One-Step Formation of WO3-Loaded TiO2 Nanotubes Composite Film for High Photocatalytic Performance
Abstract
:1. Introduction
2. Results and Discussion
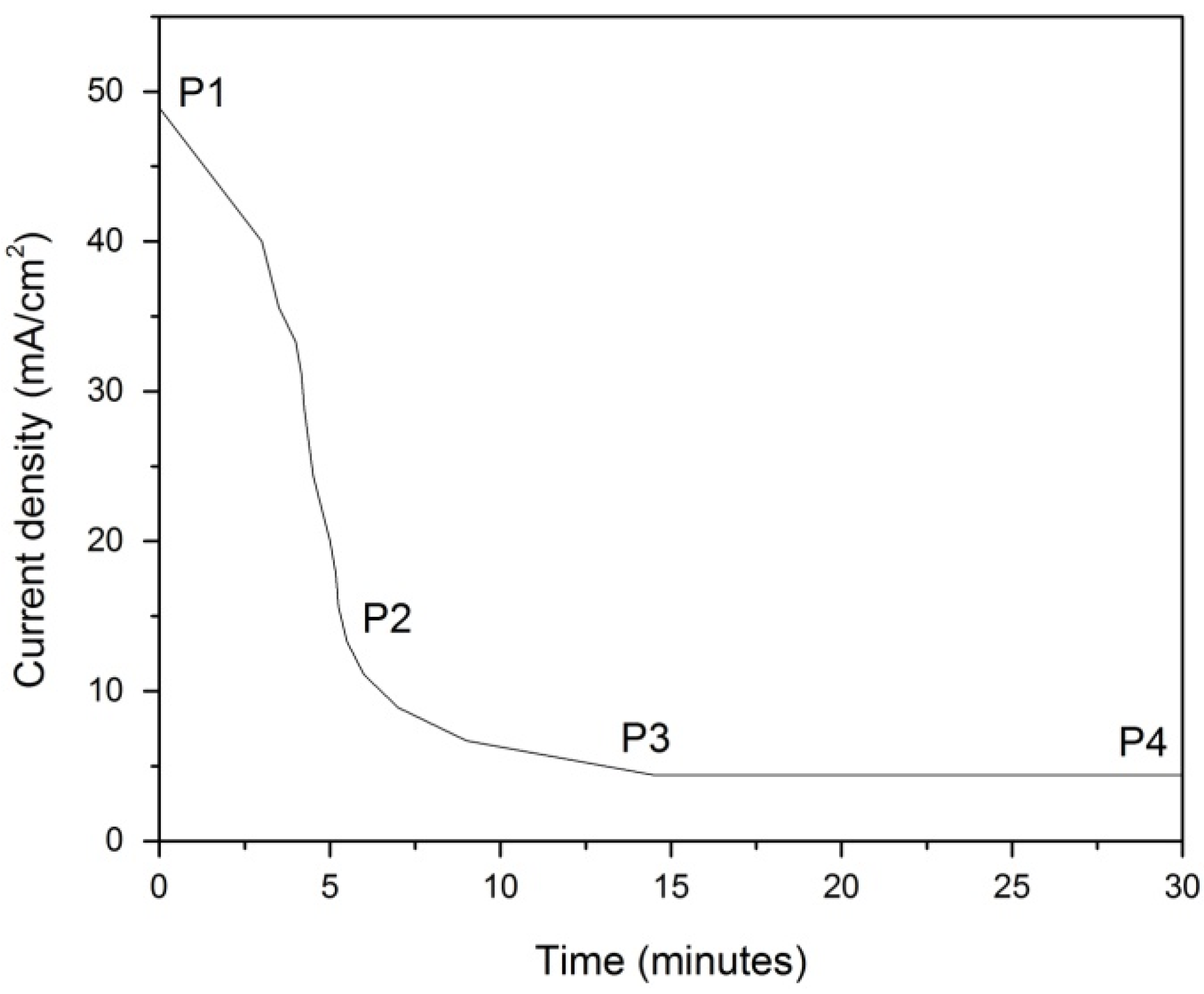
2.1. Transient and Steady State Current Density Analysis

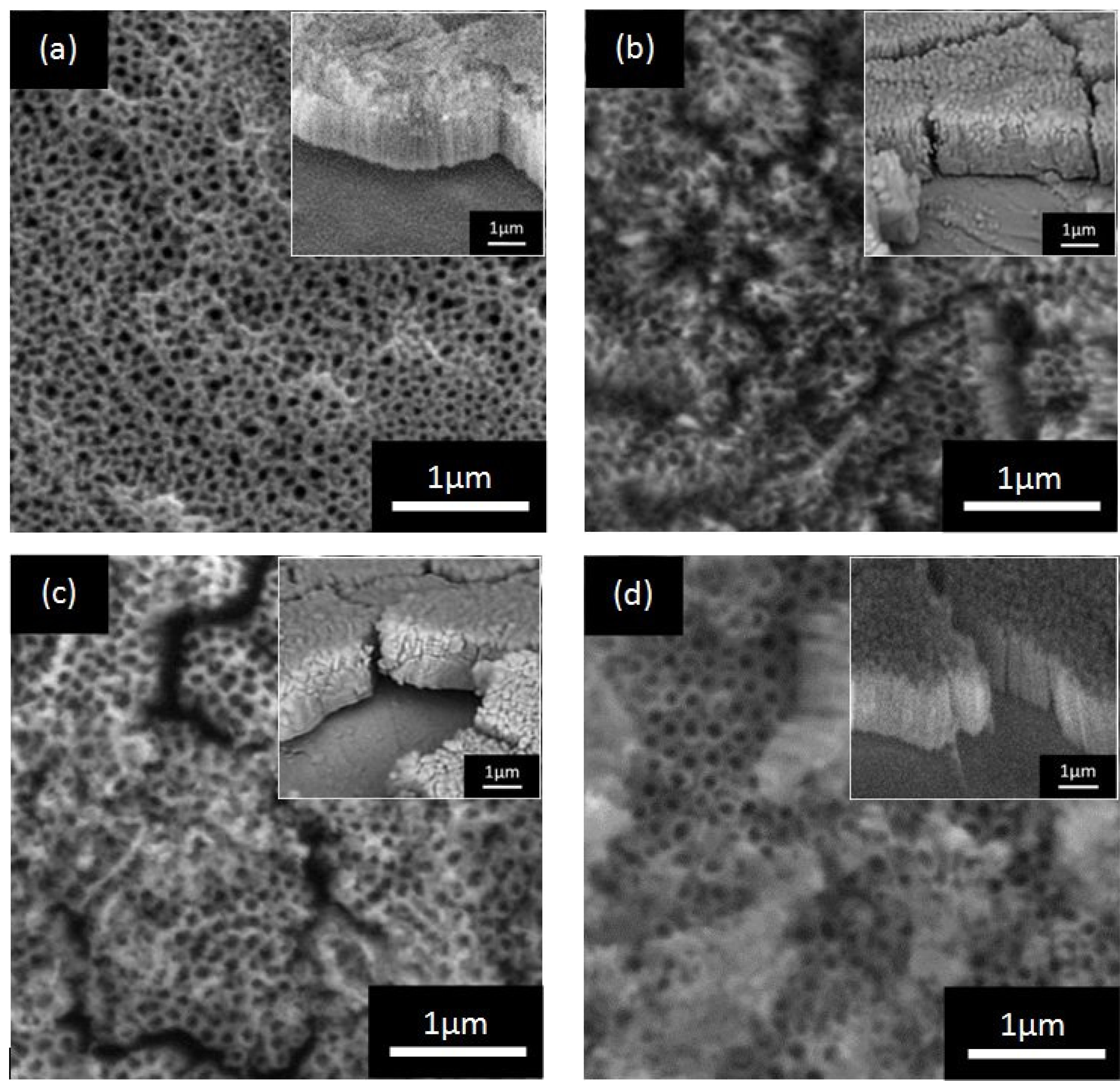
2.2. Morphological Studies and Elemental Analysis
| Voltage (V) | Diameter (nm) | Length (µm) | Wall Thickness (nm) | AR | G |
|---|---|---|---|---|---|
| 10 | 47 | 0.9 | 13 | 12.33 | 74.52 |
| 20 | 56 | 1.2 | 15 | 13.95 | 84.58 |
| 30 | 65 | 1.4 | 17 | 14.14 | 85.98 |
| 40 | 74 | 1.6 | 18 | 14.55 | 89.26 |
| Samples | Atomic % | |||
|---|---|---|---|---|
| Ti | O | W | C | |
| 10 V | 43.81 | 51.79 | 1.06 | 3.34 |
| 20 V | 48.16 | 46.95 | 1.36 | 3.53 |
| 30 V | 59.22 | 31.83 | 2.01 | 6.94 |
| 40 V | 47.38 | 44.11 | 3.29 | 5.22 |
2.3. Phase Structure Analysis

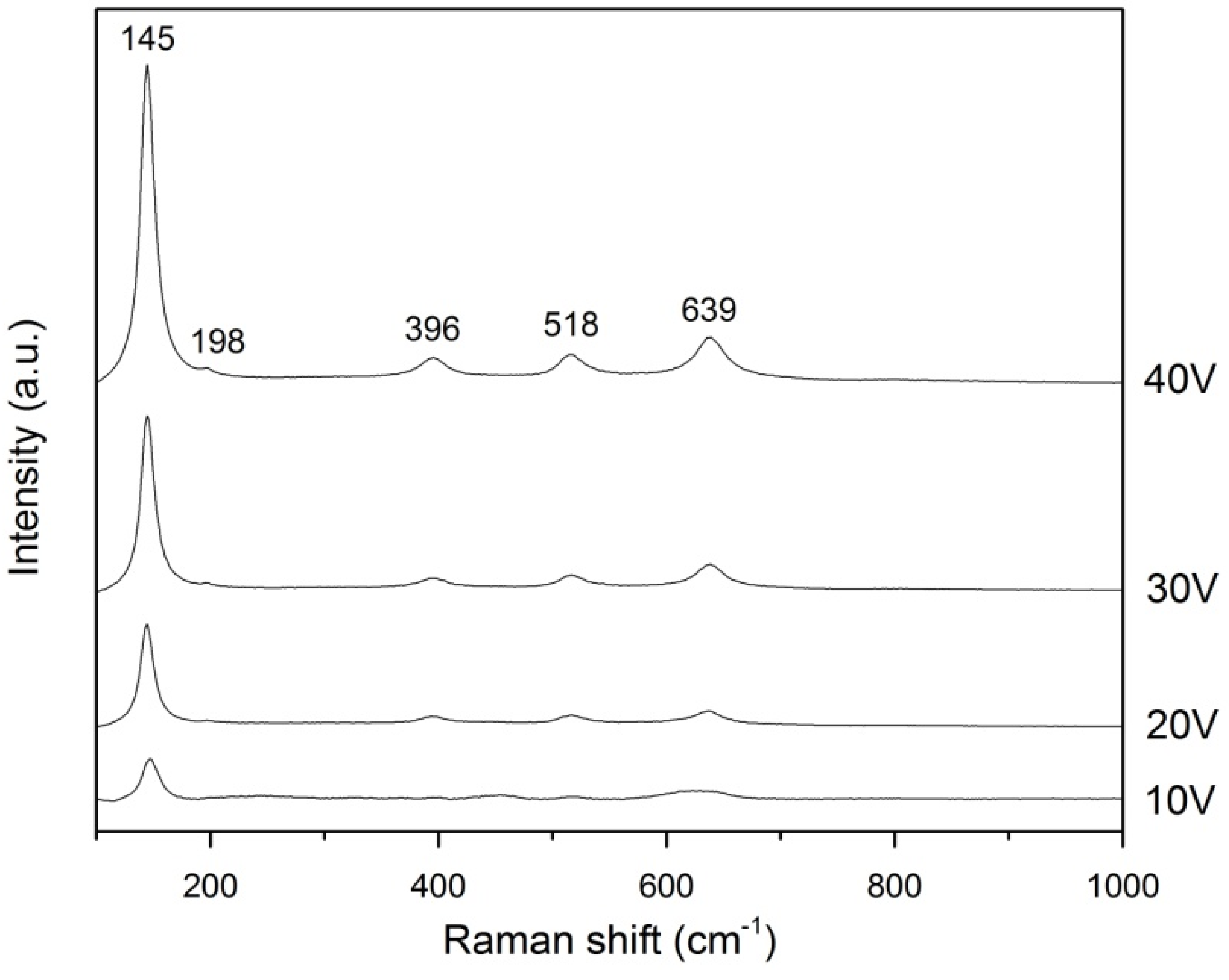
2.4. Raman Analysis
2.5. Photocatalytic Activity
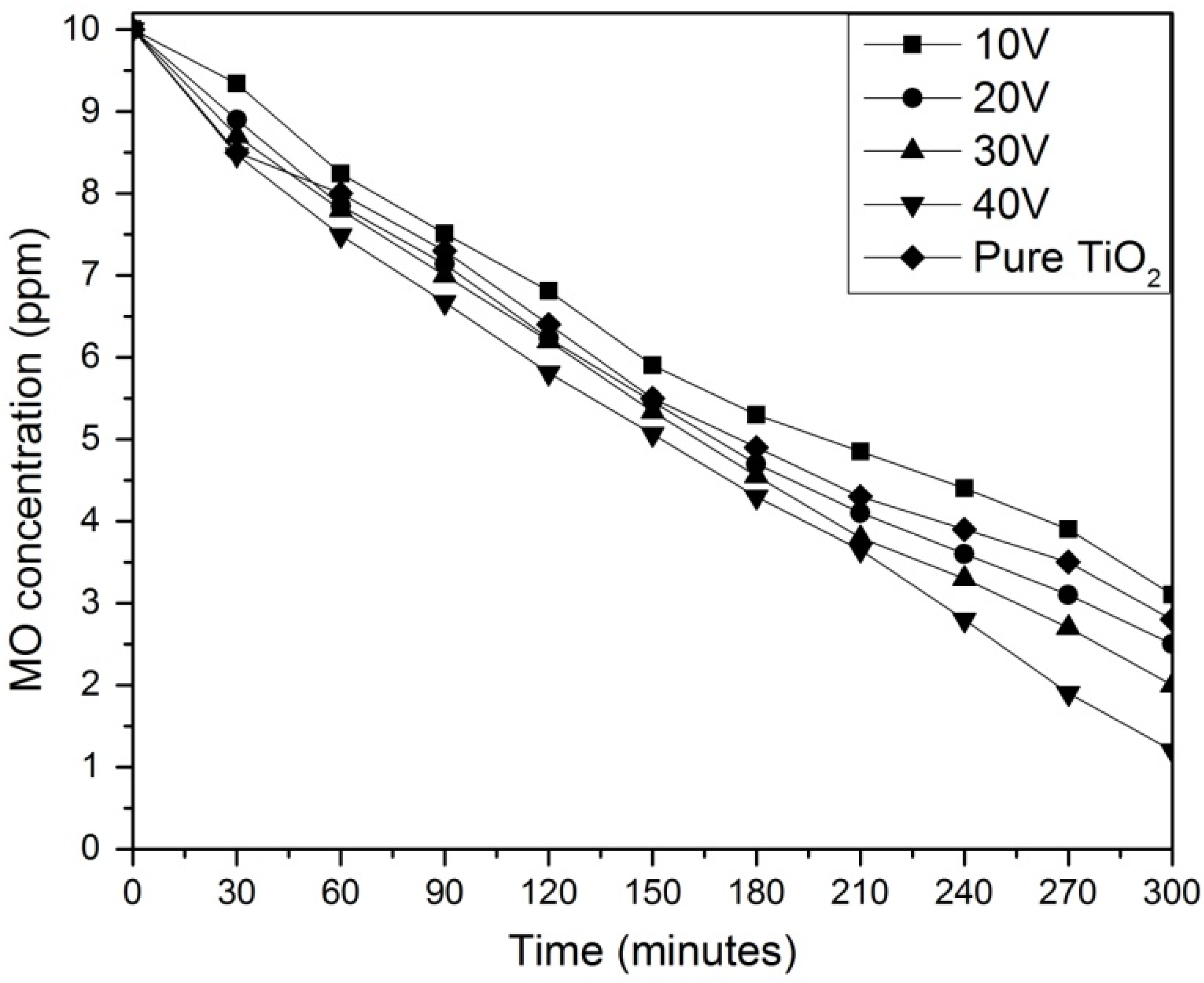
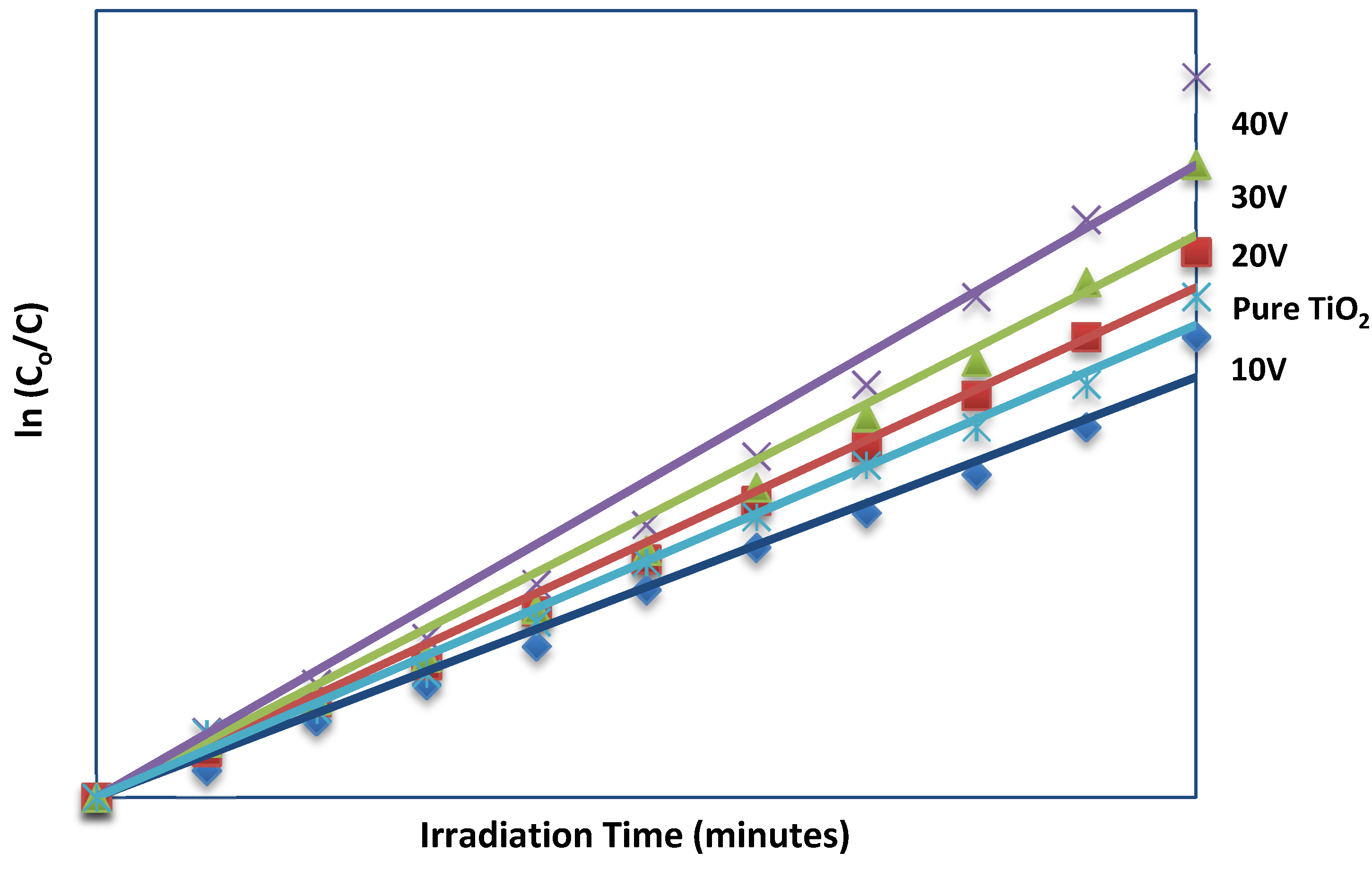
| Samples | Rate Constant (k) | R2 |
|---|---|---|
| 10 V | 0.0036 | 0.9874 |
| 20 V | 0.0043 | 0.9920 |
| 30 V | 0.0048 | 0.9759 |
| 40 V | 0.0054 | 0.9715 |
| Pure TiO2 | 0.0040 | 0.9927 |

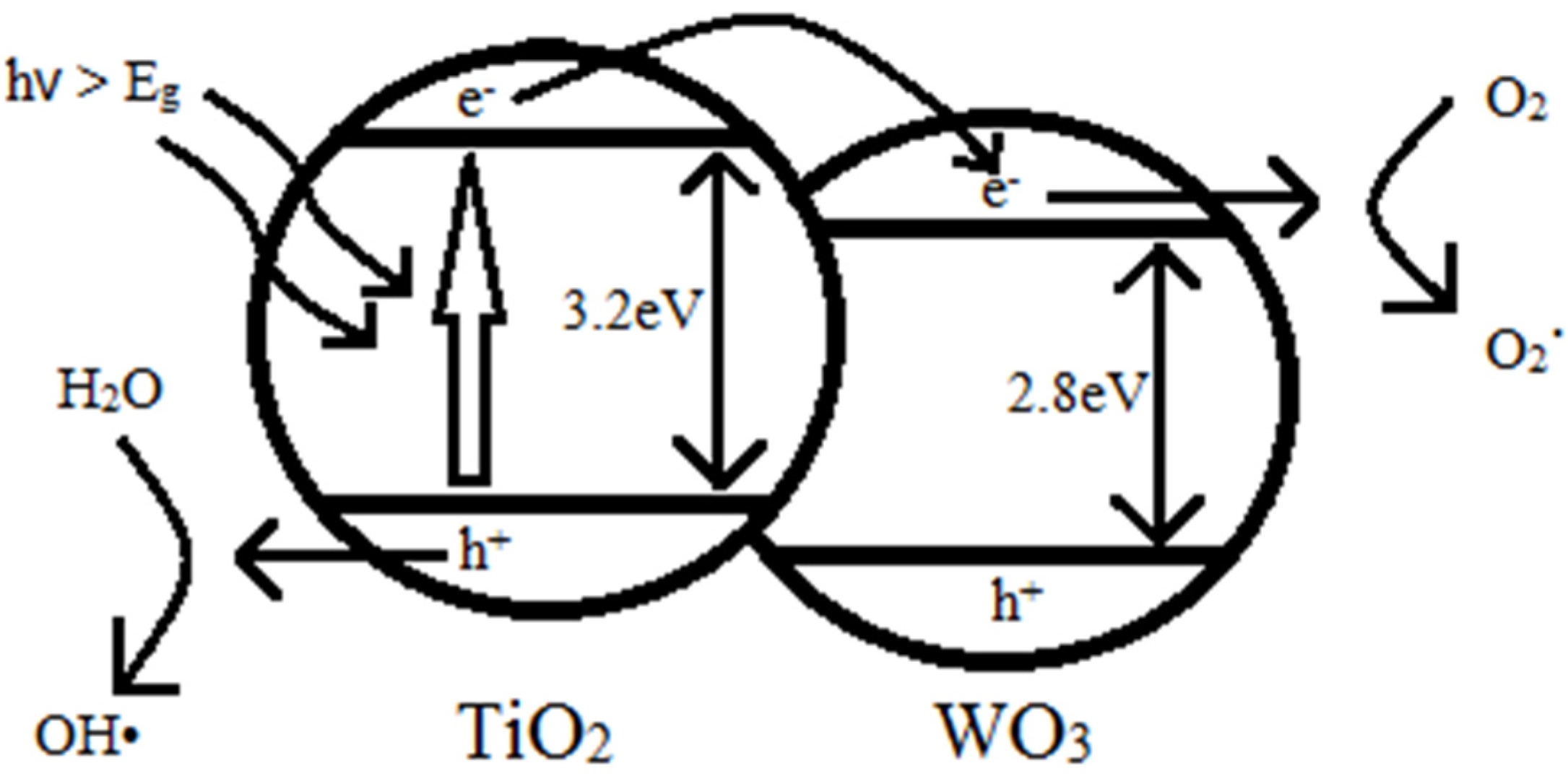
2.6. Optical Properties Analysis

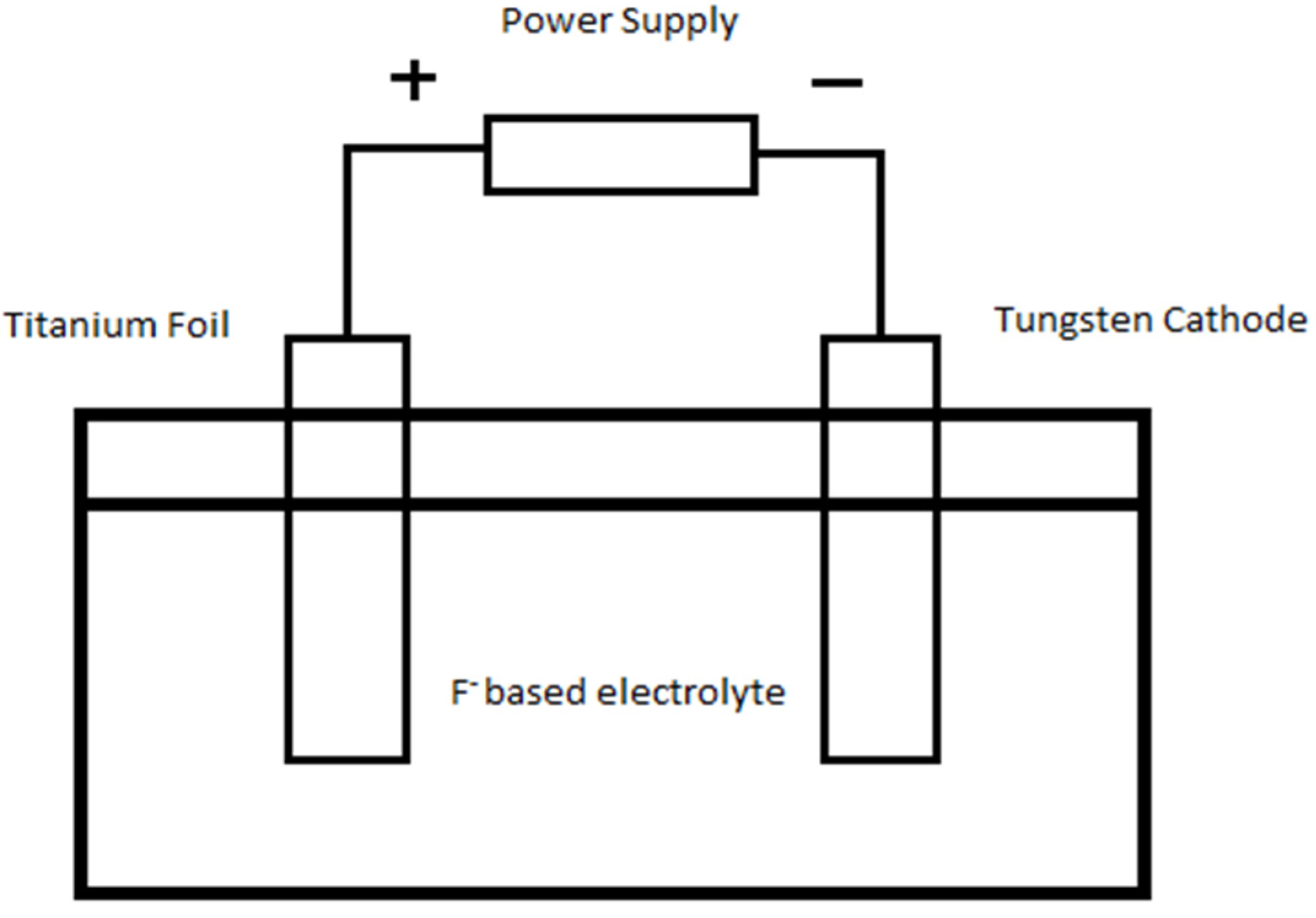
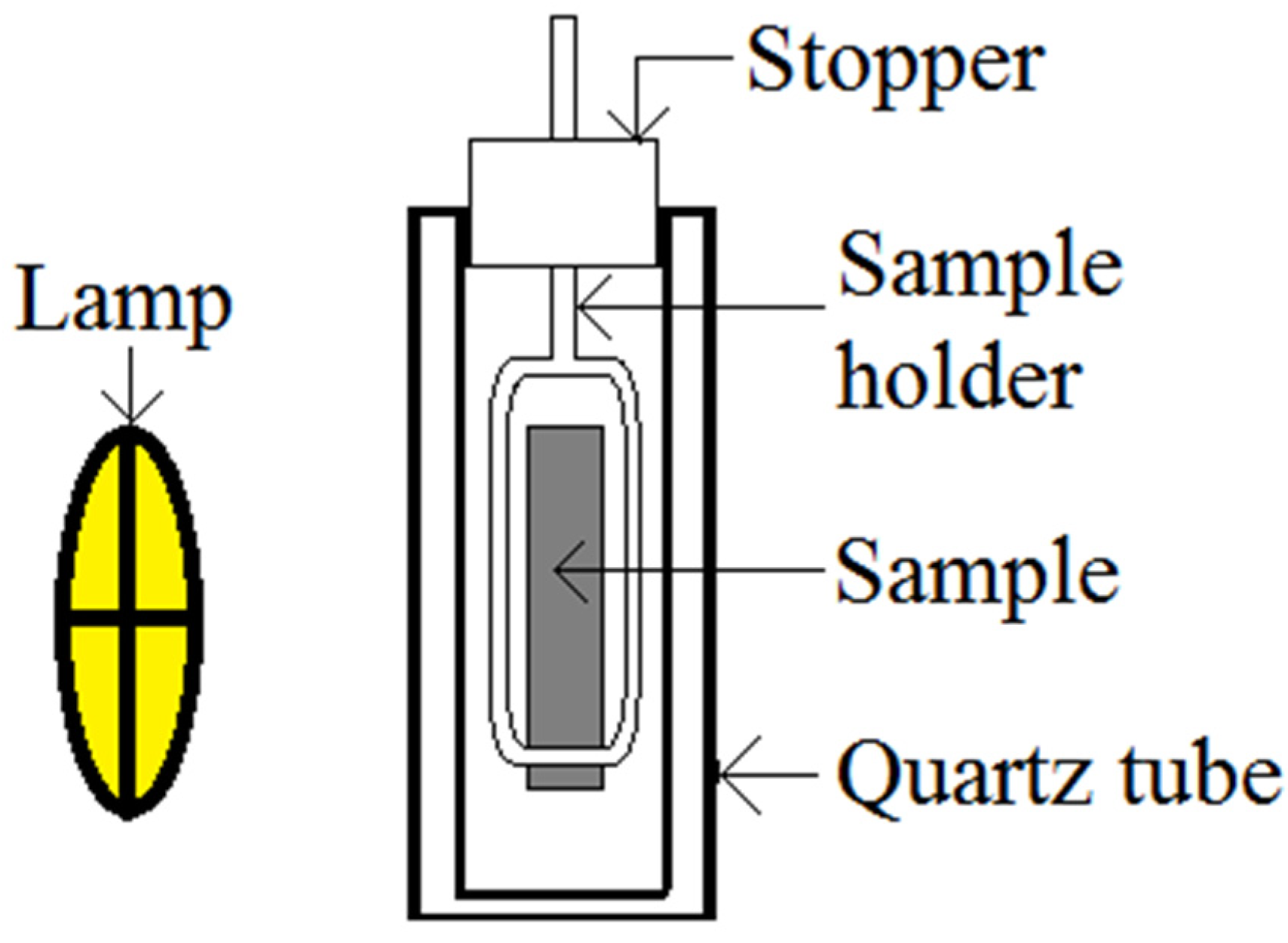
3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Olukanni, O.D.; Osuntoki, A.A.; Gbenle, G.O. Textile effluent biodegradation potentials of textile effluent-adapted and non-adapted bacteria. Afr. J. Biotechnol. 2006, 5, 1980–1984. [Google Scholar]
- Palamthodi, S.; Patil, D.; Patil, Y. Microbial degradation of textile industrial effluents. Afr. J. Biotechnol. 2013, 10, 12657–12661. [Google Scholar]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef]
- Mohapatra, S.; Misra, M.; Mahajan, V.K.; Raja, K.S. A novel method for the synthesis of titania nanotubes using sonoelectrochemical method and its application for photoelectrochemical splitting of water. J. Catal. 2007, 246, 362–369. [Google Scholar] [CrossRef]
- Liao, J.Y.; Lei, B.X.; Wang, Y.F.; Liu, J.M.; Su, C.Y.; Kuang, D.B. Hydrothermal fabrication of quasi-one-dimensional single-crystalline anatase TiO2 nanostructures on FTO glass and their applications in dye-sensitized solar cells. Chem.-A Eur. J. 2011, 17, 1352–1357. [Google Scholar] [CrossRef]
- Kitano, M.; Matsuoka, M.; Ueshima, M.; Anpo, M. Recent developments in titanium oxide-based photocatalysts. Appl. Catal. A: Gen. 2007, 325, 1–14. [Google Scholar] [CrossRef]
- Higashimoto, S.; Ushiroda, Y.; Azuma, M. Electrochemically assisted photocatalysis of hybrid WO3/TiO2 films: Effect of the WO3 structures on charge separation behavior. Top. Catal. 2008, 47, 148–154. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Nishimoto, S.; Jin, M.; Tryk, D.A.; Murakami, T.; Fujishima, A. Highly ordered TiO2 nanotube arrays with controllable length for photoelectrocatalytic degradation of phenol. J. Phys. Chem. C 2008, 112, 253–259. [Google Scholar] [CrossRef]
- Lai, Y.; Sun, L.; Chen, Y.; Zhuang, H.; Lin, C.; Chin, J.W. Effects of the structure of TiO2 nanotube array on Ti substrate on its photocatalytic activity. J. Electrochem. Soc. 2006, 153, D123–D127. [Google Scholar] [CrossRef]
- Mohamed, A.E.R.; Rohani, S. Synthesis of Titania nanotube arrays by anodization. AIDIC Conf. Ser. 2009, 9, 121–129. [Google Scholar]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.R.; Kamat, P.V. Disassembly, reassembly, and photoelectrochemistry of etched TiO2 nanotubes. J. Phys. Chem. C 2009, 113, 17967–17972. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Z. Anodic formation of ordered TiO2 nanotube arrays: Effects of electrolyte temperature and anodization potential. J. Phys. Chem. C 2009, 113, 4026–4030. [Google Scholar] [CrossRef]
- Kang, S.H.; Kim, J.Y.; Kim, H.S.; Sung, Y.E. Formation and mechanistic study of self-ordered TiO2 nanotubes on Ti substrate. J. Ind. Eng. Chem. 2008, 14, 52–59. [Google Scholar] [CrossRef]
- Raja, K.S.; Gandhi, T.; Misra, M. Effect of water content of ethylene glycol as electrolyte for synthesis of ordered titania nanotubes. Electrochem. Commun. 2007, 9, 1069–1076. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 nanotubes: Self-Organized electrochemical formation, properties and applications. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar]
- Minagar, S.; Berndt, C.C.; Wang, J.; Ivanova, E.; Wen, C. A review of the application of anodization for the fabrication of nanotubes on metal implant surfaces. Acta Biomater. 2012, 8, 2875–2888. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.W.; Sreekantan, S. Effect of applied potential on the formation of self-organized TiO2 nanotube arrays and its photoelectrochemical response. J. Nanomater. 2011, 2011. [Google Scholar] [CrossRef]
- Song, Y.Y.; Gao, Z.D.; Wang, J.H.; Xia, X.H.; Lynch, R. Multistage coloring electrochromic device based on TiO2 nanotube arrays modified with WO3 nanoparticles. Adv. Funct. Mater. 2011, 21, 1941–1946. [Google Scholar] [CrossRef]
- Grimes, C.A. Synthesis and application of highly ordered arrays of TiO2 nanotubes. J. Mater. Chem. 2007, 17, 1451–1457. [Google Scholar] [CrossRef]
- Paulose, M.; Shankar, K.; Yoriya, S.; Prakasam, H.E.; Varghese, O.K.; Mor, G.K.; Latempa, T.A.; Fitzgerald, A.; Grimes, C.A. Anodic growth of highly ordered TiO2 nanotube arrays to 134 μm in length. J. Phys. Chem. B 2006, 110, 16179–16184. [Google Scholar] [CrossRef]
- Crawford, G.; Chawla, N. Porous hierarchical TiO2 nanostructures: Processing and microstructure relationships. Acta Mater. 2009, 57, 854–867. [Google Scholar] [CrossRef]
- Issaq, H.J.; Atamna, I.Z.; Muschik, G.M.; Janini, G.M. The effect of electric field strength, buffer type and concentration on separation parameters in capillary zone electrophoresis. Chromatographia 1991, 32, 155–161. [Google Scholar] [CrossRef]
- Park, I.S.; Woo, T.G.; Lee, M.H.; Ahn, S.G.; Park, M.S.; Bae, T.S.; Seol, K.W. Effects of anodizing voltage on the anodized and hydrothermally treated titanium surface. Metals Mater. Int. 2006, 12, 505–511. [Google Scholar] [CrossRef]
- Lai, C.W.; Sreekantan, S.; Krengvirat, W.; Pei San, E. Preparation and photoelectrochemical characterization of WO3-loaded TiO2 nanotube arrays via radio frequency sputtering. Electrochem. Acta 2012, 77, 128–136. [Google Scholar] [CrossRef]
- Leghari, S.A.K.; Sajjad, S.; Chen, F.; Zhang, J. WO3/TiO2 composite with morphology change via hydrothermal template-free route as an efficient visible light photocatalyst. Chem. Eng. J. 2011, 166, 906–915. [Google Scholar] [CrossRef]
- Park, S.E.; Joo, H.; Kang, J.W. Effect of impurities in TiO2 thin films on trichloroethylene conversion. Solar Energy Mater. Solar Cells 2004, 83, 39–53. [Google Scholar] [CrossRef]
- Chafik, T.; Efstathiou, A.M.; Verykios, X.E. Effects of W6+ doping of TiO2 on the reactivity of supported Rh toward NO: Transient FTIR and mass spectroscopy studies. J. Phys. Chem. B 1997, 101, 7968–7977. [Google Scholar] [CrossRef]
- Song, H.Y.; Jiang, H.F.; Liu, X.Q.; Jiang, Y.Z.; Meng, G.Y. Preparation of WOx-TiO2 and the Photocatalytic Activity under Visible Irradiation. Key Eng. Mater. 2007, 336, 1979–1982. [Google Scholar] [CrossRef]
- Lai, C.W.; Sreekantan, S. Effect of heat treatment on WO3-loaded TiO2 nanotubes for hydrogen generation via enhanced water splitting. Mater. Sci. Semicond. Process. 2013, 16, 947–954. [Google Scholar] [CrossRef]
- MansoobáKhan, M.; OmaisháAnsari, M.; HungáHan, D.; HwanáCho, M. Band gap engineered TiO2 nanoparticles for visible light induced photoelectrochemical and photocatalytic studies. J. Mater. Chem. A 2014, 2, 637–644. [Google Scholar]
- Serpone, N.; Maruthamuthu, P.; Pichat, P.; Pelizzetti, E.; Hidaka, H. Exploiting the interparticle electron transfer process in the photocatalysed oxidation of phenol, 2-chlorophenol and pentachlorophenol: Chemical evidence for electron and hole transfer between coupled semiconductors. J. Photochem. Photobiol. A: Chem. 1995, 85, 247–255. [Google Scholar] [CrossRef]
- Yu, C.; Jimmy, C.Y.; Zhou, W.; Yang, K. WO3 coupled P-TiO2 photocatalysts with mesoporous structure. Catal. Lett. 2010, 140, 172–183. [Google Scholar] [CrossRef]
- Lai, C.W.; Sreekantan, S. Single step formation of C-TiO2 nanotubes: Influence of applied voltage and their photocatalytic activity under solar illumination. Int. J. Photoenergy 2013, 2013. [Google Scholar] [CrossRef]
- Lai, C.W.; Sreekantan, S. Optimized sputtering power to incorporate WO3 into C-TiO2 nanotubes for highly visible photoresponse performance. Nano 2012, 7. [Google Scholar] [CrossRef]
- Lai, C.W.; Sreekantan, S. Study of WO3 incorporated C-TiO2 nanotubes for efficient visible light driven water splitting performance. J. Alloys Compounds 2013, 547, 43–50. [Google Scholar] [CrossRef]
- Sreekantan, S.; Lai, C.W.; Lockman, Z. Extremely fast growth rate of TiO2 nanotube arrays in electrochemical bath containing H2O2. J. Electrochem. Soc. 2011, 158, C397–C402. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.H.; Lai, C.W.; Hamid, S.B.A. One-Step Formation of WO3-Loaded TiO2 Nanotubes Composite Film for High Photocatalytic Performance. Materials 2015, 8, 2139-2153. https://doi.org/10.3390/ma8052139
Lee WH, Lai CW, Hamid SBA. One-Step Formation of WO3-Loaded TiO2 Nanotubes Composite Film for High Photocatalytic Performance. Materials. 2015; 8(5):2139-2153. https://doi.org/10.3390/ma8052139
Chicago/Turabian StyleLee, Wai Hong, Chin Wei Lai, and Sharifah Bee Abd Hamid. 2015. "One-Step Formation of WO3-Loaded TiO2 Nanotubes Composite Film for High Photocatalytic Performance" Materials 8, no. 5: 2139-2153. https://doi.org/10.3390/ma8052139







