Gas Permeation Properties of Soluble Aromatic Polyimides Based on 4-Fluoro-4,4'-Diaminotriphenylmethane
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Polymer Synthesis
2.3. Measurements
2.4. Preparation of Dense Membranes
2.5. Gas Permeability Measurements
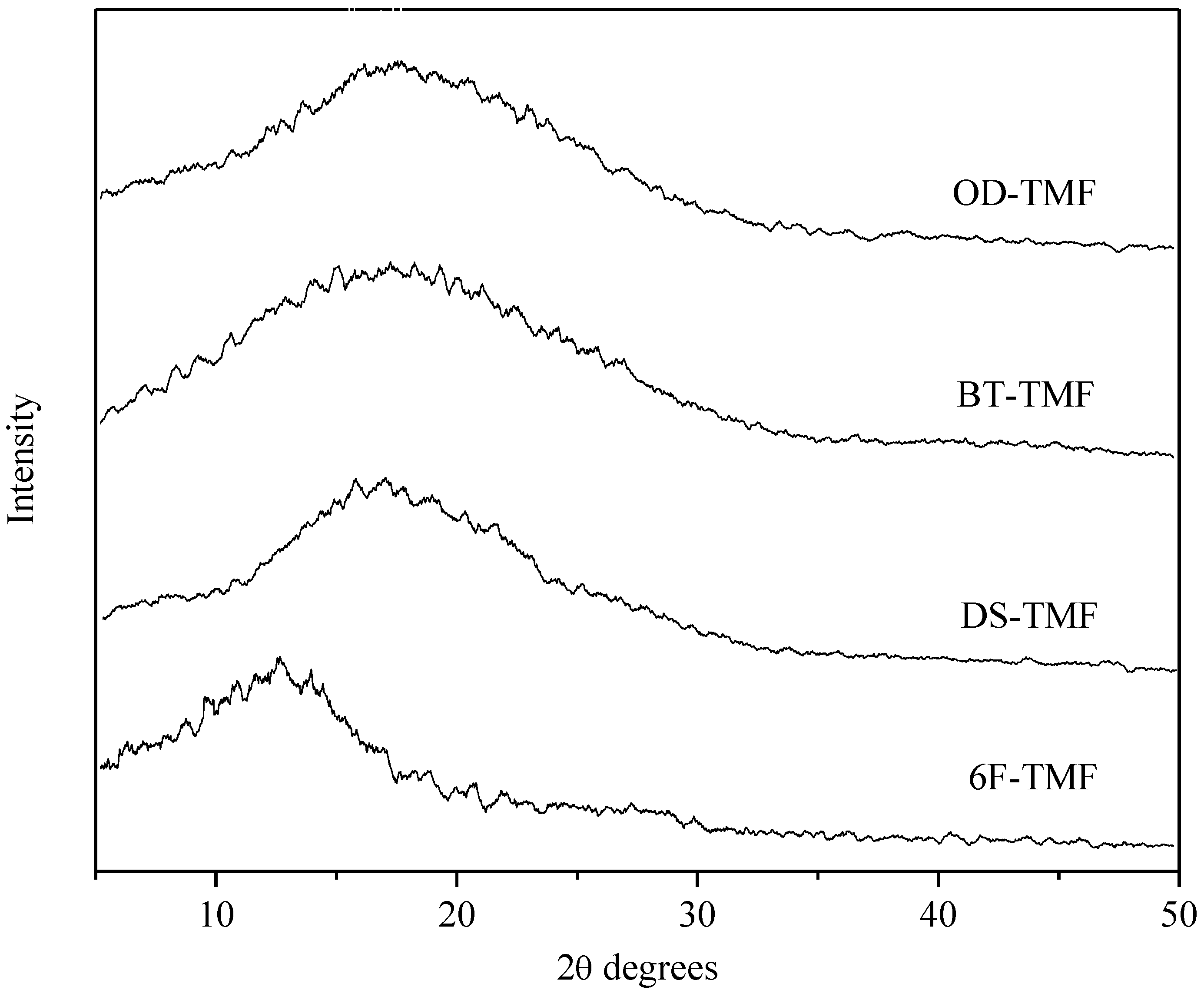
3. Results and Discussion

| Polymer | Solvent | |||||||
|---|---|---|---|---|---|---|---|---|
| CHCl3 | DMF | DMSO | NMP | Nitro-Bz | m-Cresol | DMAc | THF | |
| OD-TMF | ± | + | + | + | + | + | + | − |
| BT-TMF | ± | + | + | + | + | + | + | − |
| DS-TMF | ± | + | + | + | + | + | + | − |
| 6F-TMF | + | + | + | + | + | + | + | ± |

| Polyimide | 5% weight loss, °C | Residual weight at 600 °C, % | ηinh, dL/g | Tg, °C | Density, g/cm3 | d-Spacing, Å | FFV |
|---|---|---|---|---|---|---|---|
| OD-TMF | 517 | 68 | 1.2 | 285 | 1.328 | 4.8 | 0.173 |
| BT-TMF | 487 | 70 | 1.3 | 290 | 1.331 | 5.0 | 0.178 |
| DS-TMF | 472 | 69 | 1.0 | 316 | 1.354 | 5.2 | 0.184 |
| 6F-TMF | 503 | 72 | 1.2 | 297 | 1.379 | 6.8 | 0.205 |
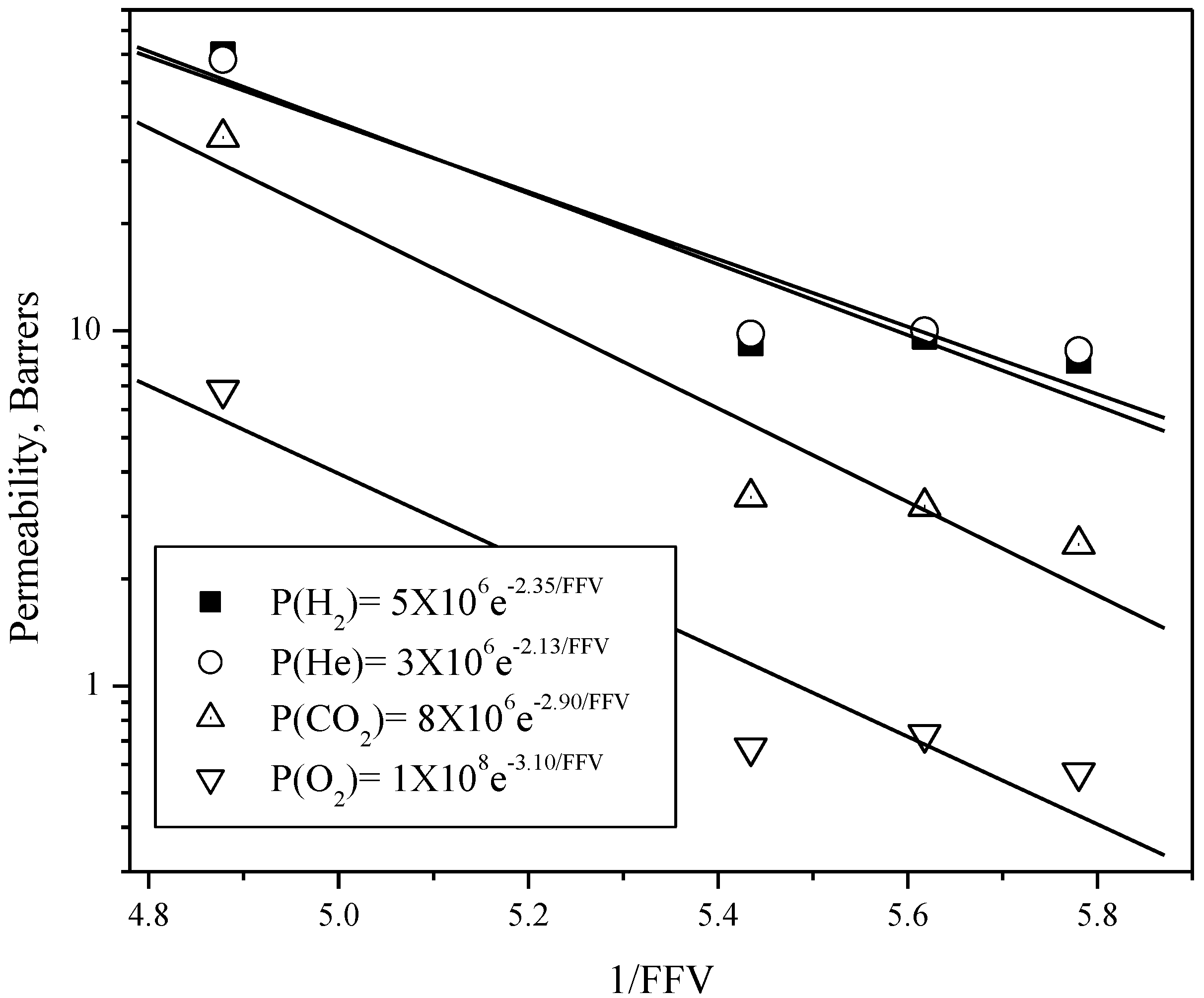
| Polyimide | H2 | He | Permeability *, P(A) | Ideal selectivity, P(A)/P(B) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| O2 | CO2 | N2 | CH4 | H2/CH4 | He/N2 | O2/N2 | CO2/CH4 | |||
| OD-TMF | 8.2 | 8.8 | 0.57 | 2.5 | 0.10 | 0.10 | 85 | 86 | 5.5 | 26 |
| BT-TMF | 9.6 | 10 | 0.73 | 3.2 | 0.14 | 0.12 | 78 | 72 | 5.2 | 26 |
| DS-TMF | 9.2 | 9.8 | 0.67 | 3.4 | 0.12 | 0.13 | 86 | 82 | 5.6 | 31 |
| 6F-TMF | 60 | 58 | 6.8 | 35 | 1.30 | 0.85 | 70 | 45 | 5.3 | 41 |
| Polyimide | Diffusivity × 108, cm2/s | Diffusivity selectivity | ||||
|---|---|---|---|---|---|---|
| D(N2) | D(O2) | D(CH4) | D(CO2) | D(O2)/D(N2) | D(CO2)/D(CH4) | |
| OD-TMF | 0.3 | 1.3 | 0.04 | 0.23 | 5.2 | 5.3 |
| BT-TMF | 0.4 | 1.1 | 0.05 | 0.22 | 2.4 | 4.4 |
| DS-TMF | 0.2 | 0.9 | 0.04 | 0.21 | 5.0 | 5.9 |
| 6F-TMF | 1.2 | 4.7 | 0.19 | 1.53 | 3.6 | 7.9 |
| Polyimide | Solubility, cm3 (STP)/cm3 atm | Solubility selectivity | ||||
| S(N2) | S(O2) | S(CH4) | S(CO2) | S(O2)/S(N2) | S(CO2)/S(CH4) | |
| OD-TMF | 0.3 | 0.3 | 1.7 | 8.3 | 1.0 | 4.8 |
| BT-TMF | 0.3 | 0.5 | 1.9 | 10.9 | 2.2 | 5.8 |
| DS-TMF | 0.5 | 0.5 | 2.2 | 11.9 | 1.1 | 5.3 |
| 6F-TMF | 0.8 | 1.1 | 3.4 | 17.4 | 1.3 | 5.2 |
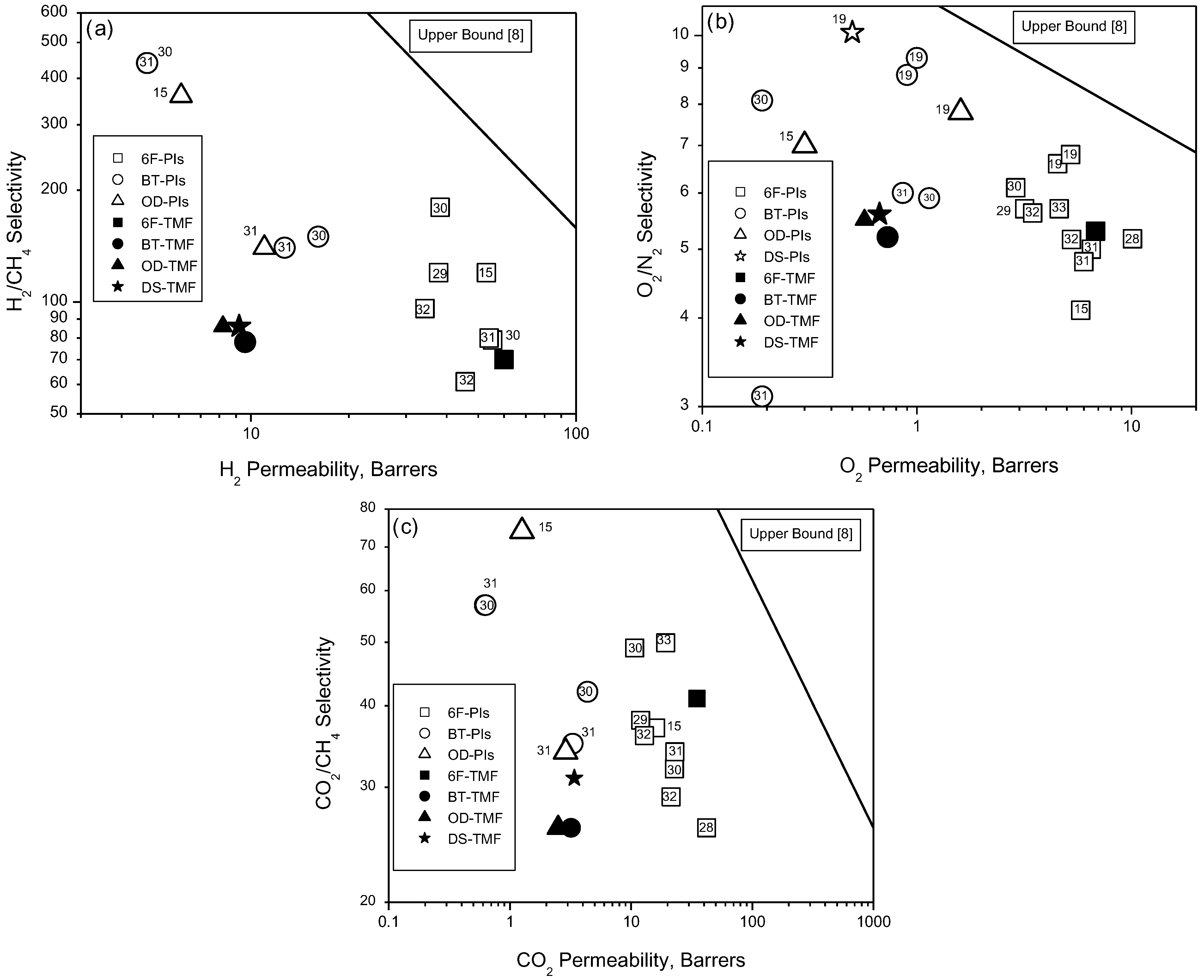
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ohya, H.; Kusdryavtsev, V.I.; Semenova, S.I. Polyimide Membranes: Application, Fabrication and Properties; Gordon and Breach Publishers: Tokyo, Japan, 1996. [Google Scholar]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane gas separation: A review/state of the art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Baker, R.W.; Lokhandwala, K. Natural gas processing with membranes: An overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- Lin, H.Q.; Freeman, B.D. Materials selection guidelines for membranes that remove CO2 from gas mixtures. J. Mol. Struct. 2005, 739, 57–74. [Google Scholar] [CrossRef]
- Stern, S.A. Polymers for gas separations—The next decade. J. Membr. Sci. 1994, 94, 1–65. [Google Scholar] [CrossRef]
- Baker, R.W. Future directions of membrane gas separation technology. Ind. Chem. Eng. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Xiao, Y.C.; Low, B.T.; Hosseini, S.S.; Chung, T.S.; Paul, D.R. The strategies of molecular architecture and modification of polyimide-based membranes for CO2 removal from natural gas—A review. Prog. Polym. Sci. 2009, 34, 561–580. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Abetz, V.; Brinkmann, T.; Dijkstra, M.; Katrin, E.; Fritsch, D.; Ohlrogge, K.; Paul, D. Developments in membrane research: From material via process design to industrial application. Adv. Eng. Mater. 2006, 8, 328–358. [Google Scholar] [CrossRef]
- Nagel, C.; Gunther-Schade, K.; Fritsch, D.; Strunskus, T.; Faupel, F. Free volume and transport properties in highly selective polymer membranes. Macromolecules 2002, 35, 2071–2077. [Google Scholar] [CrossRef]
- Budd, P.M.; McKeown, N.B.; Fritsch, D. Free volume and intrinsic microporosity in polymers. J. Mater. Chem. 2005, 15, 1977–1986. [Google Scholar] [CrossRef]
- Thran, A.; Kroll, G.; Faupel, F. Correlation between fractional free volume and diffusivity of gas molecules in glassy polymers. J. Polym. Sci. Polym. Phys. 1999, 37, 3344–3358. [Google Scholar] [CrossRef]
- Wilks, R.; Chung, W.J.; Ludovice, P.J.; Rezac, M.E.; Meakin, P.; Hill, A.J. Structural and free-volume analysis for alkyl-substituted palladium-catalyzed poly(norbornene): A combined experimental and Monte Carlo investigation. J. Polym. Sci. Polym. Phys. 2006, 44, 215–233. [Google Scholar] [CrossRef]
- Singla, S.; Beckham, H.W.; Rezac, M.E. Localized chain mobility and gas transport properties of thermoplastic aromatic polymers. J. Membr. Sci. 2002, 208, 257–267. [Google Scholar] [CrossRef]
- Kothawade, S.S.; Kulkarni, M.P.; Kharul, U.K.; Patil, A.S.; Vernekar, S.P. Synthesis, characterization, and gas permeability of aromatic polyimides containing pendant phenoxy group. J. Appl. Polym. Sci. 2008, 108, 3881–3889. [Google Scholar] [CrossRef]
- Yang, C.P.; Lin, J.H. Preparation and properties of aromatic polyamides and polyimides derived from 3,3-bis-4(4-aminophenoxy)phenyl phtalimide. J. Polym. Sci. Polym. Chem. 1994, 32, 423–433. [Google Scholar] [CrossRef]
- De Abajo, J.; de la Campa, J.G.; Lozano, A.E.; Espeso, J.; García, C. Designing aromatic polyamides and polyimides for gas separation membranes. C. Macromol. Symp. 2003, 199, 293–306. [Google Scholar] [CrossRef]
- Sasthav, J.R.; Harris, F.W. Internal plasticization of polyimides with alkyl 3,5-diaminobenzoate compounds. Polymer 1995, 36, 4911–4917. [Google Scholar] [CrossRef]
- Wang, Y.C.; Huang, S.H.; Huc, C.C.; Li, C.L.; Lee, K.R.; Liaw, D.J.; Lai, D.Y. Sorption and transport properties of gases in aromatic polyimide membranes. J. Membr. Sci. 2005, 248, 15–25. [Google Scholar] [CrossRef]
- Yampolskii, Y. Polymeric gas separation membranes. Macromolecules 2012, 45, 3298–3311. [Google Scholar] [CrossRef]
- Likhatchev, D.; Alexandrova, L.; Tlenkopatchev, M.; Martinez-Richa, A.; Vera-Graziano, R. One-step synthesis of aromatic polyimides based on 4,4'-diaminotriphenylmethane. J. Appl. Polym. Sci. 1996, 61, 815–818. [Google Scholar] [CrossRef]
- Likhatchev, D.; Alexandrova, L.; Tlenkopatchev, M.; Vilar, R.; Vera-Graziano, R. Soluble aromatic polyimides and polyamides based on 4,4'-diaminotriphenylmethane. J. Appl. Polym. Sci. 1995, 57, 37–44. [Google Scholar] [CrossRef]
- Guzman-Lucero, D.; Guzman, J.; Likhatchev, D.; Martinez-Palou, R. Microwave-assisted synthesis of 4,4'-diaminotriphenylmethanes. Tetrahedron Lett. 2005, 46, 1119–1122. [Google Scholar] [CrossRef]
- Bondi, A. Van der Waals volumes and Radii. J. Phys. Chem. 1960, 68, 441–451. [Google Scholar] [CrossRef]
- Van Krevelen, D.W.; te Nijenhuis, K. Properties of Polymers; Elsevier: Amsterdam, The Netherland, 2009. [Google Scholar]
- Ishida, H.; Wellinghoff, S.T.; Baer, E.; Koenig, J.L. Spectroscopic studies of poly(N,N’-bis(phenoxyphenyl)pyromellitimide). 1. Structures of the polyimide and three model compounds. Macromolecules 1980, 13, 826–834. [Google Scholar] [CrossRef]
- Koros, W.J.; Coleman, M.R.; Walker, D.R.B. Controlled permeability polymer membranes. Annu. Rev. Mater. Sci. 1992, 22, 47–89. [Google Scholar]
- Calle, M.; Lozano, A.; de Abajo, J.; de la Campa, J.; Alvarez, C. Desing of gas separation membranes derived of rigid aromatic polyimides. 1. Polymers from diamines containing di-tert-butyl side groups. J. Membr. Sci. 2010, 365, 145–153. [Google Scholar] [CrossRef]
- Sanders, D.; Smith, Z.P.; Ribeiro, C.P., Jr.; Guo, R.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Gas permeability, diffusivity, and free volume of thermally rearranged polymers based on 3,3'-dihydroxy-4,4'-diamino-biphenyl (HAB) and 2,2'-bis-(3,4-dicarboxyphenyl) hexafluoropropane dianhydride (6FDA). J. Membr. Sci. 2012, 409–410, 232–241. [Google Scholar] [CrossRef]
- Tanaka, K.; Kita, H.; Okano, M.; Okamoto, K.I. Permeability and permselectivity of gases in fluorinated and non-fluorinated polyimides. Polymer 1992, 33, 585–592. [Google Scholar] [CrossRef]
- Okamoto, K.I.; Tanaka, K.; Kita, H.; Ishida, M.; Kakimoto, M.; Imai, Y. Gas permeability and permselectivity of polyimides prepared from 4,4'-diaminotriphenylamine. Polym. J. 1992, 24, 451–457. [Google Scholar] [CrossRef]
- Xu, J.W.; Chng, M.L.; Chung, T.S.; He, C.B.; Wang, R. Permeability of polyimides derived from non-coplanar diamines and 4,4'-(hexafluoroisopropylidene)diphthalic anhydride. Polymer 2003, 44, 4715–4721. [Google Scholar] [CrossRef]
- Coleman, M.R.; Koros, W.J. Isomeric polyimides based on fluorinated dianhydrides and diamines for gas separation applications. J. Membr. Sci. 1990, 50, 285–297. [Google Scholar] [CrossRef]
- Stern, S.A.; Mi, Y.; Yamamoto, H.; StClair, A.K. Structure permeability relationships of polyimide membranes—Application to the separation of gas-mixtures. J. Polym. Sci. Polym. Phys. 1989, 27, 1887–1909. [Google Scholar] [CrossRef]
- McHattie, J.S.; Koros, W.J.; Paul, D.R. Gas transport properties of polysulphones: 3. Comparison of tetramethyl-substituted bisphenols. Polymer 1992, 33, 1701–1711. [Google Scholar] [CrossRef]
- Hellums, M.W.; Koros, W.J.; Paul, D.R. Gas transport in Halogen-containing aromatic polycarbonates. J. Appl. Polym. Sci. 1991, 43, 1977–1986. [Google Scholar] [CrossRef]
- Camacho-Zuñiga, C.; Ruiz-Treviño, F.A.; Zolotukhin, M.G.; del Castillo, L.F.; Guzman, J.; Chávez, J.; Torres, G.; Gileva, N.G.; Sedova, E.A. Gas transport properties of new aromatic cardo poly(aryl ether ketone)s. J. Membr. Sci. 2006, 283, 393–398. [Google Scholar] [CrossRef]
- Hougham, G.; Cassidy, P.; Johns, K.; Davidson, T. Fluoropolymers 2: Properties; Plenum press: New York, NY, USA, 1999. [Google Scholar]
- Li, N.; Fane, A.; Ho, W.; Matsuura, T. Advanced Membrane Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Alentiev, A.Y.; Shantarovich, V.P.; Merkel, T.C.; Bondar, V.I.; Freeman, B.D.; Yampolskii, Y.P. Gas and vapor sorption, permeation, and diffusion in glassy amorphous teflon AF1600. Macromolecules 2002, 35, 9513–9522. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán-Lucero, D.; Palomeque-Santiago, J.F.; Camacho-Zúñiga, C.; Ruiz-Treviño, F.A.; Guzmán, J.; Galicia-Aguilar, A.; Aguilar-Lugo, C. Gas Permeation Properties of Soluble Aromatic Polyimides Based on 4-Fluoro-4,4'-Diaminotriphenylmethane. Materials 2015, 8, 1951-1965. https://doi.org/10.3390/ma8041951
Guzmán-Lucero D, Palomeque-Santiago JF, Camacho-Zúñiga C, Ruiz-Treviño FA, Guzmán J, Galicia-Aguilar A, Aguilar-Lugo C. Gas Permeation Properties of Soluble Aromatic Polyimides Based on 4-Fluoro-4,4'-Diaminotriphenylmethane. Materials. 2015; 8(4):1951-1965. https://doi.org/10.3390/ma8041951
Chicago/Turabian StyleGuzmán-Lucero, Diego, Jorge Froylán Palomeque-Santiago, Claudia Camacho-Zúñiga, Francisco Alberto Ruiz-Treviño, Javier Guzmán, Alberto Galicia-Aguilar, and Carla Aguilar-Lugo. 2015. "Gas Permeation Properties of Soluble Aromatic Polyimides Based on 4-Fluoro-4,4'-Diaminotriphenylmethane" Materials 8, no. 4: 1951-1965. https://doi.org/10.3390/ma8041951





