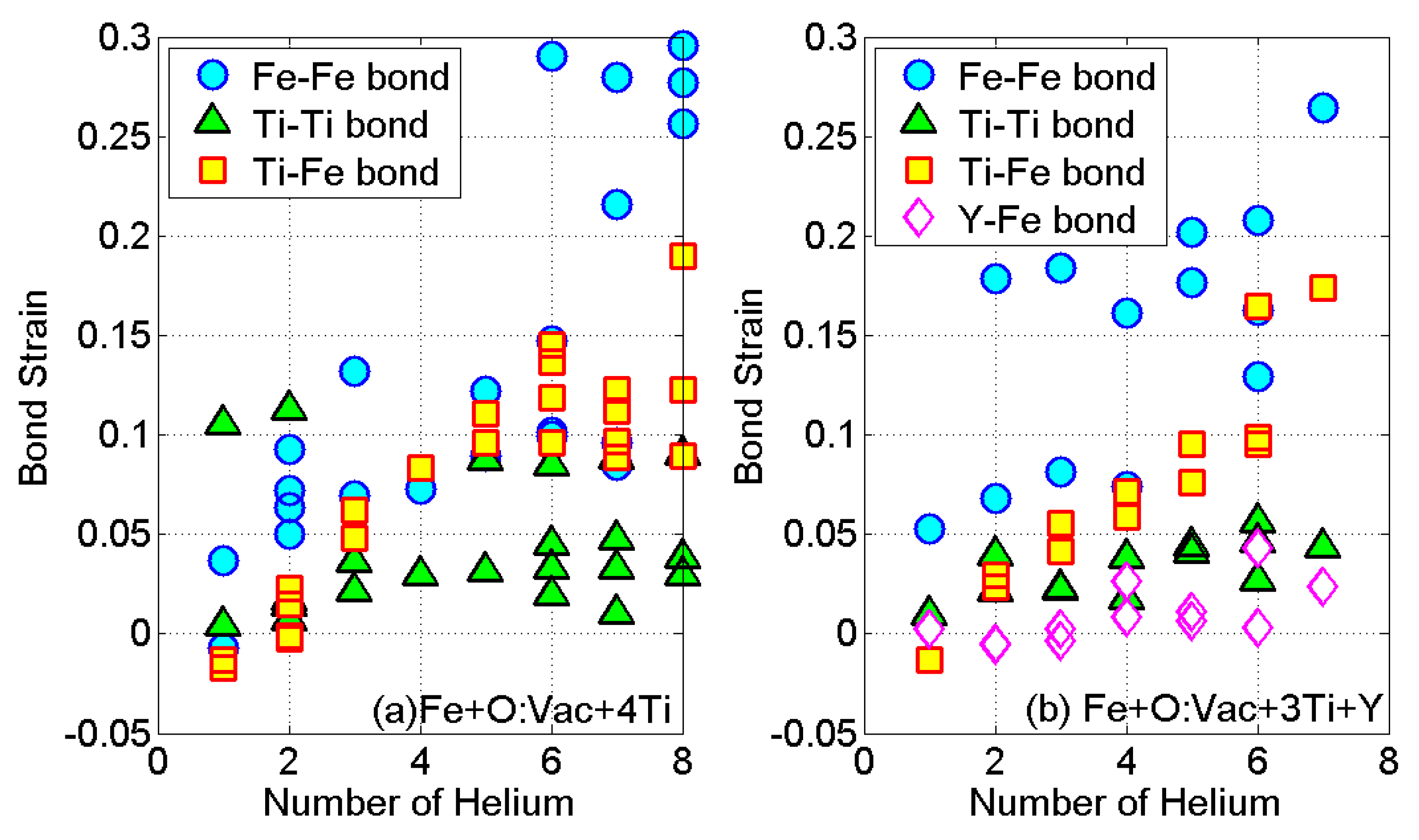
3.3. Energetics of He Clusters in 14YWT


3.4. Formation and Growth Criteria of the He Cluster in 14YWT
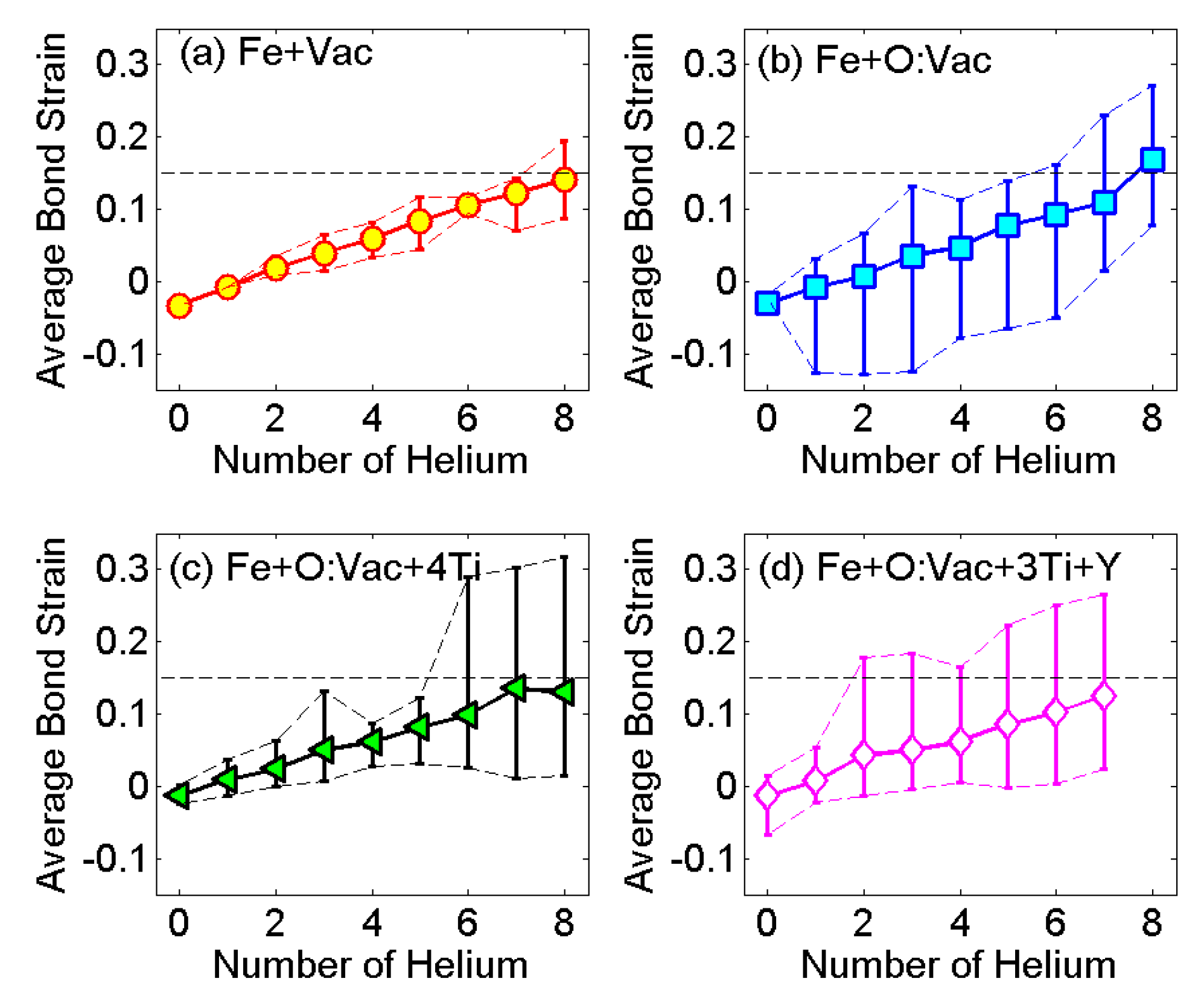
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bloom, E.E. The challenge of developing structural materials for fusion power systems. J. Nucl. Mater. 1998, 258–263, 7–17. [Google Scholar] [CrossRef]
- Schroeder, H.; Kesternich, W.; Ullmaier, H. He effects on the creep and fatigue resistance of austenitic stainless steels at high temperatures. Nucl. Eng. Des. Fusion 1985, 2, 65–95. [Google Scholar] [CrossRef]
- Braski, D.N.; Schroeder, H.; Ullmaier, H. The effect of tensile stress on the growth of He bubbles in an austenitic stainless steel. J. Nucl. Mater. 1979, 83, 265–277. [Google Scholar] [CrossRef]
- Fu, C.C.; Willaime, F. ab initio study of He in Fe: Dissolution, migration, and clustering with vacancies. Phys. Rev. B 2005, 72. [Google Scholar] [CrossRef]
- Ullmaier, H. The influence of He on the bulk properties of fusion reactor structural materials. Nucl. Fusion 1984, 24, 1039–1083. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Ghoniem, N.M. Operating temperature windows for fusion reactor structural materials. Fusion Eng. Des. 2000, 51, 55–71. [Google Scholar] [CrossRef]
- Farrell, K. Experimental effects of He on cavity formation during irradiation—A review. Radiator Eff. 1980, 53, 175–194. [Google Scholar] [CrossRef]
- Mansur, L.K.; Coghlan, W.A. Mechanisms of He interaction with radiation effects in metals and alloys: A review. J. Nucl. Mater. 1983, 119, 1–25. [Google Scholar] [CrossRef]
- Hetherly, J.; Martinez, E.; Di, Z.F.; Nastasi, M.; Caro, A. He bubble precipitation at dislocation networks. Scr. Mater. 2012, 66, 17–20. [Google Scholar] [CrossRef]
- Odette, G.R. Modeling microstructural evolution in fusion reactor environments. J. Nucl. Mater. 1985, 133, 127–133. [Google Scholar] [CrossRef]
- Odette, G.R.; Hoelzer, D.T. Irradiation-tolerant nanostructured ferritic alloys: Transforming He from a liability to an asset. J. Mater. 2010, 62, 84–92. [Google Scholar] [CrossRef]
- Demkowicz, M.J.; Misra, A.; Caro, A. The role of interface structure in controlling high He concentrations. Curr. Opin. Solid State Mater. Sci. 2012, 16, 101–108. [Google Scholar] [CrossRef]
- Kim, I.S.; Hunn, J.D.; Hashimoto, N.; Larson, D.L.; Maziasz, P.J.; Miyahara, K. Defect and void evolution in oxide dispersion strengthened ferritic steels under 3.2 MeV Fe ion irradiation with simultaneous He injection. J. Nucl. Mater. 2000, 280, 264–274. [Google Scholar] [CrossRef]
- Odette, G.R.; Alinger, M.J.; Wirth, B.D. Recent developments in irradiation-resistant steels. Annu. Rev. Mater. Res. 2008, 38, 471–503. [Google Scholar] [CrossRef]
- Miller, M.K.; Hoelzer, D.T.; Kenik, E.A.; Russell, K.F. Stability of Ferritic MA/ODS alloys at high temperatures. Intermetalics 2015, 13, 387–392. [Google Scholar] [CrossRef]
- Bentley, J.; Hoelzer, D.T. TEM characterization of tensile-tested 14YWT nanostructured ferritic alloys. Microsc. Microanal. 2008, 14, 1416–1417. [Google Scholar] [CrossRef]
- McClintock, D.A.; Hoelzer, D.T.; Sokolov, M.A.; Nanstad, M.A. Mechanical properties of neutron irradiated nanostructured ferritic alloy 14YWT. J. Nucl. Mater. 2009, 386–388, 307–311. [Google Scholar] [CrossRef]
- Kim, J.H.; Byun, T.S.; Hoelzer, D.T.; Kim, S.W.; Lee, B.H. Temperature dependence of strengthening mechanisms in the nanostructured ferritic alloy 14YWT: Part I—Mechanical and microstructural observations. Mater. Sci. Eng. A 2013, 559, 101–110. [Google Scholar] [CrossRef]
- Klueh, R.L.; Maziasz, P.J.; Kim, I.S.; Heatherly, L.; Hoelzer, D.T.; Hashimoto, N.; Kenik, E.A.; Miyahara, K. Tensile and creep properties of an oxide dispersion-strengthened ferritic steel. J. Nucl. Mater. 2002, 307–311, 773–777. [Google Scholar] [CrossRef]
- Hayashi, T.; Sarosi, P.M.; Schneibel, J.H.; Mills, M.J. Creep response and deformation processes in nanocluster-strengthened ferritic steels. Acta Mater. 2008, 56, 1407–1416. [Google Scholar] [CrossRef]
- Miller, M.K.; Kenik, E.A.; Russell, K.F.; Heatherly, L.; Hoelzer, D.T.; Maziasz, P.J. Atom probe tomography of nanoscale particles in ODS ferritic alloys. Mater. Sci. Eng. A 2003, 353, 140–145. [Google Scholar] [CrossRef]
- Jitsukawa, S.; Kimura, A.; Kohyama, A.; Klueh, R.L.; Tavassoli, A.A.; van der Schaaf, B.; Odette, G.R.; Rensman, J.W.; Victoria, M.; Petersen, C. Recent results of the reduced activation ferritic/martensitic steel development. J. Nucl. Mater. 2004, 329–333, 39–46. [Google Scholar] [CrossRef]
- El-Genk, M.S.; Tournier, J.-M. A review of refractory metal alloys and mechanically alloyed-oxide dispersion strengthened steels for space nuclear power systems. J. Nucl. Mater. 2005, 340, 93–112. [Google Scholar] [CrossRef]
- Pareige, P.; Miller, M.K.; Stoller, R.E.; Hoelzer, D.T.; Cadel, E.; Radiguet, B. Stability of nanometer-sized oxide clusters in mechanically-alloyed steel under ion-induced displacement cascade damage conditions. J. Nucl. Mater. 2007, 360, 136–142. [Google Scholar] [CrossRef]
- Miller, M.K.; Fu, C.L.; Krcmar, M.; Hoelzer, D.T.; Liu, C.T. Vacancies as a constitutive element for the design of nanocluster-strengthened ferritic steels. Front. Mater. Sci. China 2009, 3, 9–14. [Google Scholar] [CrossRef]
- Ukai, S.; Nishida, T.; Okada, H.; Okuda, T.; Fujiwara, M.; Asabe, K. Development of oxide dispersion strengthened ferritic steels for FBR core application, (I). J. Nucl. Sci. Technol. 1997, 34, 256–263. [Google Scholar] [CrossRef]
- He, J.; Wan, F.; Sridharan, K.; Allen, T.R.; Certain, A.; Shutthanandan, V.; Wu, Y.Q. Stability of nanoclusters in 14YWT oxide dispersion strengthened steel under heavy ion-irradiation by atom probe tomography. J. Nucl. Mater. 2014, 455, 41–45. [Google Scholar] [CrossRef]
- Miller, M.K.; Russell, K.F.; Hoelzer, D.T. Characterization of precipitates in MA/ODS ferritic alloys. J. Nucl. Mater. 2006, 351, 261–268. [Google Scholar] [CrossRef]
- Miller, M.K.; Parish, C.M. Role of alloying elements in nanostructured ferritic steels. Mater. Sci. Technol. 2011, 27, 729–734. [Google Scholar] [CrossRef]
- Larson, D.J.; Maziasz, P.J.; Kim, I.S.; Miyahara, K. Three-dimensional atom probe observation of nanoscale titanium-oxygen clustering in an oxide-dispersion-strengthened Fe-12Cr-3W-0.4 Ti + Y2O3 ferritic alloy. Scr. Mater. 2001, 44, 359–364. [Google Scholar] [CrossRef]
- Hsiung, L.L.; Fluss, M.J.; Tumey, S.J.; Choi, B.W.; Serruys, Y.; Willaime, F.; Kimura, A. Formation mechanism and the role of nanoparticles in Fe-Cr ODS steels developed for radiation tolerance. Phys. Rev. B 2010, 82. [Google Scholar] [CrossRef]
- Edmondson, P.D.; Parish, C.M.; Zhang, Y.; Hallen, A.; Miller, M.K. He bubble distributions in a nanostructured ferritic alloy. J. Nucl. Mater. 2013, 434, 210–216. [Google Scholar] [CrossRef]
- Xing, W.; Chen, X.; Liu, P.; Wang, X.; Zhang, P.; Li, D.; Li, Y. First principles studies of hydrogen behavior interacting with oxygen-enriched nanostructured particles in the ODS steels. Int. J. Hydrog. Energy 2014, 39, 18506–18519. [Google Scholar] [CrossRef]
- Lewis, M.B.; Farrell, K. Migration behavior of He under displacive irradiation in stainless steel, nickel, iron and zirconium. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 1986, 16, 163–170. [Google Scholar] [CrossRef]
- Vassen, R.; Trinkaus, H.; Jung, P. He desorption from Fe and V by atomic diffusion and bubble migration. Phys. Rev. B 1991, 44, 4206–4213. [Google Scholar] [CrossRef]
- Zu, X.T.; Yang, L.; Gao, F.; Peng, S.M.; Heinisch, H.L.; Long, X.G.; Kurtz, R.J. Properties of helium defects in bcc and fcc metals investigated with density functional theory. Phys. Rev. B 2009, 80. [Google Scholar] [CrossRef]
- Fu, C.L.; Krcmar, M.; Painter, G.S.; Chen, X.-Q. Vacancy mechanism of high oxygen solubility and nucleation of stable oxygen-enriched clusters in Fe. Phys. Rev. Lett. 2007, 99. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Victoria, M.; Chen, J.; van Swygenhoven, H. He-vacancy cluster in a single bcc iron crystal lattice. J. Phys. Conden. Matter 2011, 23. [Google Scholar] [CrossRef] [PubMed]
- Seletskaia, T.; Osetsky, Y.N.; Stoller, R.S.; Stocks, G.M. Calculation of He defect clustering properties in iron using a multi-scale approach. J. Nucl. Mater. 2006, 351, 109–118. [Google Scholar] [CrossRef]
- Naveen Kumar, N.; Martinez, E.; Dutta, B.K.; Dey, G.K.; Caro, A. Nodal effects in α-iron dislocation mobility in the presence of He bubbles. Phys. Rev. B 2013, 87. [Google Scholar] [CrossRef]
- Zhao, H.; Fu, C.L.; Krcmar, M.; Miller, M.K. Effect of strain on the stabilization of oxygen-enriched nanoclusters in Fe-based alloys. Phys. Rev. B 2011, 84. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Zhang, X.; Geng, W.T.; Lu, G. Helium bubble nucleation and growth in α-Fe: Insights from first-principles simulations. J. Phys. Conden. Matter 2014, 26. [Google Scholar] [CrossRef] [PubMed]
- Terentyev, D.; Juslin, N.; Nordlund, K.; Sandberg, N. Fast three dimensional migration of He clusters in bcc Fe and Fe-Cr alloys. J. Appl. Phys. 2009, 105. [Google Scholar] [CrossRef]
- Seletskaia, T.; Osetsky, Y.; Stoller, R.E.; Stocks, G.M. Magnetic Interactions Influence the Properties of He Defects in Iron. Phys. Rev. Lett. 2005, 94. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.C.; Willaime, F. Interaction between He and self-defects in α-iron from first principles. J. Nucl. Mater. 2007, 367–370, 244–250. [Google Scholar] [CrossRef]
- Wilson, W.D.; Bisson, C.L.; Baskes, M.I. Self-trapping of He in metals. Phys. Rev. B 1981, 24, 5616–5624. [Google Scholar] [CrossRef]
- Clatterbuck, D.M.; Chrzan, D.C.; Morris, J.W., Jr. The ideal strength of iron in tension and shear. Acta Mater. 2003, 51, 2271–2283. [Google Scholar] [CrossRef]
- Mills, G.; Jonsson, H.; Schenter, G.K. Reversible work transition state theory: Application to dissociative adsorption of hydrogen. Surf. Sci. 1995, 324, 305–337. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, Y.; Zhao, H.; Hoelzer, D.T.; Yun, D. Energetic Study of Helium Cluster Nucleation and Growth in 14YWT through First Principles. Materials 2016, 9, 17. https://doi.org/10.3390/ma9010017
Gan Y, Zhao H, Hoelzer DT, Yun D. Energetic Study of Helium Cluster Nucleation and Growth in 14YWT through First Principles. Materials. 2016; 9(1):17. https://doi.org/10.3390/ma9010017
Chicago/Turabian StyleGan, Yingye, Huijuan Zhao, David T. Hoelzer, and Di Yun. 2016. "Energetic Study of Helium Cluster Nucleation and Growth in 14YWT through First Principles" Materials 9, no. 1: 17. https://doi.org/10.3390/ma9010017





