Biofilm and Dental Biomaterials
Abstract
:1. Introduction
2. Oral Biofilm
3. Oral Microorganisms and Oral Infections

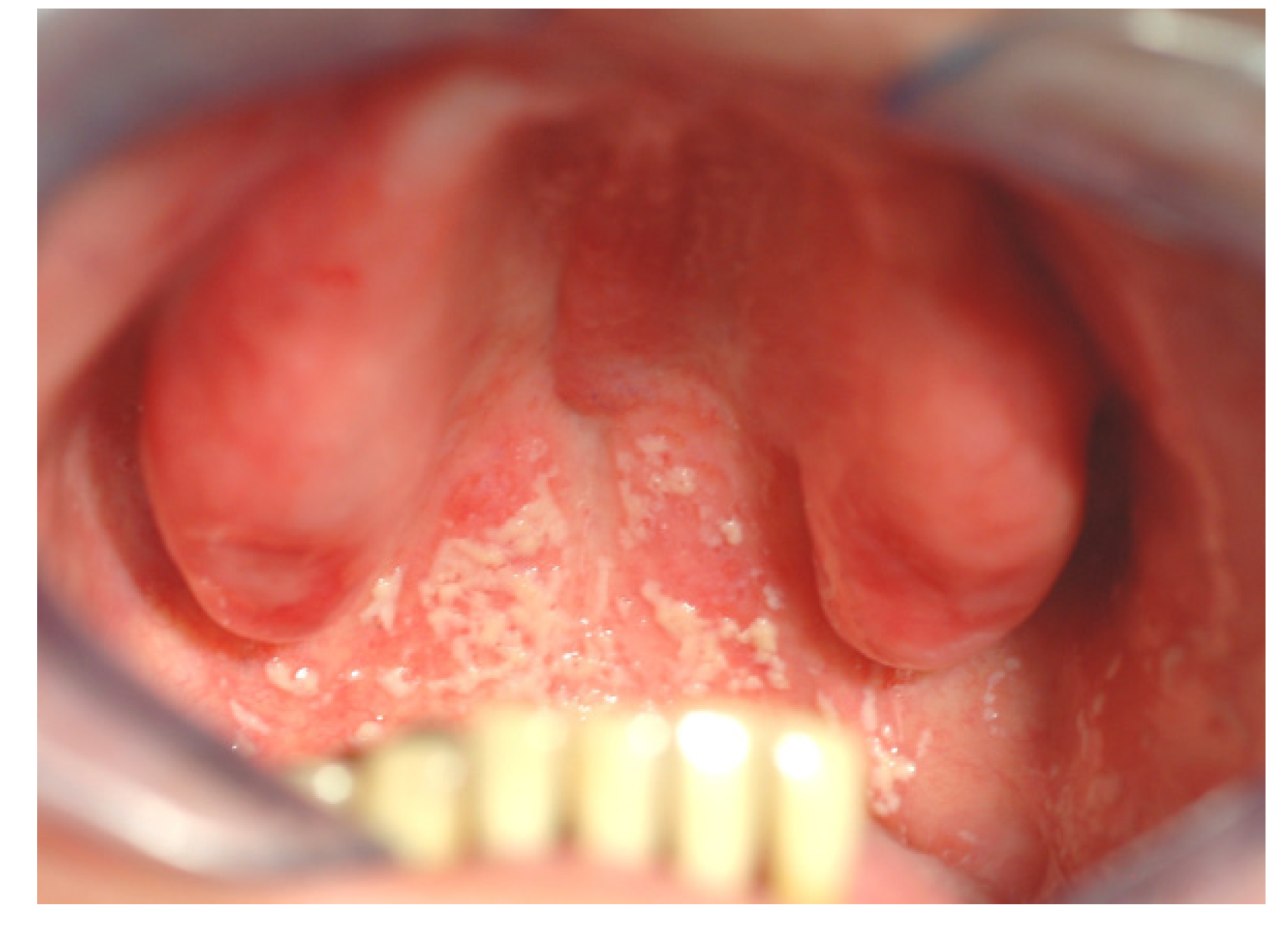
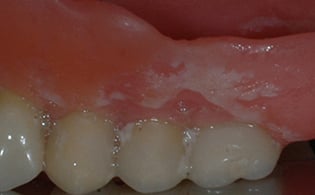
4. Biomaterials’ Effect on Biofilm

4.1. Surface Energy
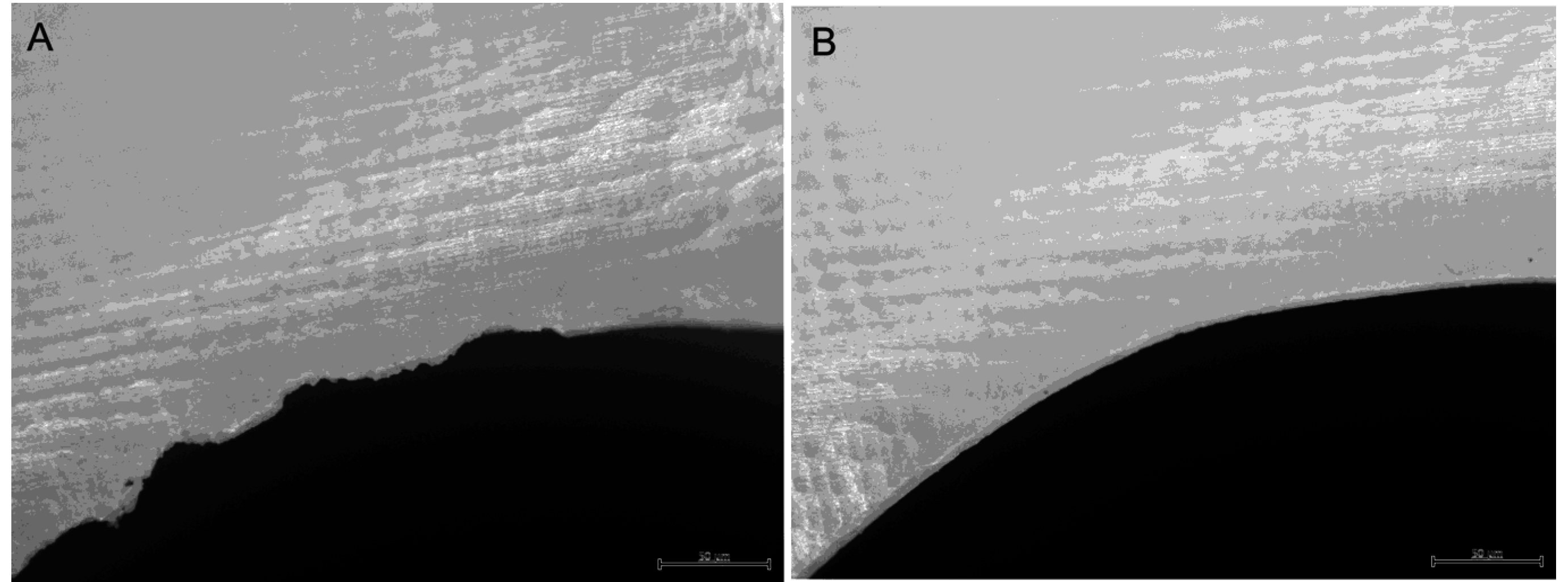
4.2. Surface Roughness
4.3. Chemical Composition
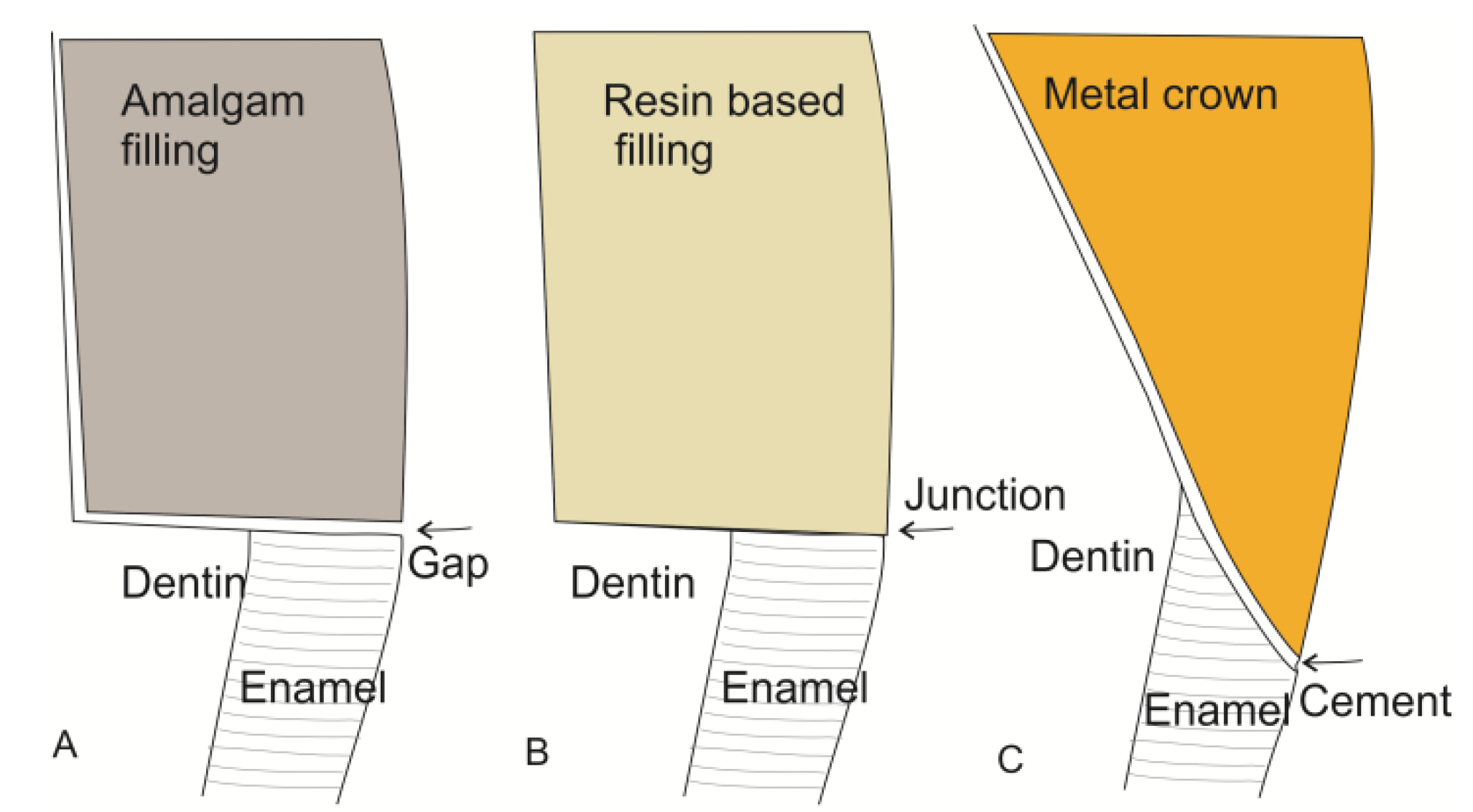
4.4. Dental Restorations

5. Future Aspects and Current Research
Author Contributions
Conflicts of interest
References
- Avila, M.; Ojcius, D.M.; Yilmaz, O. The oral microbiota: Living with a permanent guest. DNA Cell Biol. 2009, 28, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Filoche, S.; Wong, L.; Sissons, C.H. Oral biofilms: Emerging concepts in microbial ecology. J. Dent. Res. 2010, 89, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Mjör, I.A.; Moorhead, J.E.; Dahl, J.E. Reasons for replacement of restorations in permanent teeth in general dental practice. Int. Dent. J. 2000, 50, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Hasan, J.; Crawford, R.J.; Ivanova, E.P. Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms and device-associated infections. Emerg. Infect. Dis. 2001, 7, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J. Oral Microbiology and Immunology; ASM Press: Washington, DC, USA, 2006. [Google Scholar]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kolltveit, K.M.; Tronstad, L.; Olsen, I. Systemic diseases caused by oral infection. Clin. Microbiol. Rev. 2000, 13, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Sbordone, L.; Bortolaia, C. Oral microbial biofilms and plaque-related diseases: Microbial communities and their role in the shift from oral health to disease. Clin. Oral Investig. 2003, 7, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Plaque as a biofilm: Pharmacological principles of drug delivery and action in the sub- and supragingival environment. Oral Dis. 2003, 9, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.W. The role of erosion in tooth wear: Aetiology, prevention and management. Int. Dent. J. 2005, 55, 277–284. [Google Scholar] [PubMed]
- Von Fraunhofer, J.A.; Loewy, Z.G. Factors involved in microbial colonization of oral prostheses. Gen. Dent. 2009, 57, 136–143. [Google Scholar] [PubMed]
- Sachdeo, A.; Haffajee, A.D.; Socransky, S.S. Biofilms in the edentulous oral cavity. J. Prosthodont. 2008, 17, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Paranhos Hde, F.; da Silva, C.H.; Venezian, G.C.; Macedo, L.D.; de Souza, R.F. Distribution of biofilm on internal and external surfaces of upper complete dentures: The effect of hygiene instruction. Gerodontology 2007, 24, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.D.; Wilson, M. The effects of surface roughness and type of denture acrylic on biofilm formation by streptococcus oralis in a constant depth film fermentor. J. Appl. Microbiol. 2001, 91, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Busscher, H.J.; Rinastiti, M.; Siswomihardjo, W.; van der Mei, H.C. Biofilm formation on dental restorative and implant materials. J. Dent. Res. 2010, 89, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.J., Jr. Supragingival and subgingival plaque: Paradigm of biofilms. Compend. Contin. Educ. Dent. 2010, 31, 104–106. [Google Scholar] [PubMed]
- Francolini, I.; Donelli, G. Prevention and control of biofilm-based medical-device-related infections. FEMS Immunol. Med. Microbiol. 2010, 59, 227–238. [Google Scholar] [PubMed]
- Leknes, K.N. The influence of anatomic and iatrogenic root surface characteristics on bacterial colonization and periodontal destruction: A review. J. Periodontol. 1997, 68, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Coulthwaite, L.; Verran, J. Potential pathogenic aspects of denture plaque. Br. J. Biomed. Sci. 2007, 64, 180–189. [Google Scholar] [PubMed]
- Nyvad, B.; Crielaard, W.; Mira, A.; Takahashi, N.; Beighton, D. Dental caries from a molecular microbiological perspective. Caries Res. 2013, 47, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Debelian, G.J.; Olsen, I.; Tronstad, L. Systemic diseases caused by oral microorganisms. Endod. Dent. Traumatol. 1994, 10, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Sumi, Y.; Kagami, H.; Ohtsuka, Y.; Kakinoki, Y.; Haruguchi, Y.; Miyamoto, H. High correlation between the bacterial species in denture plaque and pharyngeal microflora. Gerodontology 2003, 20, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, C.J.; Jin, L.; Samaranayake, L.P. Biofilm lifestyle of candida: A mini review. Oral. Dis. 2008, 14, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Martinez, J.P.; Lopez-Ribot, J.L. Candida biofilms on implanted biomaterials: A clinically significant problem. FEMS Yeast Res. 2006, 6, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D.; Martin, M.V. Oral Microbiology, 5th ed.; Elsevier: Edinburgh, Scotland, UK, 2009. [Google Scholar]
- Marsh, P.D.; Percival, R.S.; Challacombe, S.J. The influence of denture-wearing and age on the oral microflora. J. Dent. Res. 1992, 71, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Chiappelli, F.; Bauer, J.; Spackman, S.; Prolo, P.; Edgerton, M.; Armenian, C.; Dickmeyer, J.; Harper, S. Dental needs of the elderly in the 21st century. Gen. Dent. 2002, 50, 358–363. [Google Scholar] [PubMed]
- Adamczyk, E.; Spiechowicz, E. Plaque accumulation on crowns made of various materials. Int. J. Prosthodont. 1990, 3, 285–291. [Google Scholar] [PubMed]
- Addy, M.; Bates, J.F. Plaque accumulation following the wearing of different types of removable partial dentures. J. Oral Rehabil. 1979, 6, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.; Weber, H. Plaque retention on teeth restored with full-ceramic crowns: A comparative study. J. Prosthet. Dent. 1986, 56, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Jameson, L.M.; Malone, W.F. Crown contours and gingival response. J. Prosthet. Dent. 1982, 47, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, C.F. Excessive crown contours facilitate endemic plaque niches. J. Prosthet. Dent. 1976, 35, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Bollen, C.M.L.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Subramani, K.; Jung, R.E.; Molenberg, A.; Hammerle, C.H. Biofilm on dental implants: A review of the literature. Int. J. Oral Maxillofac. Implants 2009, 24, 616–626. [Google Scholar] [PubMed]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implants Res. 2006, 17, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Lyngstadaas, S.P.; Ellingsen, J.E.; Taxt-Lamolle, S.; Haugen, H.J. The influence of surface nanoroughness, texture and chemistry of tizr implant abutment on oral biofilm accumulation. Clin. Oral Implants Res. 2015, 26, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Aykent, F.; Yondem, I.; Ozyesil, A.G.; Gunal, S.K.; Avunduk, M.C.; Ozkan, S. Effect of different finishing techniques for restorative materials on surface roughness and bacterial adhesion. J. Prosthet. Dent. 2010, 103, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Vyas, V.; Patil, R.; Sharma, V.; Scopelliti, P.E.; Bongiorno, G.; Podestà, A.; Lenardi, C.; Gade, W.N.; Milani, P. Quantitative characterization of the influence of the nanoscale morphology of nanostructured surfaces on bacterial adhesion and biofilm formation. PLoS One 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, R.; Powers, J. Craig’s Restorative Dental Materials, 13th ed.; Elsevier: Philadelphia, PA, USA, 2012. [Google Scholar]
- Auschill, T.M.; Arweiler, N.B.; Brecx, M.; Reich, E.; Sculean, A.; Netuschil, L. The effect of dental restorative materials on dental biofilm. Eur. J. Oral Sci. 2002, 110, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Gotfredsen, K.; Carlsson, G.E.; Jokstad, A.; Arvidson Fyrberg, K.; Berge, M.; Bergendal, B.; Bergendal, T.; Ellingsen, J.E.; Gunne, J.; Hofgren, M.; et al. Implants and/or teeth: Consensus statements and recommendations. J. Oral Rehabil. 2008, 35, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Felton, D.A.; Kanoy, B.E.; Bayne, S.C.; Wirthman, G.P. Effect of in vivo crown margin discrepancies on periodontal health. J. Prosthet. Dent. 1991, 65, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Jansen, V.K.; Conrads, G.; Richter, E.J. Microbial leakage and marginal fit of the implant-abutment interface. Int. J. Oral. Maxillofac. Implants 1997, 12, 527–540. [Google Scholar] [PubMed]
- Ong, C.T.; Ivanovski, S.; Needleman, I.G.; Retzepi, M.; Moles, D.R.; Tonetti, M.S.; Donos, N. Systematic review of implant outcomes in treated periodontitis subjects. J. Clin. Periodontol. 2008, 35, 438–462. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.; Lang, N.P. Comparative biology of chronic and aggressive periodontitis vs. Peri-implantitis. Periodontology 2000, 53, 167–181. [Google Scholar] [CrossRef]
- Boscato, N.; Radavelli, A.; Faccio, D.; Loguercio, A.D. Biofilm formation of candida albicans on the surface of a soft denture-lining material. Gerodontology 2009, 26, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Rocha, E.P.; Luvizuto, E.R.; Sabotto, S.F. Biofilm formation and caries incidence with removable partial dentures. Dent. Today 2008, 27, 60–62. [Google Scholar] [PubMed]
- Zlataric, D.K.; Celebic, A.; Valentic-Peruzovic, M. The effect of removable partial dentures on periodontal health of abutment and non-abutment teeth. J. Periodontol. 2002, 73, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Radford, D.R.; Challacombe, S.J.; Walter, J.D. Denture plaque and adherence of candida albicans to denture-base materials in vivo and in vitro. Crit. Rev. Oral Biol. Med. 1999, 10, 99–116. [Google Scholar] [CrossRef] [PubMed]
- McHenry, K.R.; Johansson, O.E.; Christersson, L.A. The effect of removable partial denture framework design on gingival inflammation: A clinical model. J. Prosthet. Dent. 1992, 68, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.S.; Marchini, L.; Bernardes, L.A.; Paulino, L.C.; Nobrega, F.G. Biofilm microbial communities of denture stomatitis. Oral Microbiol. Immunol. 2008, 23, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Cenci, T.; Del Bel Cury, A.A.; Crielaard, W.; Ten Cate, J.M. Development of candida-associated denture stomatitis: New insights. J. Appl. Oral Sci. 2008, 16, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Tomsett, K.; Wickes, B.L.; Lopez-Ribot, J.L.; Redding, S.W. Denture stomatitis: A role for candida biofilms. Oral Surg. Oral Med .Oral Pathol. Oral Radiol. Endod. 2004, 98, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Redding, S.; Bhatt, B.; Rawls, H.R.; Siegel, G.; Scott, K.; Lopez-Ribot, J. Inhibition of candida albicans biofilm formation on denture material. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Shenoy, V.; Shetty, T. Resilient liners: A review. J. Indian Prosthodont. Soc. 2013, 13, 155–164. [Google Scholar] [PubMed]
- Nikawa, H.; Jin, C.; Makihira, S.; Egusa, H.; Hamada, T.; Kumagai, H. Biofilm formation of candida albicans on the surfaces of deteriorated soft denture lining materials caused by denture cleansers in vitro. J. Oral Rehabil. 2003, 30, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Cenci, T.; da Silva, W.J.; Cenci, M.S.; Cury, A.A. Temporal changes of denture plaque microbiologic composition evaluated in situ. Int. J. Prosthodont. 2010, 23, 239–242. [Google Scholar] [PubMed]
- Mutluay, M.M.; Oguz, S.; Floystrand, F.; Saxegaard, E.; Dogan, A.; Bek, B.; Ruyter, I.E. A prospective study on the clinical performance of polysiloxane soft liners: One-year results. Dent. Mater. J. 2008, 27, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, I.; Takahashi, Y.; Shimizu, H. Mechanical properties of injection-molded thermoplastic denture base resins. Acta Odontol. Scand. 2011, 69, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Sarrett, D.C. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dent. Mater. 2005, 21, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, A.; Buchalla, W.; Attin, T. Review on fluoride-releasing restorative materials—fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2007, 23, 343–362. [Google Scholar]
- Wang, Z.; Shen, Y.; Haapasalo, M. Dental materials with antibiofilm properties. Dent. Mater. 2014, 30. [Google Scholar] [CrossRef]
- Beyth, N.; Farah, S.; Domb, A.J.; Weiss, E.I. Antibacterial dental resin composites. React. Funct. Polym. 2014, 75, 81–88. [Google Scholar] [CrossRef]
- Weng, Y.; Howard, L.; Guo, X.; Chong, V.; Gregory, R.; Xie, D. A novel antibacterial resin composite for improved dental restoratives. J. Mater. Sci. Mater. Med. 2012, 23, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Weir, M.D.; Xu, H.H.K.; Kraigsley, A.M.; Lin, N.J.; Lin-Gibson, S.; Zhou, X. Antibacterial and physical properties of calcium–phosphate and calcium–fluoride nanocomposites with chlorhexidine. Dent. Mater. 2012, 28, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Kasraei, S.; Sami, L.; Hendi, S.; AliKhani, M.-Y.; Rezaei-Soufi, L.; Khamverdi, Z. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on streptococcus mutans and lactobacillus. Restor Dent Endod 2014, 39, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Elsaka, S.E. Antibacterial activity and adhesive properties of a chitosan-containing dental adhesive. Quintessence Int. 2012, 43, 603–613. [Google Scholar] [PubMed]
- Polydorou, O.; Rogatti, P.; Bolek, R.; Wolkewitz, M.; Kümmerer, K.; Hellwig, E. Elution of monomers from three different bonding systems and their antibacterial effect. Odontology 2013, 101, 1–7. [Google Scholar] [CrossRef]
- Pizzo, G.; Giammanco, G.M.; Cumbo, E.; Nicolosi, G.; Gallina, G. In vitro antibacterial activity of endodontic sealers. J. Dent. 2006, 34, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Wataha, J.C. Predicting clinical biological responses to dental materials. Dent. Mater. 2012, 28, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Gnanadhas, D.P.; Marathe, S.A.; Chakravortty, D. Biocides—resistance, cross-resistance mechanisms and assessment. Expert Opin. Investig. Drugs 2013, 22, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; AlSalhi, M.S.; Siddiqui, M.K.J. Silver nanoparticle applications and human health. Clin. Chim. Acta 2010, 411, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Øilo, M.; Bakken, V. Biofilm and Dental Biomaterials. Materials 2015, 8, 2887-2900. https://doi.org/10.3390/ma8062887
Øilo M, Bakken V. Biofilm and Dental Biomaterials. Materials. 2015; 8(6):2887-2900. https://doi.org/10.3390/ma8062887
Chicago/Turabian StyleØilo, Marit, and Vidar Bakken. 2015. "Biofilm and Dental Biomaterials" Materials 8, no. 6: 2887-2900. https://doi.org/10.3390/ma8062887