1. Introduction
Hydrogen (H
2) is an attractive energy carrier to effectively utilize natural energy resources such as solar, hydro, and wind energy. However, it is difficult to produce the high condensation state because H
2 is in the gaseous phase under ambient conditions and the critical point is −240 °C. Thus, hydrogen storage techniques are necessary to compactly store and transport hydrogen and have been investigated. Hydrogen storage in materials is one of attractive techniques because they can store hydrogen as atomic state, resulting in the highly compact state. In 2007, a target of hydrogen storage materials for fuel cell vehicle is determined by New Energy and Industrial Technology Development Organization (NEDO) in Japan [
1]. In this policy, more than 5.5 mass% of the reversible hydrogen capacity and less than 150 °C for the operating temperature are required, where these values are based on material. To achieve the above targets, the hydrogen storage materials based on light elements, lithium (Li), sodium (Na), magnesium (Mg), boron (B), carbon (C), and nitrogen (N), attract much interest because these materials can realize the high gravimetric hydrogen density, which is potentially higher than the target value [
2,
3,
4,
5,
6,
7,
8,
9,
10,
11]. Among them, the amide-imide system is recognized as an attractive hydrogen storage system. The reaction of typical Li system is described as follows [
12]:
in this system, lithium amide (LiNH
2) phase in the hydrogenated state is changed to imide phase (Li
2NH) after the hydrogen desorption. Although about 6.0 mass% of hydrogen is reversibly stored, the hydrogen desorption requires more than 200 °C due to the high enthalpy change, Δ
H = 67 kJ·mol
−1 H
2 [
13,
14,
15]. To decrease the reaction temperature, the lithium hydride (LiH) and magnesium amide (Mg(NH
2)
2) system has been proposed. Systems with different molar ratio were reported at almost the same time by Luo
et al. [
16], Leng
et al. [
17], Xiong
et al. [
18], and Nakamori
et al. [
19]. The representative three reactions are described as follows:
the detailed reaction process of the 8LiH-3Mg(NH
2)
2 system below 200 °C is expressed as follows [
20]:
where
x: 0 <
x < 1.7. First, 3 mol of H
2 is desorbed by Equation (5) with the flat plateau in the pressure-composition isotherm (PCI) [
21]. After that, 3LiMgN
2H
3 (=3LiNH
2-3MgNH) continuously reacts with LiH and desorbs H
2. The compositions of Li and H in the product defined as Li
1+xMgN
2H
3-x are non-stoichiometrically varied in this process, resulting in the slope in the PCI [
21,
22]. Under vacuum condition at 200 °C, a single phase of 3Li
2.7MgN
2H
1.3 (=4Li
2NH-Mg
3N
2) is finally formed, and then total 6.9 mass% of hydrogen can be desorbed [
23], where this single phase would disproportionate into Li
2NH and Mg
3N
2 under higher temperature condition [
17]. Thus, this system is recognized as a potential hydrogen storage material to achieve the above practical properties. However, improvement of the hydrogen storage properties is still necessary. The cyclic hydrogen absorption and desorption properties were investigated by Ikeda
et al. [
24]. They reported that the initial hydrogen desorption capacity was 4.6 mass% and decayed to 3.6 mass% after 300 cycles at 200 °C. They claimed that the NH
3 emission during the dehydrogenation is the origin of decrease in the hydrogen capacity. On the other hand, the detailed properties at 150 °C, as the target temperature, have not been clarified yet. At lower temperatures, the kinetic control would be main issue to utilize the essential hydrogen storage properties because hydrogen is desorbed by the solid-solid reaction and the hydrogen absorption proceeds with the phase separation. Namely, the crystalline size of solid materials and the contact between two solid phases would be important factors to realize suitable reaction rate at 150 °C. Furthermore, it is expected that the sintering and/or crystallization of solid materials by heating for the hydrogen desorption and absorption processes slows the reaction rates.
In this work, the various synthesis and rehydrogenation processes of the 8LiH-3Mg(NH2)2 system are investigated to understand the essential hydrogen storage properties as fundamental research. From the obtained experimental results, the feasibility of achieving 5.5 mass% H2 desorption at 150 °C is discussed.
2. Results and Discussion
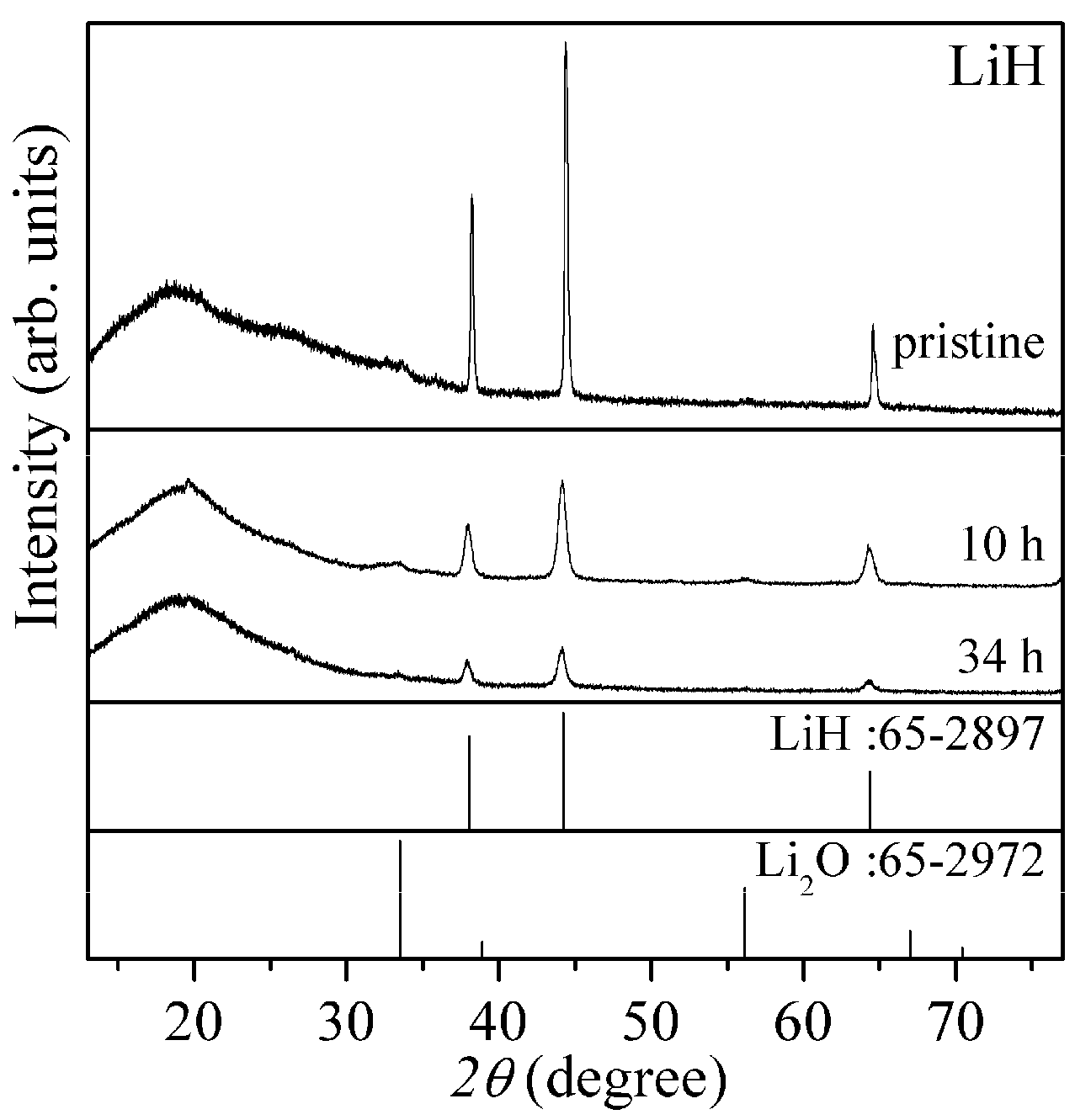
Figure 1 shows X-ray diffraction (XRD) patterns of the pristine and ball-milled LiH with the patterns of LiH and Li
2O in database as reference. Here, broad peaks around 20° and 25° are caused by a grease to spread the powder sample and a polyimide sheet to cover the sample for avoiding the oxidation.
The diffraction peaks observed in the case of pristine LiH were high intensity and sharp. After the ball-milling as pre-treatment, the peaks were clearly lowered and broadened, suggesting that the crystalline size was reduced and the structural disorder was induced. The milling effects became slightly strong for 34 h.
Figure 1.
XRD (X-ray diffraction) patterns of the pristine and ball-milled LiH (10 h and 34 h). XRD patterns of LiH and Li2O in database are also shown as reference.
Figure 1.
XRD (X-ray diffraction) patterns of the pristine and ball-milled LiH (10 h and 34 h). XRD patterns of LiH and Li2O in database are also shown as reference.
Mg(NH
2)
2 used in this work was synthesized by the method reported before [
15,
25]. Mg(NH
2)
2 and 10 h milled LiH were mixed with a 3:8 molar ratio by ball-milling for 2 and 20 h, and the property of each mixture was compared with the samples synthesized from the pristine LiH (see also
Figure S1).
Figure 2a shows XRD patterns of 2 and 20 h milled mixtures using the pristine and pretreated LiH (10 h milled). The only LiH phase was observed without impurity phases such as oxides and the peak intensity and shape were almost similar for all the samples. Mg(NH
2)
2 should be of nano-structure or amorphous, as no diffraction peaks appeared. From the XRD results, clear difference was not found. On the other hand, the hydrogen desorption profiles obtained by thermogravimetry-mass spectroscopy (TG-MS) showed the different behaviors as shown in
Figure 2b. Here, the hydrogen desorption as shoulder around 250–300 °C would be caused by the variation of reaction path from Equations (3) to (6) with the phase separation [
17]. The hydrogen desorption peaks corresponding to the samples milled for 2 h were slightly broad and weak compared with those of the sample milled for 20 h. Moreover, the weight loss corresponding to the 20 h milled sample was closer to the theoretical value, 6.9 mass%. As described above, it is known that the small amount of NH
3 originated in the decomposition of Mg(NH
2)
2 is released with the H
2 desorption. When the mixing state between LiH and Mg(NH
2)
2 is poor, the NH
3 desorption amount should be increased. Thus, these results indicate that the mixing state between two solid phases started by the pre-treated LiH was much better in the 2 h milled mixtures. However, for the 20 h milled mixtures, the hydrogen desorption profile was clearly sharpened, suggesting that the two materials with the better mixing state easily reacted. In this case, the effect of pre-treatment was not clear because of the same TG-MS profiles in our experimental accuracy. It is expected that the crystalline size of LiH is reduced enough to form the good contact between LiH and Mg(NH
2)
2 during the 20 h milling. The weight loss corresponding to the hydrogen desorption was almost consistent with the theoretical value due to the faster reaction rate. Although it is clarified that the pre-treatment of LiH is effective to improve the kinetics of hydrogen desorption, it is not necessary when the mixing time is long enough.
Figure 2.
(a) XRD patterns and (b) TG-MS (thermogravimetry-mass spectroscopy) profiles of the 8LiH-3Mg(NH2)2 samples synthesized from the pristine or pre-treated LiH (10 h milling) by ball-milling for 2 h and 20 h. XRD patterns of LiH, Mg(NH2)2, Li2O, and MgO in database are shown as reference.
Figure 2.
(a) XRD patterns and (b) TG-MS (thermogravimetry-mass spectroscopy) profiles of the 8LiH-3Mg(NH2)2 samples synthesized from the pristine or pre-treated LiH (10 h milling) by ball-milling for 2 h and 20 h. XRD patterns of LiH, Mg(NH2)2, Li2O, and MgO in database are shown as reference.
In order to examine an effect of the longer mixing time of LiH and Mg(NH
2)
2, the sample was prepared by milling for 40 h. In
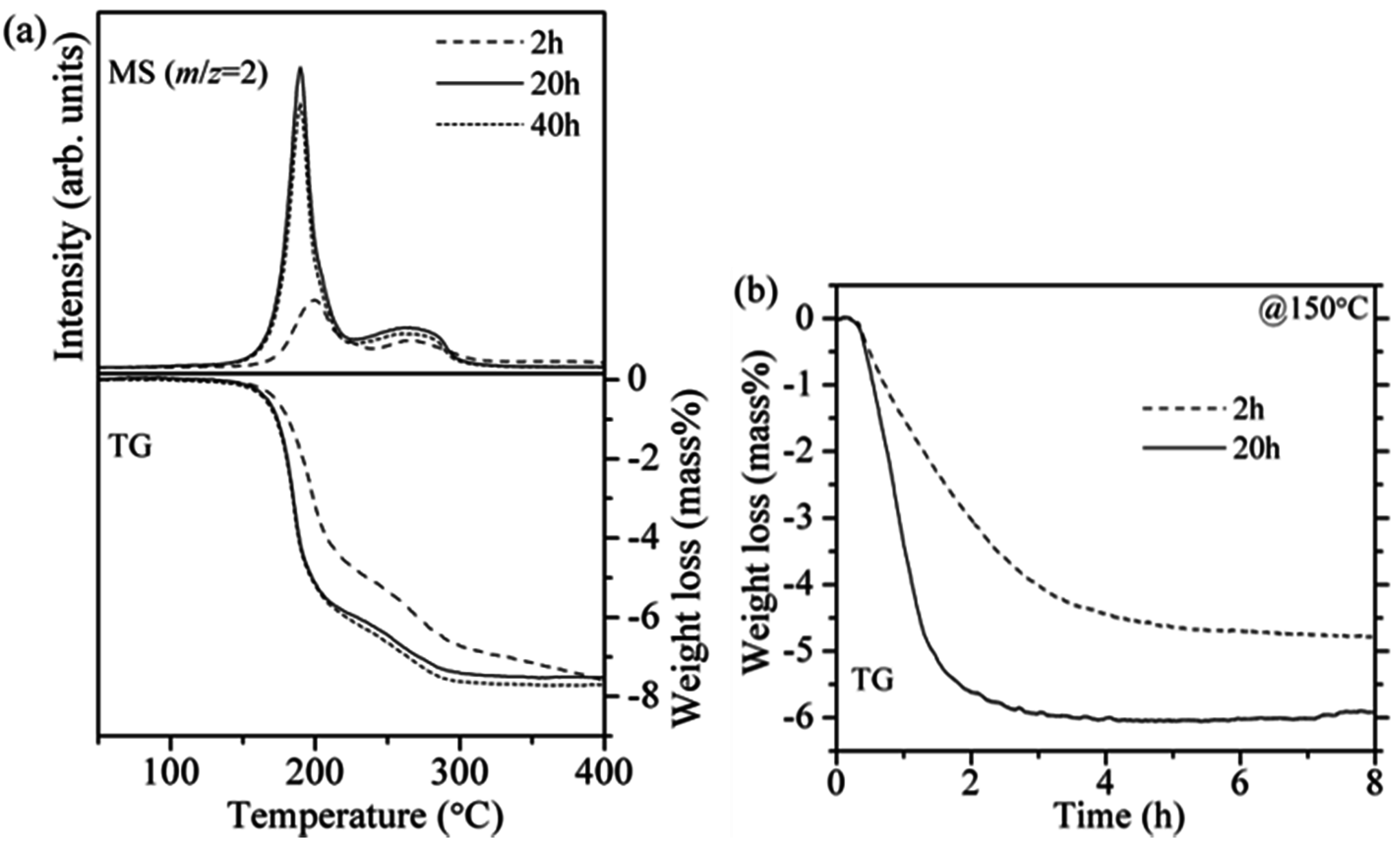
Figure 3a, the TG-MS results are compared, in which LiH used here was not pre-treated. The reaction rate of the 20 h milled sample was obviously faster than that of the 2 h milled sample. However, no clear difference between the samples prepared by the 20 h and 40 h milling was found in the TG-MS profiles, indicating that the reduction of crystalline size was saturated and the close contact of two materials was realized by milling for 20 h.
Figure 3b shows isothermal TG profiles of the 2 and 20 h milled 8LiH-3Mg(NH
2)
2 samples at 150 °C for 8 h. For the 2 h milled sample, the weight loss due to the hydrogen desorption gradually proceeded and the hydrogen desorption amount was less than 5.0 mass%, even after 8 h. On the other hand, the 20 h milled sample revealed faster reaction rate, and then the hydrogen desorption amount reached to about 6.0 mass% within 3 h. To accurately evaluate the potential amount of hydrogen desorption, the NH
3 emission should be considered. In previous reports, about 0.05 mol% of NH
3 was essentially released during all the hydrogen absorption and desorption cycles at 200 °C [
24]. In fact, the small amount of NH
3 was also observed in MS measurement around 200 °C [
26]. On the other hand, the MS signal corresponding to NH
3 was quite low intensity and out of an apparatus resolution at 150 °C. Therefore, the contribution of NH
3 emission to the weight loss in the isothermal TG measurement at 150 °C performed in this work was negligible.
Figure 3.
(a) TG-MS profiles of the 8LiH-3Mg(NH2)2 samples synthesized by ball-milling for 2, 20, and 40 h; (b) Isothermal TG profiles at 150 °C of the 2 h and 20 h milled samples.
Figure 3.
(a) TG-MS profiles of the 8LiH-3Mg(NH2)2 samples synthesized by ball-milling for 2, 20, and 40 h; (b) Isothermal TG profiles at 150 °C of the 2 h and 20 h milled samples.
For the LiH-LiNH
2 system, 1 mol% titanium chloride (TiCl
3) shows excellent catalytic effect and lowers the peak temperature of hydrogen desorption in the MS analysis [
27,
28]. On the basis of previous works, the effects of TiCl
3 on the 8LiH-3Mg(NH
2)
2 system was investigated.
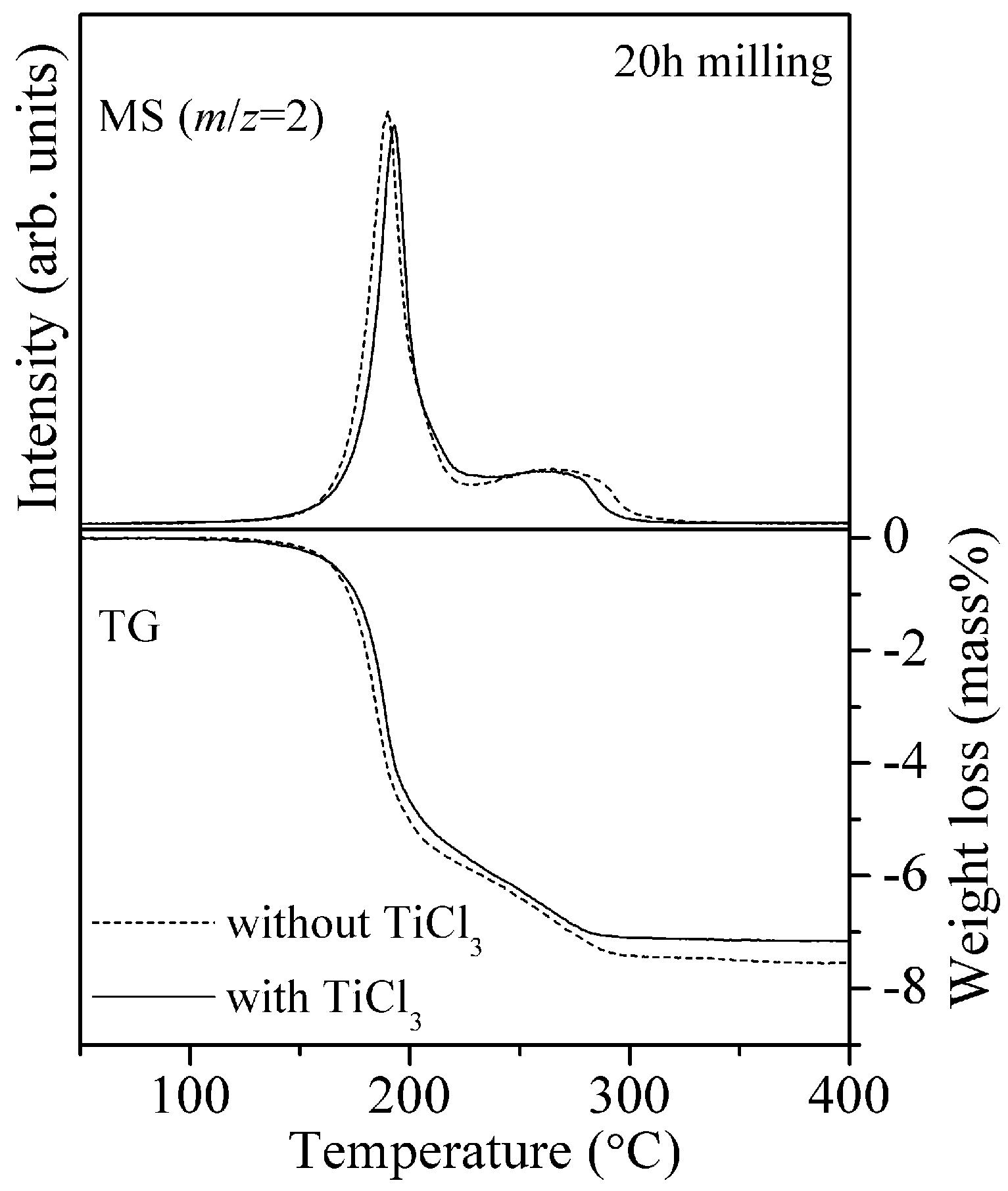
Figure 4 shows the results of TG-MS measurement for the 8LiH-3Mg(NH
2)
2 samples with and without TiCl
3. The peak temperature of hydrogen desorption was located around 190 °C for both samples, and the profiles were almost same. The TG profiles were also similar, where the slight difference of weight loss at 400 °C would be caused by the TiCl
3 addition. Thus, it was clarified that TiCl
3 has no significant catalytic effects on the 8LiH-3Mg(NH
2)
2 system, suggesting that the reaction processes are possibly different from the LiH and LiNH
2 system.
Figure 4.
TG-MS profiles of the 8LiH-3Mg(NH2)2 samples with and without 0.54 mol% TiCl3 synthesized by the ball-milling for 20 h.
Figure 4.
TG-MS profiles of the 8LiH-3Mg(NH2)2 samples with and without 0.54 mol% TiCl3 synthesized by the ball-milling for 20 h.
From the above results, the 8LiH-3Mg(NH
2)
2 sample synthesized from the pristine LiH by ball-milling for 20 h is chosen as the starting material for experiments to investigate the rehydrogenation processes. The synthesized 8LiH-3Mg(NH
2)
2 was dehydrogenated at 200 °C under dynamic vacuum condition for 24 h. The dehydrogenated sample was analyzed by the TG-MS measurement to quantitatively evaluate the progress of reaction. As a result, the weight loss with heating up to 400 °C was less than 0.5 mass% (see
Figure S2), suggesting that the dehydrogenation was almost completed in this condition. The rehydrogenation was performed at 200 °C under 10.0 MPa of H
2 for 12 h. After the thermochemical rehydrogenation, it is expected that the crystallization and sintering of the solid particles are induced. To reduce the crystalline size and recover the close contact between LiH and Mg(NH
2)
2, the rehydrogenated sample was ball-milled under three different temperature conditions, at room temperature, 100 °C, and −79 °C, where the products were named “rehy + mill”. Here, the results of sample prepared at room temperature is omitted in the discussion below because the properties are almost same as those prepared at 100 °C and −79 °C.
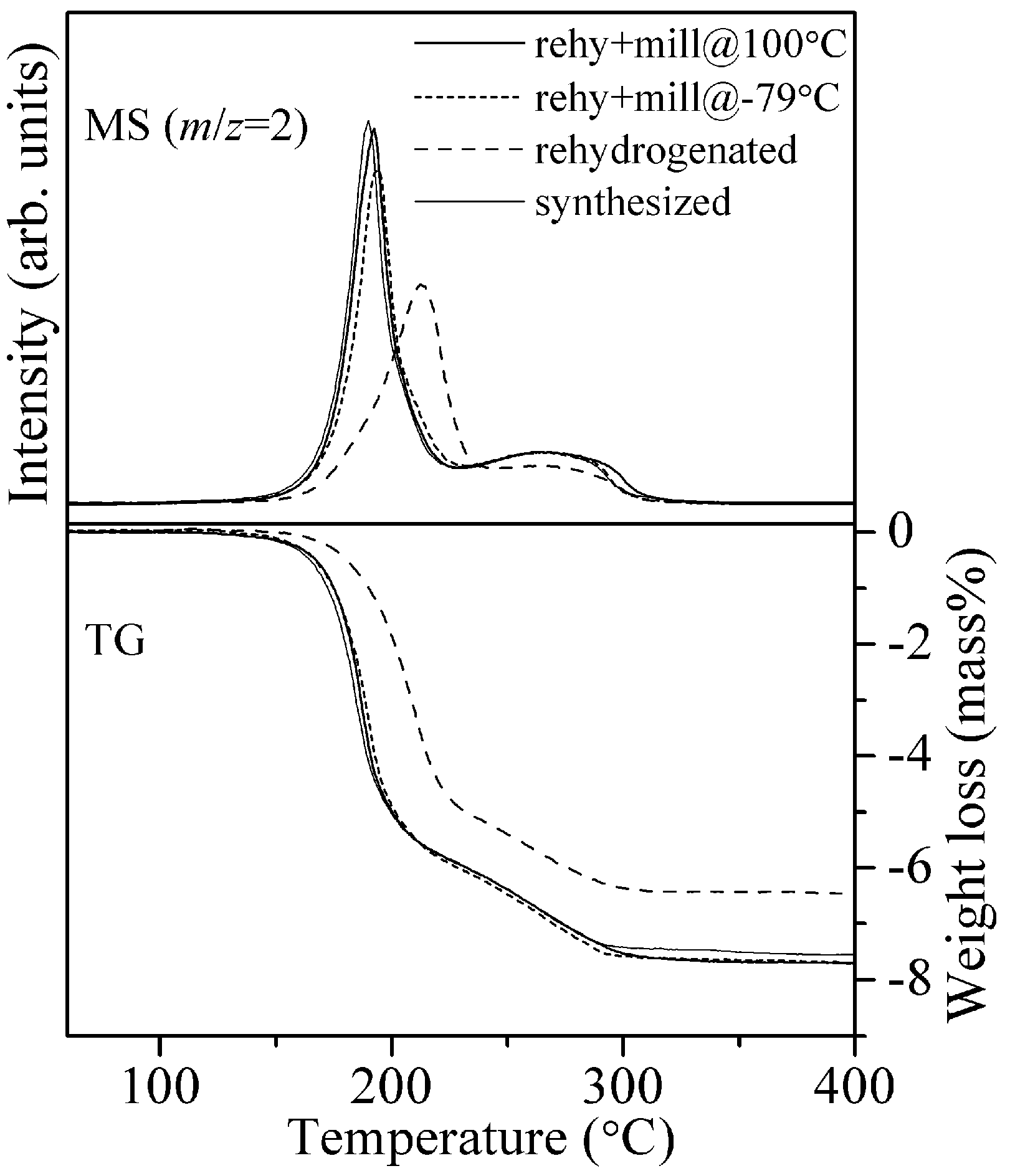
Figure 5 representatively shows the TG-MS profiles of the rehy + mill@100 °C and rehy + mill@ −79 °C samples with the profiles of the samples after synthesis and thermochemical rehydrogenation. In addition, all the information obtained by TG-MS measurements is listed in
Table 1.
Figure 5.
TG-MS profiles of the 8LiH-3Mg(NH2)2 samples after the synthesis, the thermochemical rehydrogenation, and the ball-milling at 100 °C and −79 °C for the rehydrogenated sample.
Figure 5.
TG-MS profiles of the 8LiH-3Mg(NH2)2 samples after the synthesis, the thermochemical rehydrogenation, and the ball-milling at 100 °C and −79 °C for the rehydrogenated sample.
Table 1.
Peak temperature and weight loss obtained by TG-MS to 400 °C, weight loss obtained by isothermal TG measurements at 150 °C for 8 h, and time required to desorb 5.5 mass% of hydrogen at 150 °C of each sample.
Table 1.
Peak temperature and weight loss obtained by TG-MS to 400 °C, weight loss obtained by isothermal TG measurements at 150 °C for 8 h, and time required to desorb 5.5 mass% of hydrogen at 150 °C of each sample.
| Sample | TG-MS (to 400 °C) | Isothermal TG (150 °C, 8 h) |
|---|
| Peak temperature (°C) | Weight loss (mass%) | Weight loss (mass%) | Time to 5.5 mass% (h) |
|---|
| Synthesized | 190 | 7.5 | 6.0 | 1.8 |
| Rehydrogenated | 213 | 6.5 | 4.0 | > 8.0 |
| Rehy + mill@100 °C | 192 | 7.7 | 5.8 | 3.2 |
| Rehy + mill@–79 °C | 193 | 7.7 | 5.8 | 2.5 |
| Mill-rehy@150 °C | 198 | 7.9 | 5.7 | 4.7 |
| Mill-rehy@RT | 174 | 4.7 | – | – |
The peak temperature of hydrogen desorption of the synthesized sample was located at 190 °C. After the rehydrogenation without milling treatment, the peak was significantly shifted, to a temperature higher than 200 °C. Moreover, it was noted that the weight loss was only 6.5 mass%. The decrease in hydrogen capacity for the sample rehydrogenated by the only thermochemical reaction is, possibly, caused by the following reasons. During the heating processes for de/rehydrogenation, the crystalline size of solid materials is increased by sintering, resulting in the longer diffusion distance of atoms and the poor contact between the generated hydride and amide. In this case, the unreacted parts should remain after the TG-MS measurement. As another possibility, it seemed that the rehydrogenation was not completed under the conditions. Because both the rehy + mill samples revealed better hydrogen desorption properties, where the MS and TG profiles were almost same as those of the synthesized sample, the additional milling processes under H2 pressure is able to recover the close contact of both materials due to the reduction of crystalline size.
The above results indicate that the small crystalline sizes should be preserved to prevent the decay of the essential hydrogen desorption properties. Thus, the rehydrogenation was performed by the mechanochemical reaction using ball-milling under H
2 pressure, where the mechanochemically rehydrogenated samples are named “mill-rehy”. The dehydrogenated sample was milled under two conditions: room temperature under 6.0 MPa of H
2 and 150 °C under 15.0 MPa of H
2 for 5 h. At 150 °C, the high pressure was applied to thermodynamically suppress the hydrogen desorption. The TG-MS profiles of mill-rehy@RT and mill-rehy@150 °C are shown in
Figure 6.
Figure 6.
TG-MS profiles of the 8LiH-3Mg(NH2)2 samples after synthesis, thermochemical rehydrogenation, and mechanochemical rehydrogenation at room temperature and 150 °C.
Figure 6.
TG-MS profiles of the 8LiH-3Mg(NH2)2 samples after synthesis, thermochemical rehydrogenation, and mechanochemical rehydrogenation at room temperature and 150 °C.
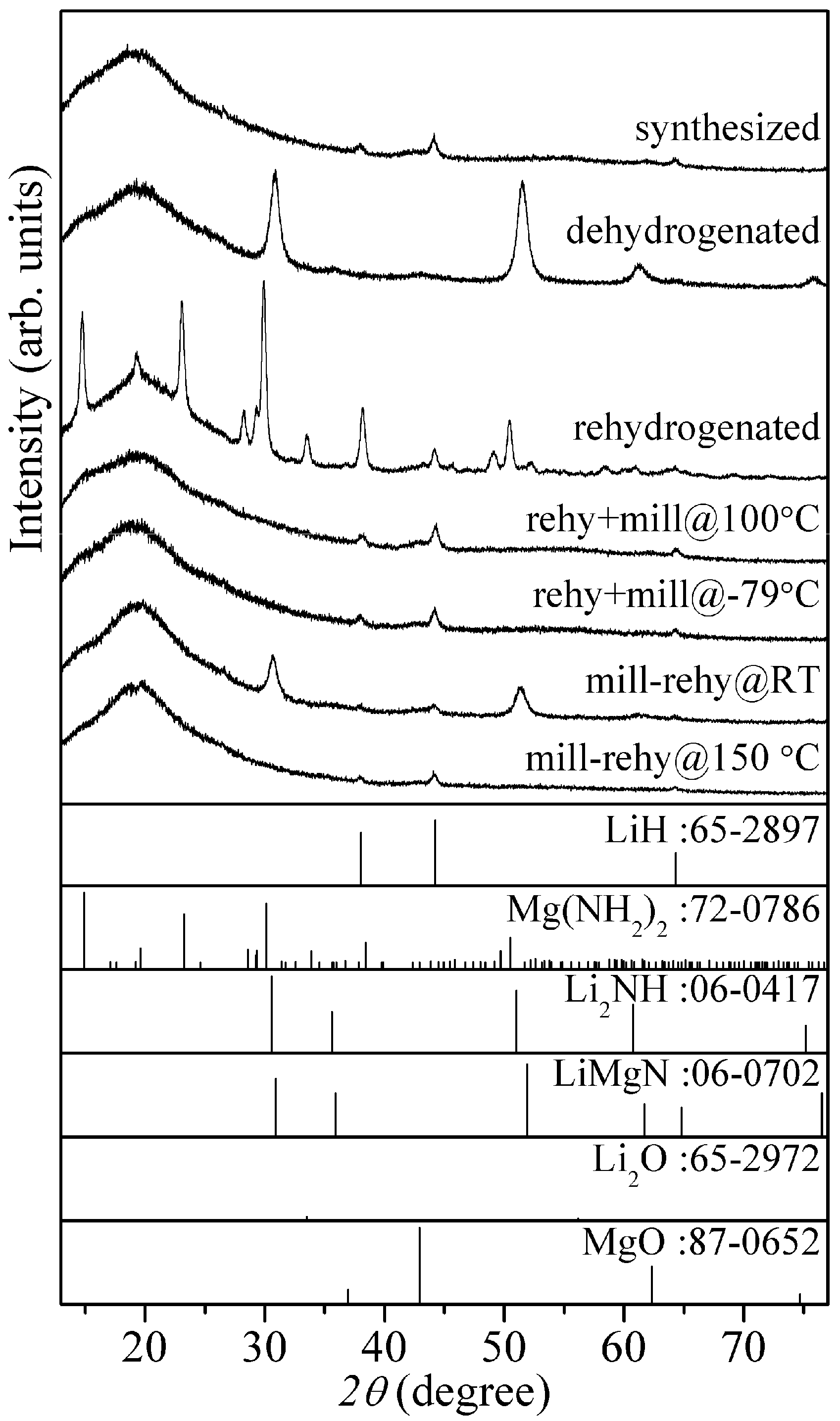
Figure 7 shows XRD patterns of 8LiH-3Mg(NH
2)
2 sample for all the processes. As already described above, the synthesized sample included the low crystalline LiH and Mg(NH
2)
2. After the dehydrogenation at 200 °C, the diffraction peaks were still broad shape and would be originated in the similar structure to Li
2NH and/or LiMgN, which should be dehydrogenated state defined as Li
1+xMgN
2H
3−x. In the XRD pattern of the thermochemical rehydrogenated sample, the sharp peaks corresponding to LiH and Mg(NH
2)
2 phases were clearly observed, suggesting that the crystallization of them proceeded during the rehydrogenation. Because the peaks assigned to the dehydrogenated phases completely disappeared, the poor hydrogen desorption properties of the rehydrogenated sample observed in the TG-MS measurement would be caused by the formation of worse mixing state between both materials due to the thermal crystallization. By the ball-milling at 100 °C and −79 °C after the thermochemical rehydrogenation, Mg(NH
2)
2 was changed to nano-structural or amorphous phase. The shapes of peaks corresponding to LiH were similar to those of the synthesized sample. From these results, it is indicated that the low crystalline size are recovered. In fact, the hydrogen desorption properties was improved by the milling processes as shown in
Figure 5. In the XRD pattern of mill-rehy@RT, the dehydrogenated phases remained although the crystalline Mg(NH
2)
2 was not observed. This result is consistent with the low hydrogen desorption capacity observed in the TG-MS measurement. After the mechanochemical rehydrogenation at 150 °C, the XRD pattern was similar to the synthesized and rehy + mill samples.
Figure 7.
XRD patterns of the 8LiH-3Mg(NH2)2 samples after the synthesis, dehydrogenation, and various rehydrogenation processes. XRD patterns of LiH, Mg(NH2)2, Li2NH, LiMgN, Li2O, and MgO in database are shown as reference.
Figure 7.
XRD patterns of the 8LiH-3Mg(NH2)2 samples after the synthesis, dehydrogenation, and various rehydrogenation processes. XRD patterns of LiH, Mg(NH2)2, Li2NH, LiMgN, Li2O, and MgO in database are shown as reference.
For the samples having better hydrogenated states, rehy + mill@100 °C, rehy + mill@−79 °C, and mill-rehy@150 °C, the isothermal TG measurements were performed at 150 °C. The obtained results are shown in
Figure 8 with the results of synthesized and rehydrogenated samples. Furthermore, the weight loss observed for 8 h and the time required to desorb 5.5 mass% of H
2, which is the target value of hydrogen capacity, are listed in
Table 1. The synthesized sample showed the fastest reaction rate, and about 5.5 mass% of H
2 can be desorbed within 1.8 h. However, the hydrogen desorption of the thermochemically rehydrogenated sample was only 4.0 mass%, even after reaction for 8 h. The TG profiles of the rehy + mill samples were similar to each other and revealed relatively faster hydrogen desorption. As a result, 5.5 mass% of H
2 was obtained within 3.2 h. Although the reaction rate of the mechanochemically rehydrogenated sample was slightly slower, the hydrogen desorption amount can reach 5.5 mass% after 4.7 h. The difference between the rehy + mill and mill-rehy samples might be caused by the temperature of the rehydrogenation processes, namely, 150 °C might be too high to realize the suitable crystalline size and mixing state by competing with the thermal crystallization. By further optimizing the treatment conditions, the hydrogen desorption properties of mechanochemically rehydrogenated sample would be improved.
Figure 8.
Isothermal TG profiles at 150 °C of the 8LiH-3Mg(NH2)2 samples after the synthesis, and various rehydrogenation processes.
Figure 8.
Isothermal TG profiles at 150 °C of the 8LiH-3Mg(NH2)2 samples after the synthesis, and various rehydrogenation processes.
All the rehydrogenated samples showed about 5.8 mass% for 8 h, although the initial reaction rate was different for each sample, and then the reaction stage should be in the non-stoichiometric variation and roughly described as follows:
from the above experimental results, it is clarified that the crystalline size and contact between LiH and Mg(NH
2)
2 are important factors to realize the suitable reaction kinetics. Furthermore, it is demonstrated that the 8LiH-3Mg(NH
2)
2 system is the potential hydrogen storage material to achieve 5.5 mass% of H
2 desorption at 150 °C, even after the rehydrogenation by preserving the suitable crystalline size and mixing state. The reaction mechanism was proposed in previous literature, reported by Isobe
et al. [
20] and Nakamura
et al. [
23]. In the hydrogen desorption Reaction (5), H
+ in the non-stoichiometric Li
1+xMgN
2H
3−x phase and Li
+ in LiH are diffused and exchanged each other via thorough the interface between the solid phases, and then Li-rich/H-poor imide phase are generated. The H
2 desorption proceeds by combining H
+ diffused from Li
1+xMgN
2H
3−x with H
− in LiH. This proposed mechanism is quite reasonable from the points of view of charge and mass balance in the system. Considering the above hydrogen desorption mechanism, it is expected that the shorter diffusion distance of the ions and the larger number of interface between both materials will realize the fast hydrogen desorption. Therefore, the experimental facts in this work strongly support the reaction mechanism.
3. Experimental Section
Commercial LiH (99.4%, Alfa Aesar, Lancashire, UK) was used as the starting material. Mg(NH
2)
2 was synthesized by ball-milling MgH
2 (95%, Gelest Inc., Morrisville, PA, USA) under 0.6 MPa of NH
3, where the ball-milling was performed at room temperature by using a planetary ball-mill apparatus (P5, Fritsch, Idar-Oberstein, Germany) with 250 rpm for 10 h. The purity of synthesized Mg(NH
2)
2 was evaluated by the weight loss with thermal decomposition (NH
3 desorption) to 500 °C and estimated to be about 95%. Here, the synthesized Mg(NH
2)
2 possesses low crystallinity like nano-structural or amorphous phase because the crystalline size is decreased by the mechanical energy applied during the ball-milling process. To know the effect of starting crystalline size, LiH was pre-treated by the ball-milling (P7, Fritsch, Idar-Oberstein, Germany) under 1.0 MPa of H
2 for 10 h and 34 h. The pristine or milled LiH was mixed with Mg(NH
2)
2 by the ball-milling under 1.0 MPa of H
2 for 2, 20, and 40 h, where the molar ratio of Li and Mg was 8:3 (73:27 mol%). In addition, the catalyzed samples were also prepared, in which 0.54 mol% TiCl
3 (98%) was added into the above mixing process for 20 h. The amount of TiCl
3 was chosen to be 2 mol% for Mg(NH
2)
2 based on previous reports for the LiH-LiNH
2 system [
27,
29]. For all the ball-milling process, 30 min interval is taken into every 1 h milling. The dehydrogenation of the samples was carried out at 200 °C for 24 h under dynamic vacuum condition. For the rehydrogenation, various procedures were performed as follows (see
Figure S1). As the conventional thermochemical rehydrogenation, the dehydrogenated sample was heat-treated at 200 °C for 12 h under 10.0 MPa of H
2. In addition, the rehydrogenated samples were ball-milled at room temperature under 1 MPa of H
2 for 20 h, at 100 °C under 10 MPa of H
2 for 5 h, and at −79 °C under 1 MPa of H
2 for 2 h, where the products were named rehy + mill@RT, rehy + mill@100 °C, and rehy + mill@ −79 °C, respectively. For the ball-milling at −79 °C, the milling pot was cooled by the jacket filled with dry ice. Furthermore, the rehydrogenation was performed by the mechanochemical reaction. The dehydrogenated sample was milled at room temperature under 6.0 MPa of H
2 for 5 h by a vibrating ball-mill apparatus (RM-10, Seiwa Giken, Hiroshima, Japan) and at 150 °C under 15.0 MPa of H
2 for 5 h by a high-pressure type of vibrating ball-mill apparatus (RM-40, Seiwa Giken, Hiroshima, Japan). These rehydrogenated samples are named mill-rehy@RT and mill-rehy@150 °C. All the sample treatment was performed in a glove box filled with purified Ar (MP-P60W, Miwa MFG, Ibaraki, Osaka, Japan) because the Li and Mg compounds are easily oxidized in the air.
To identify the solid materials and discuss the structural properties, X-ray diffraction (XRD) measurement (RINT-2100, CuKα radiation, Rigaku, Akishima, Japan) was performed, where the samples were covered by a polyimide sheet (Kapton®, Du Pont-Toray Co., Ltd., Chuo-ku, Tokyo, Japan) to avoid sample oxidation. The hydrogen desorption properties were evaluated by thermogravimetry (TG, TG8120, Rigaku, Akishima, Japan) and mass spectroscopy (MS, M-QA200TS, Anelva, Kawasaki, Japan), which are placed into a glove box to measure essential properties of the samples without the influence of oxidation. For the TG-MS measurements, He gas was flowed as a carrier gas, suggesting that the H2 partial pressure around the sample would be kept to low level. The heating rate was fixed to be 5 °C·min−1. To evaluate the reaction rate and determine the utilizable hydrogen amount at 150 °C, an isothermal TG experiment was carried out, where the heating rate from room temperature to 150 °C was 5 °C·min−1 and the total measurement time was 8 h.