Effects of Different pH-Values on the Nanomechanical Surface Properties of PEEK and CFR-PEEK Compared to Dental Resin-Based Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
| Materials | Organic Matrix | Filler | Content of Filler |
|---|---|---|---|
| PEEK 450G | Polyetheretherketone | None | None |
| PEEK 450CA30 | Polyetheretherketone | Short carbon fiber: 6–9 µm (diameter) | 30 wt % |
| CHARISMA | Bis-GMA(2,2-Bis[4-(2hydroxy-3-methacryloxypropyl-1-oxy) phenyl]pro-pane) | Barium-aluminaborosilicate, Silica: 0.02–0.07 µm | 64 wt % |
| PMMA | Poly(methyl methacrylate) | None | None |
| Components | Concentration (mg L−1) |
|---|---|
| NaCl | 125.6 |
| KCl | 963.9 |
| KSCN | 189.2 |
| KH2PO4 | 654.5 |
| Urea | 200.0 |
| NaSO4·10H2O | 763.2 |
| NH4Cl | 178.0 |
| CaCl2·2H2O | 227.8 |
| NaHCO3 | 630.8 |
2.2. Nanoindentation and Nanoscratching
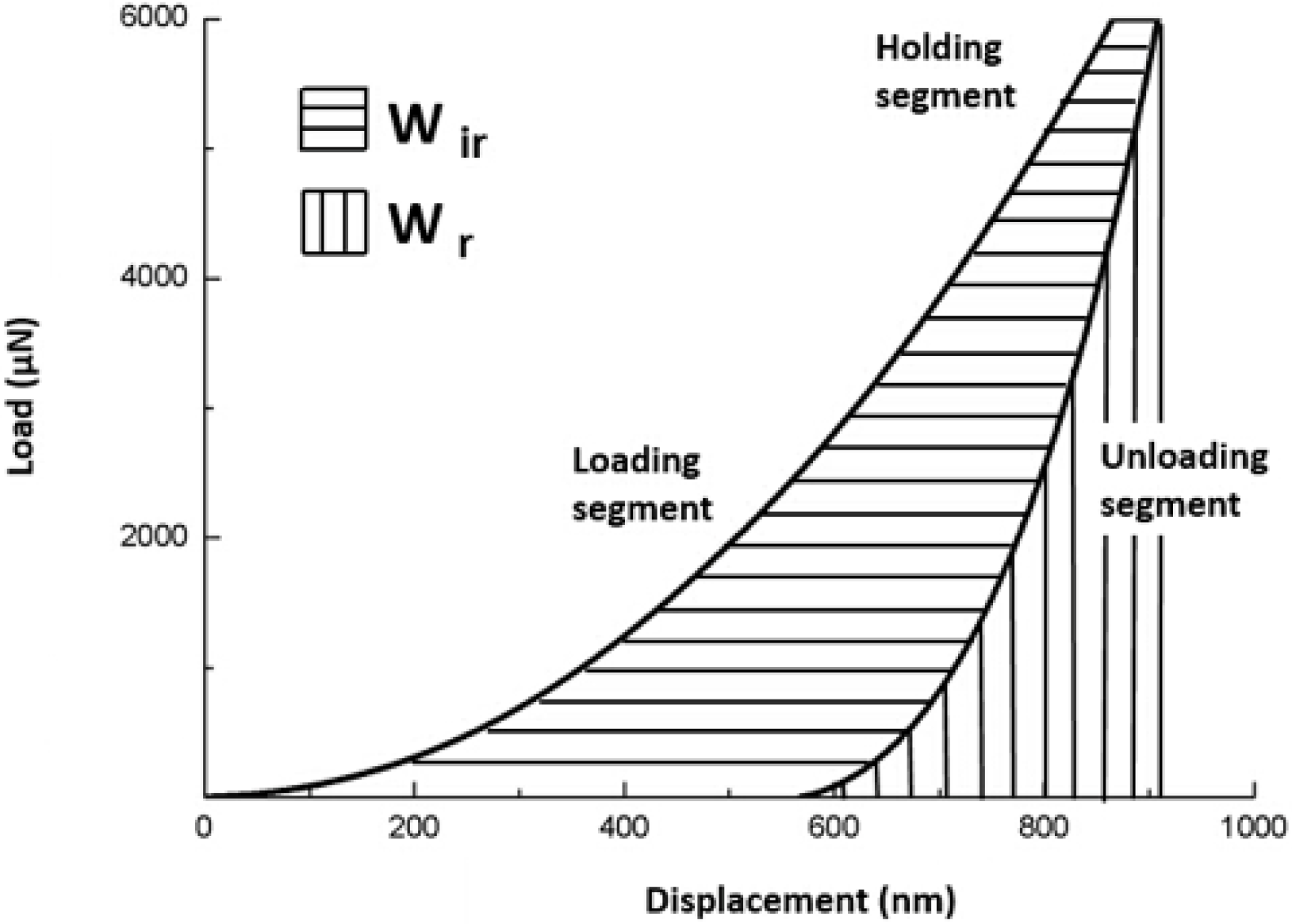
2.2.1. Nanoindentation Test Protocol
2.2.2. Nanoindentation Creep and Recovery Test Protocol

2.2.3. Nanoscratching Test Protocol
3. Statistical Analysis
4. Results
4.1. Nanoindentation Results
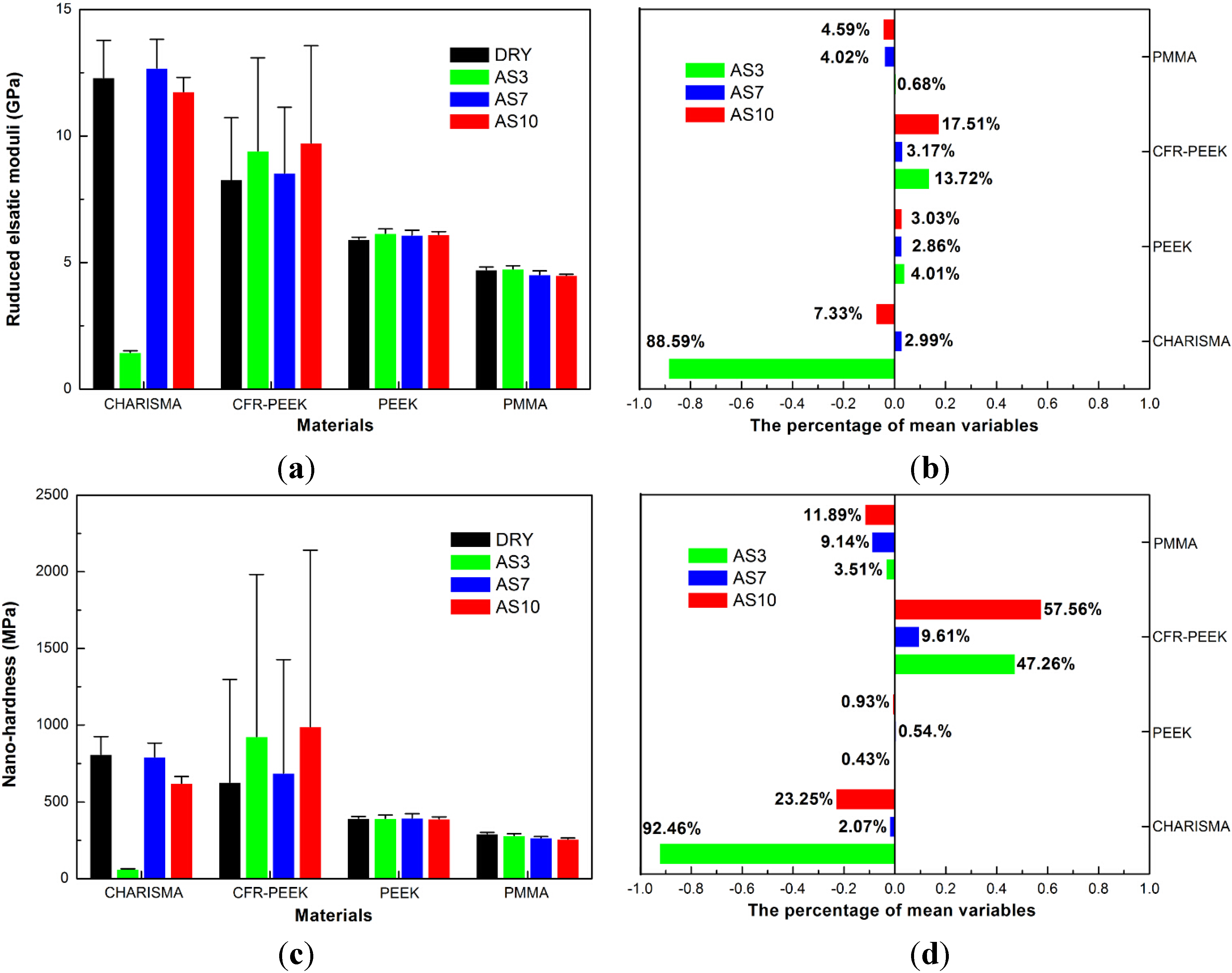
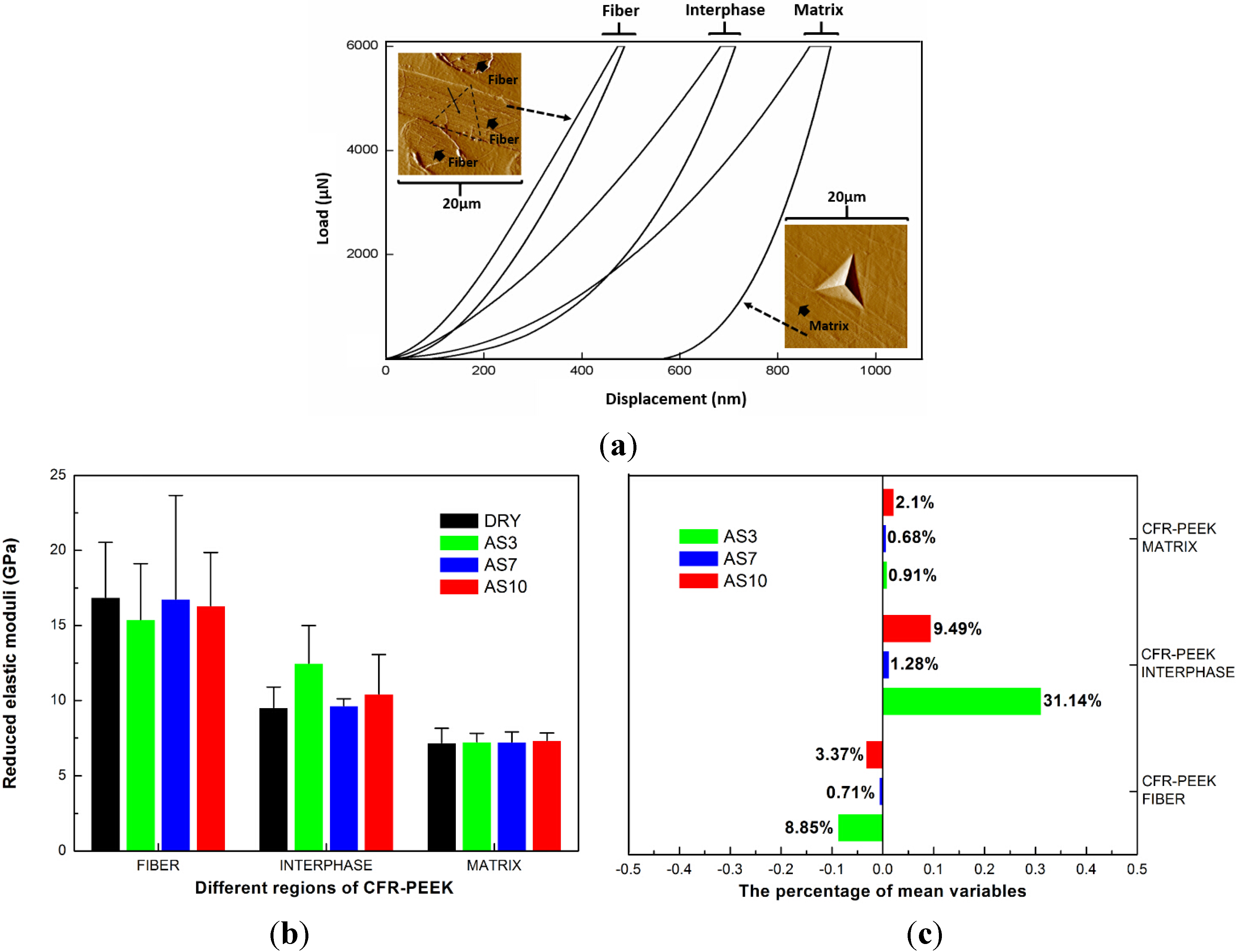
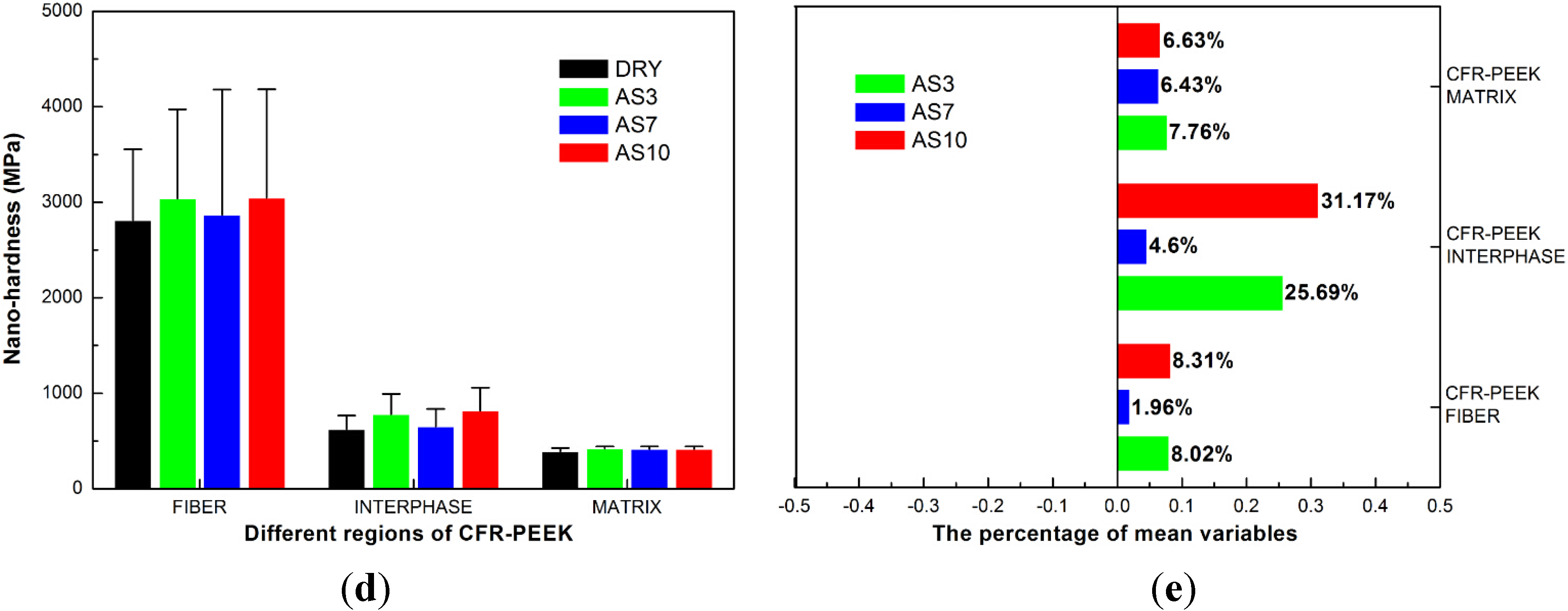

| Materials (Treatment Group) | Nanoindentation Creep (nm) | Nanoindentation Recovery (nm) | Percentage of Nanoindentation Recovery (%) |
|---|---|---|---|
| CHARISMA (DRY) | 98.41(21.59) a | 55.45(24.08) a | 0.56 |
| CHARISMA (AS3) | 337.78(14.98) b | 233.98(11.87) b | 0.69 |
| CHARISMA (AS7) | 112.27(35.15) a | 58.56(20.14) a | 0.52 |
| CHARISMA (AS10) | 66.97(11.49) c | 57.46(12.62) a | 0.86 |
| PEEK (DRY) | 64.43(25.88) e | 78.36(32.23) e | 1.22 |
| PEEK (AS3) | 59.23(20.96) e | 58.38(31.13) e | 0.99 |
| PEEK (AS7) | 74.11(27.59) e | 72.17(31.65) e | 0.97 |
| PEEK (AS10) | 53.63(6.56) e | 45.89(6.95) f | 0.86 |
| CFR-PEEK (DRY) | 76.67(20.79) i | 92.91(30.61) i | 1.21 |
| CFR-PEEK (AS3) | 103.34(32.85) j | 31.28(17.46) j | 0.30 |
| CFR-PEEK (AS7) | 52.79(12.4) k | 27.24(9.68) j | 0.52 |
| CFR-PEEK (AS10) | 71.39(18.60) i | 23.05(9.76) j | 0.32 |
| PMMA (DRY) | 153.73(19.39) m | 144.54(20.99) m | 0.94 |
| PMMA (AS3) | 162.35(5.98) m | 125.34(7.27) n | 0.77 |
| PMMA (AS7) | 150.61(4.55) m | 122.55(4.52) n,o | 0.81 |
| PMMA (AS10) | 161.49(7.01) m | 128.96(7.18) n,p | 0.79 |
4.2. Nanoscratch Results
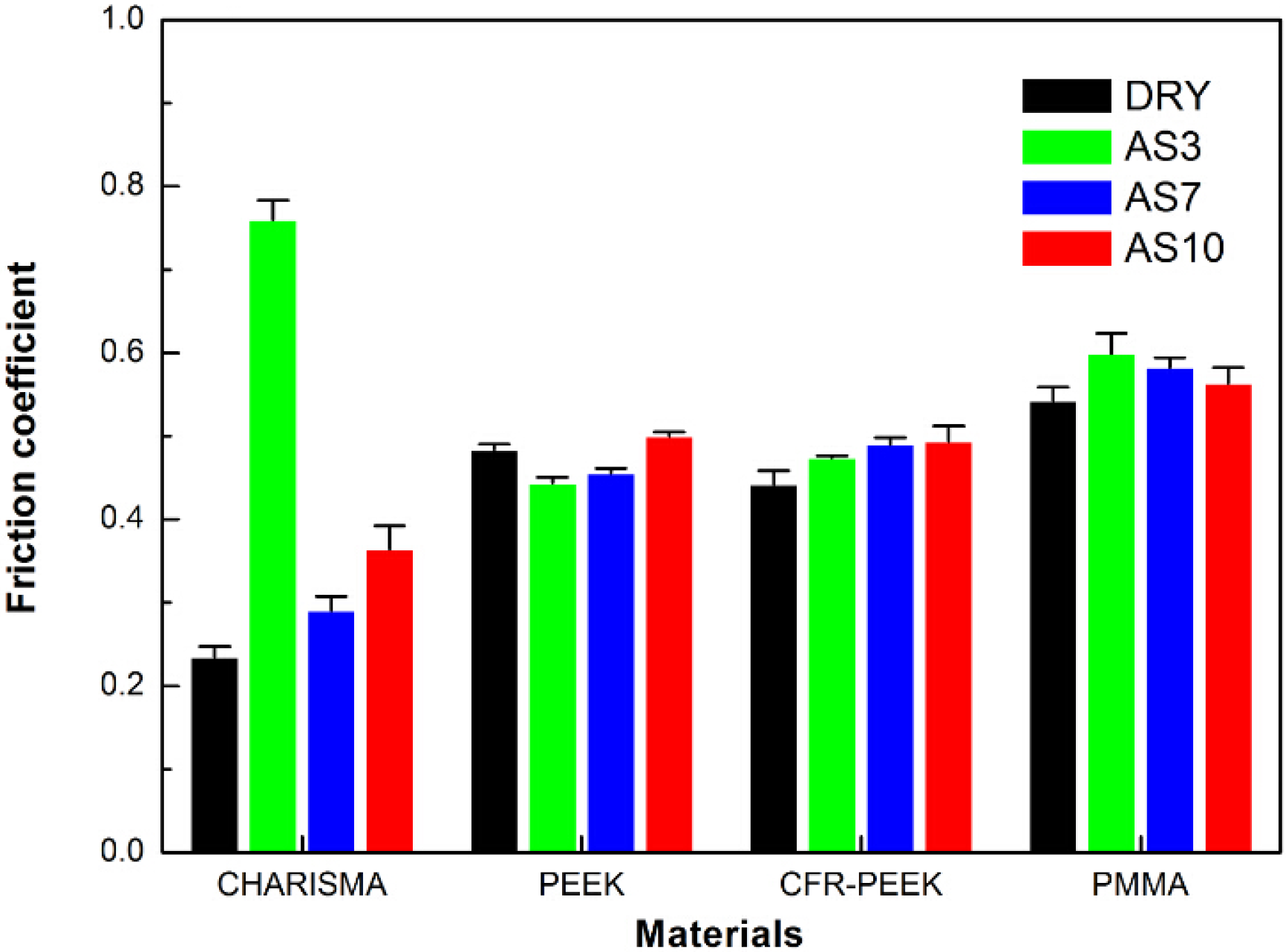
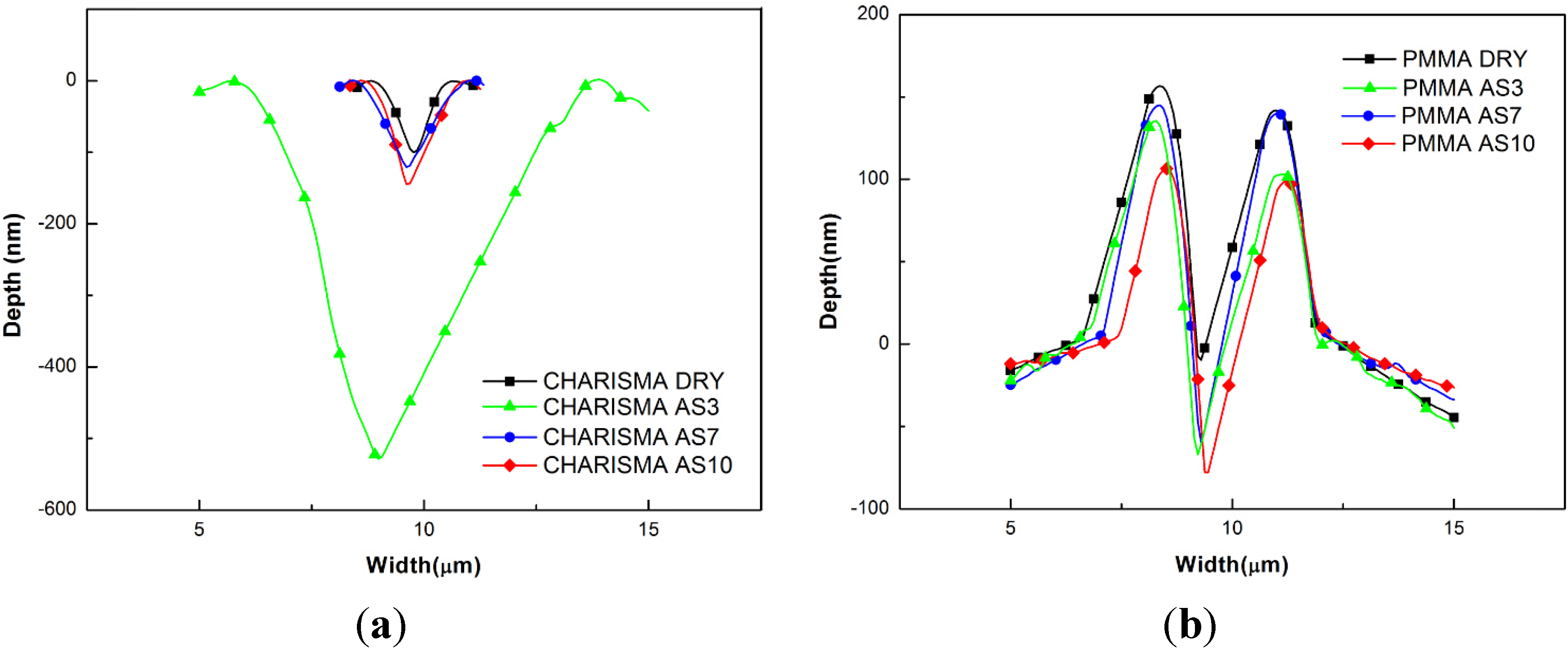
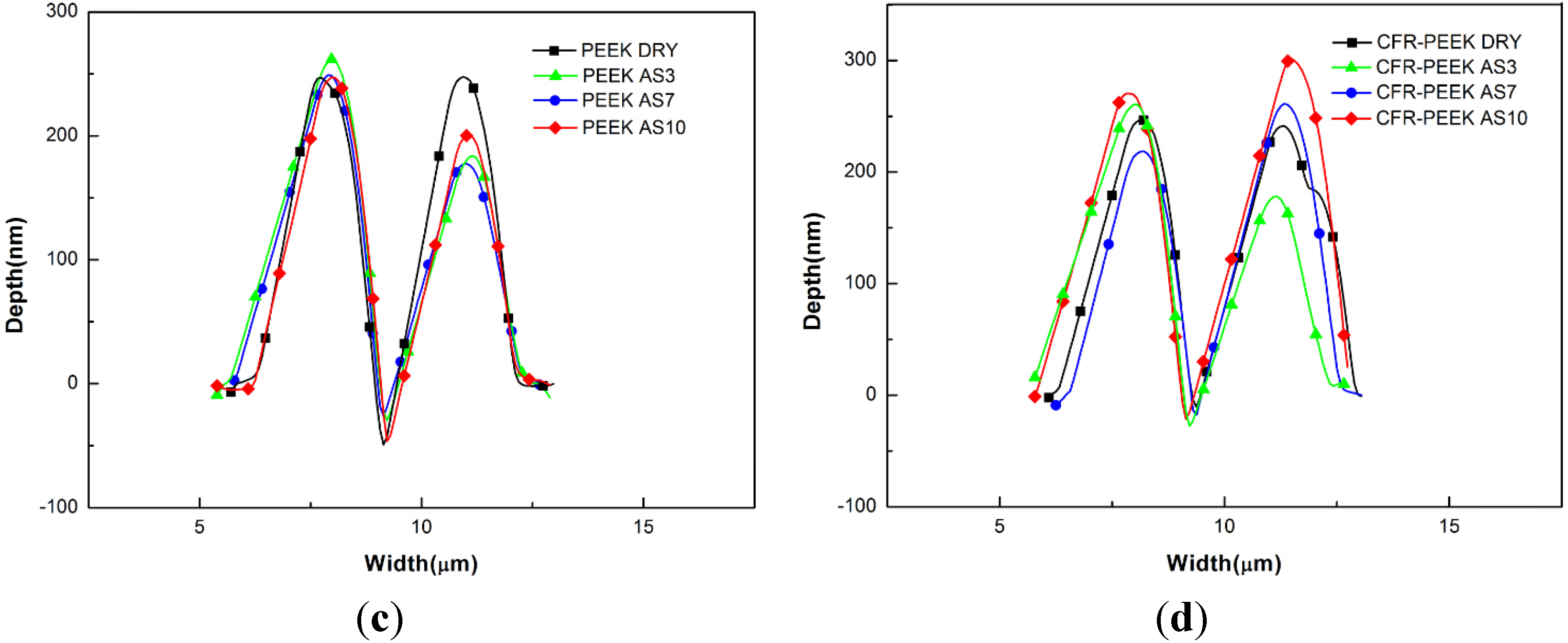
5. Discussion
5.1. The Reduced Elastic Moduli and Nanohardness
5.2. The Nanoindentation Viscoelasticity
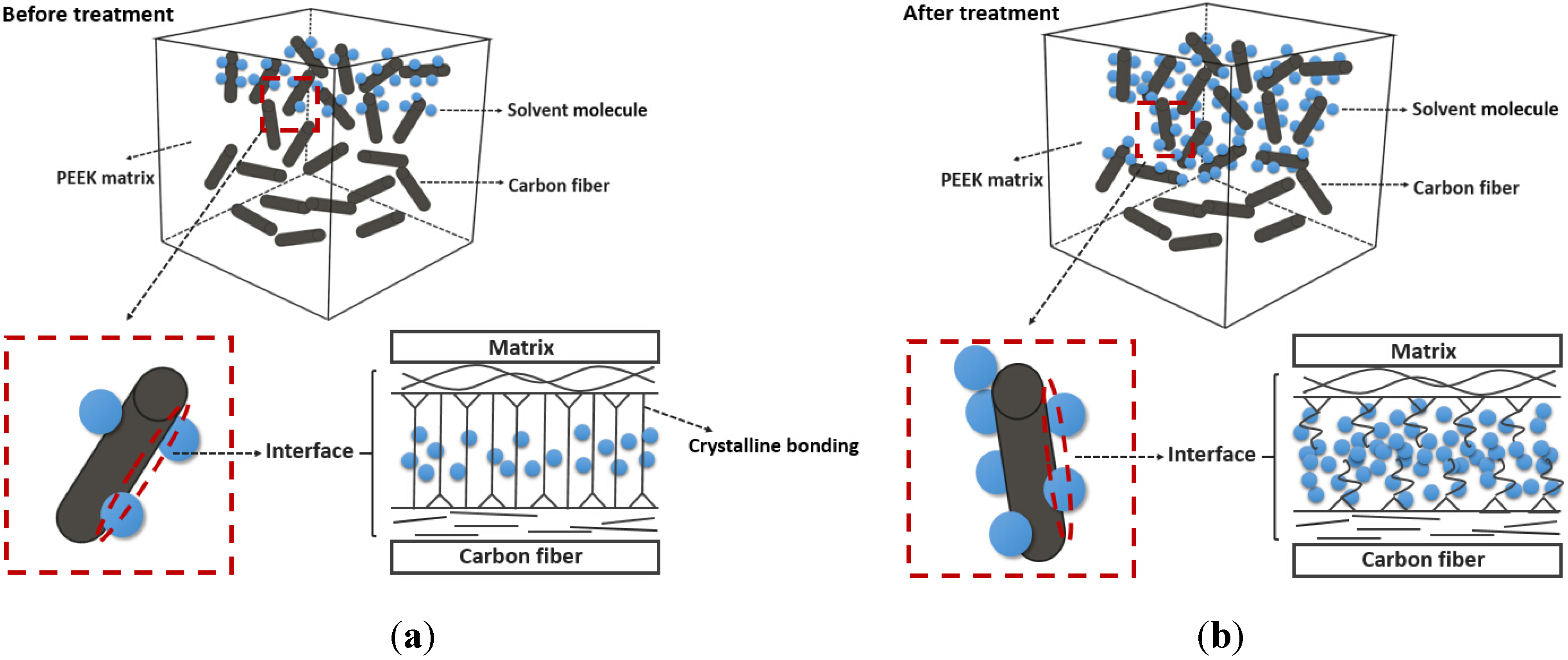
5.3. The Nanoscratch Property
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hallmann, L.; Mehl, A.; Sereno, N.; Hämmerle, C.H.F. The improvement of adhesive properties of PEEK through different pre-treatments. Appl. Surf. Sci. 2012, 258, 7213–7218. [Google Scholar] [CrossRef]
- Godara, A.; Raabe, D.; Green, S. The influence of sterilization processes on the micromechanical properties of carbon fiber-reinforced PEEK composites for bone implant applications. Acta. Biomater. 2007, 3, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Goodson, M.; Farr, D.; Keith, D.; Banks, R. Use of two-piece polyetheretherketone (PEEK) implants in orbitozygomatic reconstruction. Br. J. Oral Maxillofac. Surg. 2012, 50, 268–269. [Google Scholar] [CrossRef] [PubMed]
- Kasliwal, M.; O’Toole, J. Clinical experience using polyetheretherketone (PEEK) intervertebral structural cage for anterior cervical corpectomy and fusion. J. Clin. Neurosci. 2014, 21, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, P. Polyetheretherketone (PEEK) cages for cervical interbody replacement. Apollo Med. 2013, 10, 233–236. [Google Scholar] [CrossRef]
- Zhou, J.; Li, X.L.; Dong, J.; Zhou, X.G.; Fang, T.L.; Lin, H.; Ma, Y.Q. Three-level anterior cervical discectomy and fusion with self-locking stand-alone polyetheretherketone cages. J. Clin. Neurosci. 2011, 18, 1505–1509. [Google Scholar] [CrossRef] [PubMed]
- Koutouzis, T.; Richardson, J.; Lundgren, T. Comparative soft and hard tissue responses to titanium and polymer healing abutments. J. Oral Implantol. 2011, 37, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Bayer, S.; Komor, N.; Kramer, A.; Albrecht, D.; Mericske-Stern, R.; Enkling, N. Retention force of plastic clips on implant bars: A randomized controlled trial. Clin. Oral Implants Res. 2012, 23, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Schwitalla, A.; Muller, W.D. PEEK dental implants: A review of the literature. J. Oral Implantol. 2013, 39, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Stawarczyk, B.; Beuer, F.; Wimmer, T.; Jahn, D.; Sener, B.; Roos, M.; Schmidlin, P.R. Polyetheretherketone-a suitable material for fixed dental prostheses? J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Stawarczyk, B.; Keul, C.; Beuer, F.; Roos, M.; Schmidlin, P.R. Tensile bond strength of veneering resins to PEEK: Impact of different adhesives. Dent. Mater. J. 2013, 32, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Bettencourt, A.F.; Neves, C.B.; de Almeida, M.S.; Pinheiro, L.M.; Oliveira, S.A.; Lopes, L.P.; Castro, M.F. Biodegradation of acrylic based resins: A review. Dent. Mater. 2010, 26, E171–E180. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Shepherd, D.E.T.; Dearn, K.D. Strength of poly-ether-ether-ketone: Effects of sterilisation and thermal ageing. Polym. Test. 2013, 32, 1001–1005. [Google Scholar] [CrossRef]
- Kern, M.; Lehmann, F. Influence of surface conditioning on bonding to polyetheretherketon (PEEK). Dent. Mater. 2012, 28, 1280–1283. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M.; Gómez-Fatou, M.A.; Ania, F.; Flores, A. Nanoindentation in polymer nanocomposites. Prog. Mater. Sci. 2015, 67, 1–94. [Google Scholar] [CrossRef]
- Jee, A.Y.; Lee, M. Comparative analysis on the nanoindentation of polymers using atomic force microscopy. Polym. Test. 2010, 29, 95–99. [Google Scholar] [CrossRef]
- Gal, J.Y.; Fovet, Y.; Adib-Yadzi, M. About a synthetic saliva for in vitro studies. Talanta 2001, 53, 1103–1115. [Google Scholar] [CrossRef]
- Vouvoudi, E.C.; Sideridou, I.D. Effect of food/oral-simulating liquids on dynamic mechanical thermal properties of dental nanohybrid light-cured resin composites. Dent. Mater. 2013, 29, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Cilli, R.; Pereira, J.C.; Prakki, A. Properties of dental resins submitted to pH catalysed hydrolysis. J. Dent. 2012, 40, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Ebenstein, D.M.; Pruitt, L.A. Nanoindentation of biological materials. Nano Today 2006, 1, 26–33. [Google Scholar] [CrossRef]
- Powles, R.C.; McKenzie, D.R.; Meure, S.J.; Swain, M.V.; James, N.L. Nanoindentation response of PEEK modified by mesh-assisted plasma immersion ion implantation. Surf. Coat. Tech. 2007, 201, 7961–7969. [Google Scholar] [CrossRef]
- El-Safty, S.; Silikas, N.; Akhtar, R.; Watts, D.C. Nanoindentation creep versus bulk compressive creep of dental resin-composites. Dent. Mater. 2012, 28, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Z.; Gu, P.; Zhang, Z. Indentation and scratch behavior of nano-SiO2/polycarbonate composite coating at the micro/nano-scale. Wear 2010, 269, 21–25. [Google Scholar] [CrossRef]
- Ebadian, B.; Razavi, M.; Soleimanpour, S.; Mosharraf, R. Evaluation of tissue reaction to some denture-base materials: An animal study. J. Contemp. Dent. Pract. 2008, 9, 67–74. [Google Scholar] [PubMed]
- Santerre, J.; Shajii, L.; Leung, B. Relation of dental composite formulations to their degradation and the release of hydrolyzed polymeric-resin-derived products. Crit. Rev. Oral Biol. Med. 2001, 12, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, J.; Vreven, J.; Leloup, G. Dynamic and static moduli of elasticity of resin-based materials. Dent. Mater. 2002, 18, 64–71. [Google Scholar] [CrossRef]
- Prakki, A.; Cilli, R.; Mondelli, R.F.; Kalachandra, S.; Pereira, J.C. Influence of pH environment on polymer based dental material properties. J. Dent. 2005, 33, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Molazemhosseini, A.; Tourani, H.; Naimi-Jamal, M.R.; Khavandi, A. Nanoindentation and nanoscratching responses of PEEK based hybrid composites reinforced with short carbon fibers and nano-silica. Polym. Test. 2013, 32, 525–534. [Google Scholar] [CrossRef]
- Ray, B.C. Temperature effect during humid ageing on interfaces of glass and carbon fibers reinforced epoxy composites. J. Colloid Interface Sci. 2006, 298, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.L.; Kim, J.K. Cooling rate influences in carbon fibre/PEEK composites. Part I. Crystallinity and interface adhesion. Compos. A Appl. Sci. Manuf. 2000, 31, 517–530. [Google Scholar] [CrossRef]
- Gao, S.L.; Kim, J.K. Cooling rate influences in carbon fibre/PEEK composites. Part II: Interlaminar fracture toughness. Compos. A Appl. Sci. Manuf. 2001, 32, 763–774. [Google Scholar] [CrossRef]
- Gao, S.L.; Kim, J.K. Cooling rate influences in carbon fibre/PEEK composites. Part III: Impact damage performance. Compos. A Appl. Sci. Manuf. 2001, 32, 775–785. [Google Scholar] [CrossRef]
- Sethi, S.; Ray, B.C. Environmental effects on fibre reinforced polymeric composites: Evolving reasons and remarks on interfacial strength and stability. Adv. Colloid Interface Sci. 2015, 217, 43–67. [Google Scholar] [CrossRef] [PubMed]
- Moghbelli, E.; Banyay, R.; Sue, H.J. Effect of moisture exposure on scratch resistance of PMMA. Tribol. Int. 2014, 69, 46–51. [Google Scholar] [CrossRef]
- Schambron, T.; Lowe, A.; McGregor, H.V. Effects of environmental ageing on the static and cyclic bending properties of braided carbon fibre/PEEK bone plates. Compos. B Eng. 2008, 39, 1216–1220. [Google Scholar] [CrossRef]
- Yu, W.; Wang, X.; Tang, Q.; Guo, M.; Zhao, J. Reinforcement of denture base PMMA with ZrO2 nanotubes. J. Mech. Behav. Biomed. Mater. 2014, 32, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Assunção, W.G.; Gomes, É.A.; Barão, V.A.R.; Barbosa, D.B.; Delben, J.A.; Tabata, L.F. Effect of storage in artificial saliva and thermal cycling on Knoop hardness of resin denture teeth. J. Prosthodont. Res. 2010, 54, 123–127. [Google Scholar] [CrossRef] [PubMed]
- N’Diaye, M.; Pascaretti-Grizon, F.; Massin, P.; Baslé, M.F.; Chappard, D. Water Absorption of Poly(methyl methacrylate) Measured by Vertical Interference Microscopy. Langmuir 2012, 28, 11609–11614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baroudi, K.; Silikas, N.; Watts, D.C. Time-dependent visco-elastic creep and recovery of flowable composites. Eur. J. Oral Sci. 2007, 115, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, T.; Briscoe, B.J.; Luckham, P.F. Surface plasticization of poly(ether ether ketone). Eur. Polym. J. 2011, 47, 2244–2258. [Google Scholar] [CrossRef]
- McBagonluri, F.; Garcia, K.; Hayes, M.; Verghese, K.; Lesko, J. Characterization of fatigue and combined environment on durability performance of glass/vinyl ester composite for infrastructure applications. Int. J. Fatigue 2000, 22, 53–64. [Google Scholar] [CrossRef]
- Miller, D.C.; Carloni, J.D.; Johnson, D.K.; Pankow, J.W.; Gjersing, E.L.; To, B.; Packard, C.E.; Kennedy, C.E.; Kurtz, S.R. An investigation of the changes in poly(methyl methacrylate) specimens after exposure to ultra-violet light, heat, and humidity. Sol. Energ. Mat. Solar C 2013, 111, 165–180. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Gao, S.; Xu, B.; Yu, H. Effects of Different pH-Values on the Nanomechanical Surface Properties of PEEK and CFR-PEEK Compared to Dental Resin-Based Materials. Materials 2015, 8, 4751-4767. https://doi.org/10.3390/ma8084751
Gao S, Gao S, Xu B, Yu H. Effects of Different pH-Values on the Nanomechanical Surface Properties of PEEK and CFR-PEEK Compared to Dental Resin-Based Materials. Materials. 2015; 8(8):4751-4767. https://doi.org/10.3390/ma8084751
Chicago/Turabian StyleGao, Shuai, Shanshan Gao, Baohua Xu, and Haiyang Yu. 2015. "Effects of Different pH-Values on the Nanomechanical Surface Properties of PEEK and CFR-PEEK Compared to Dental Resin-Based Materials" Materials 8, no. 8: 4751-4767. https://doi.org/10.3390/ma8084751





