Covalent Binding of Heparin to Functionalized PET Materials for Improved Haemocompatibility
Abstract
:1. Introduction
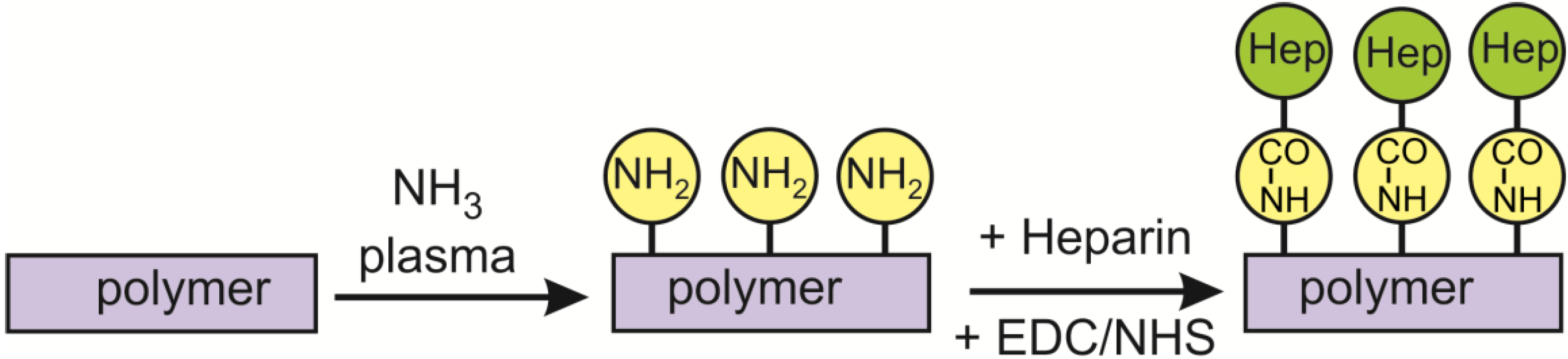
2. Results and Discussion
2.1. Functionalization of the Polymer Surface with Amino Groups
| Gas | C | N | O |
|---|---|---|---|
| untreated | 74.7 | - | 25.3 |
| NH3 | 64.7 | 10.6 | 24.7 |
| NH3 + Ar | 65.8 | 9.6 | 24.5 |
| N2 | 61.2 | 2.9 | 35.8 |
| N2 + H2 | 64.5 | 4.2 | 31.4 |
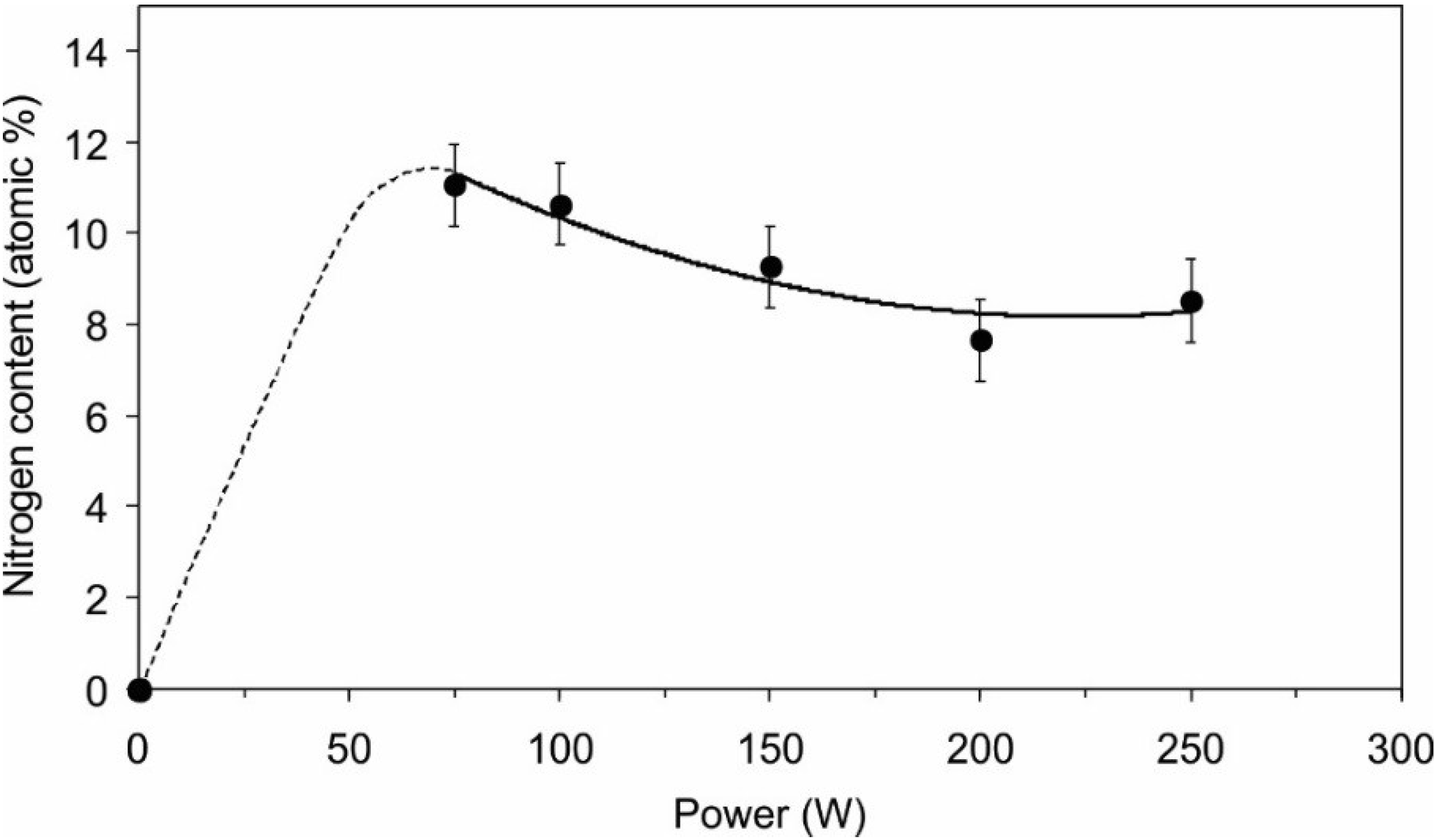
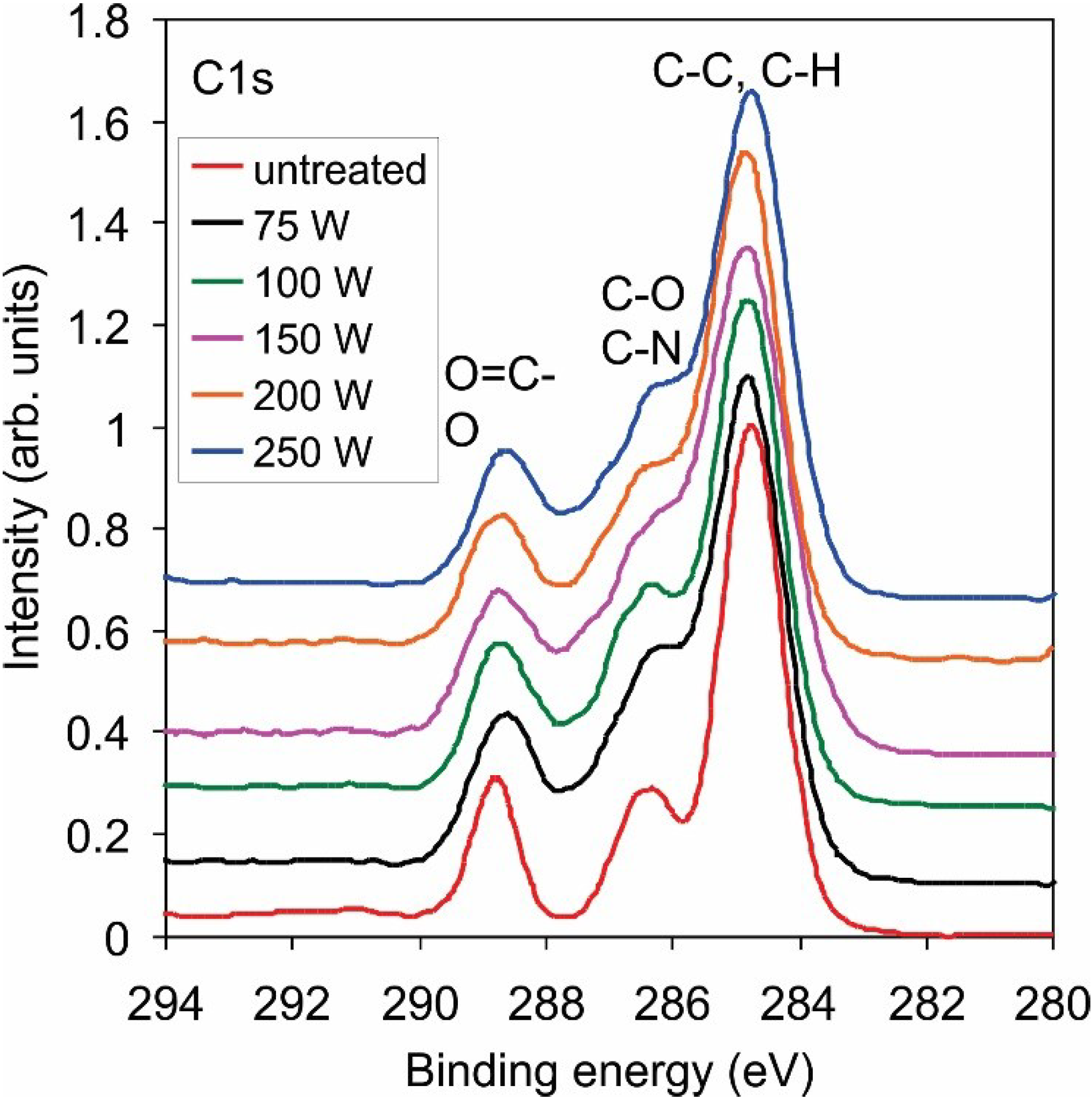
| Power (W) | C | N | O | Cl |
|---|---|---|---|---|
| untreated | 71.8 | 27.9 | 0.3 | |
| 75 | 69.8 | 2.6 | 25.5 | 2.1 |
| 100 | 69.9 | 3.5 | 24.8 | 1.9 |
| 150 | 69.1 | 3.5 | 25.6 | 1.8 |
| 150 | 69.5 | 3.7 | 24.5 | 2.3 |
| 200 | 71.2 | 2.9 | 23.9 | 2.1 |
| 250 | 70.6 | 3.2 | 24.4 | 1.8 |


| Power | NH2/% |
|---|---|
| untreated | 0.4 |
| 75 W | 3.8 |
| 100 W | 3.4 |
| 150 W | 3.7 |
| 200 W | 3.7 |
| 250 W | 3.1 |
| NH3 + Ar | 4.3 |
| N2 | 0.8 |
| N2 + H2 | 1.3 |
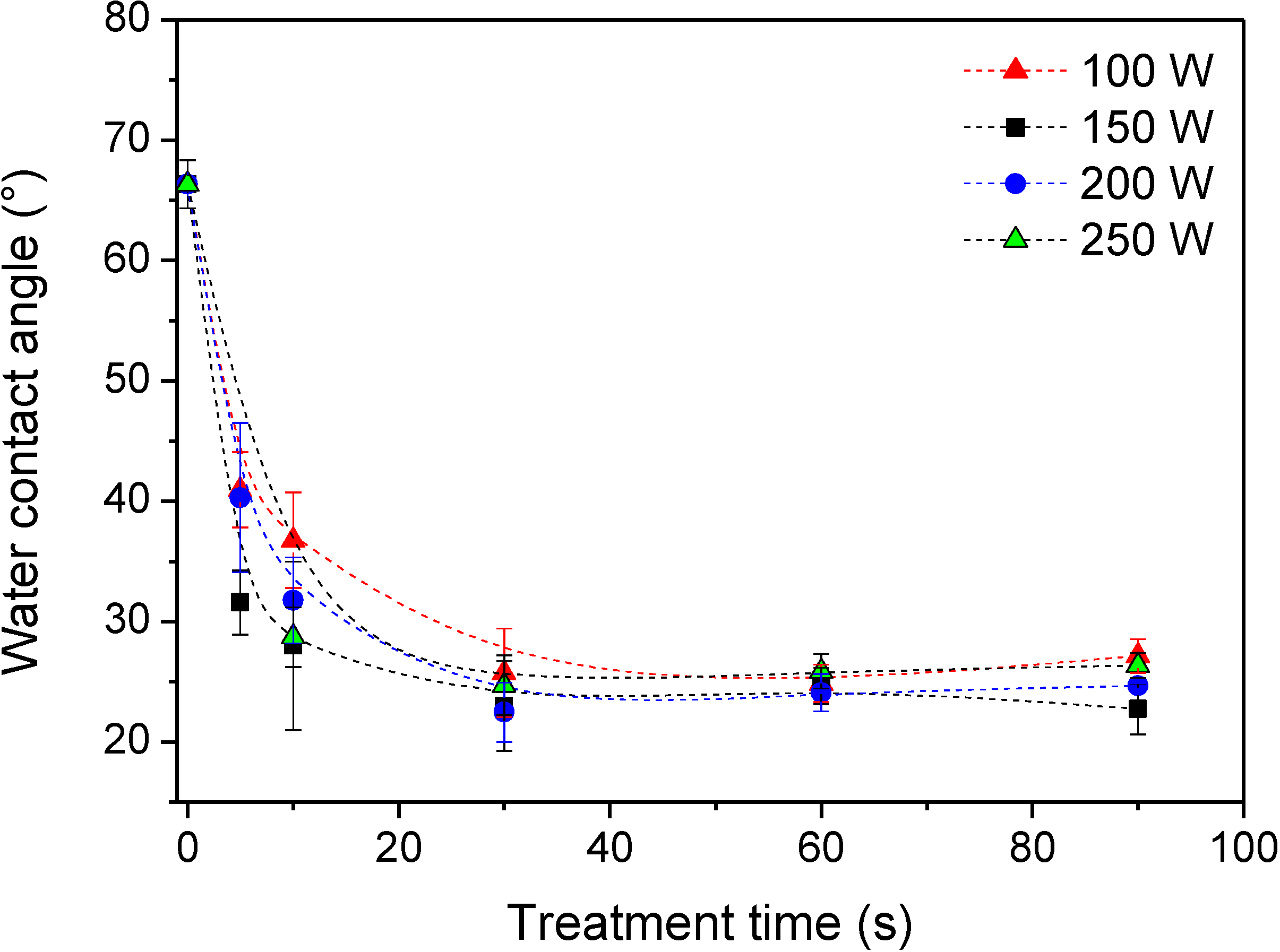
2.2. Covalent Binding of Heparin
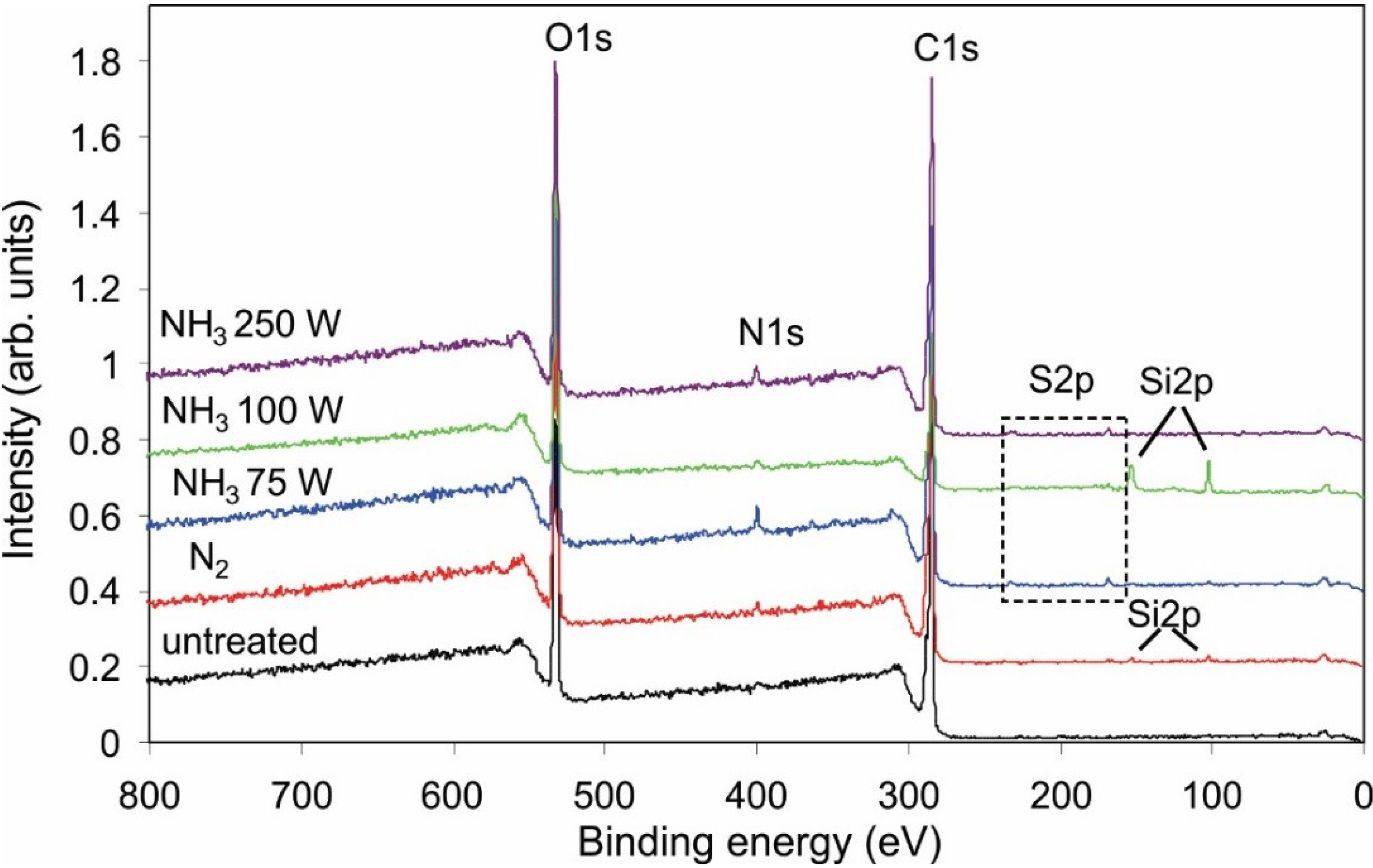
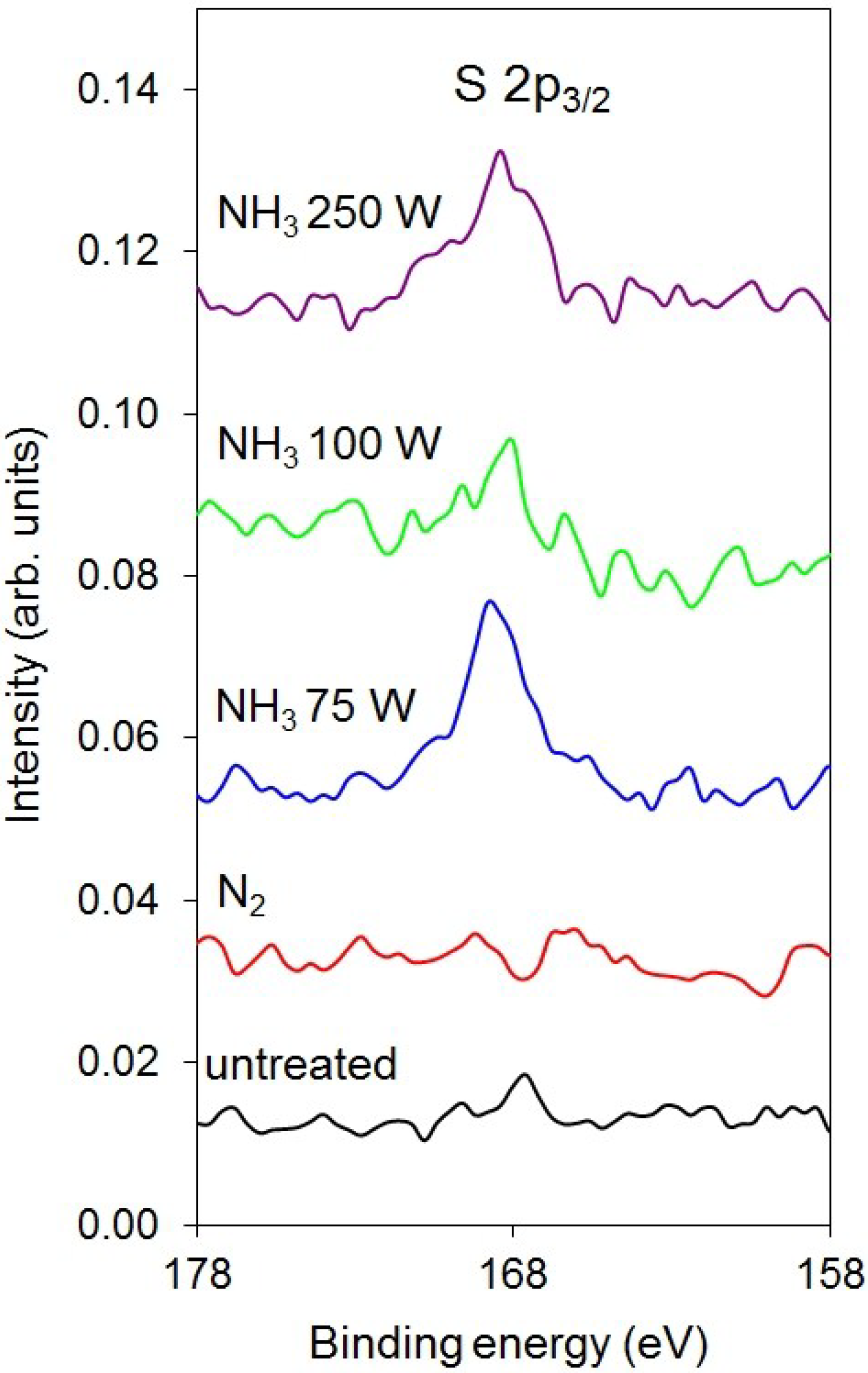
| Sample | C | N | O | S |
|---|---|---|---|---|
| untreated | 75.1 | - | 24.9 | - |
| N2-100 W | 71.3 | 1.5 | 27.2 | - |
| NH3-75 W | 70.4 | 2.9 | 26.0 | 0.7 |
| NH3-100 W | 63.3 | 2.2 | 33.8 | 0.7 |
| NH3-250 W | 70.9 | 2.0 | 26.5 | 0.6 |
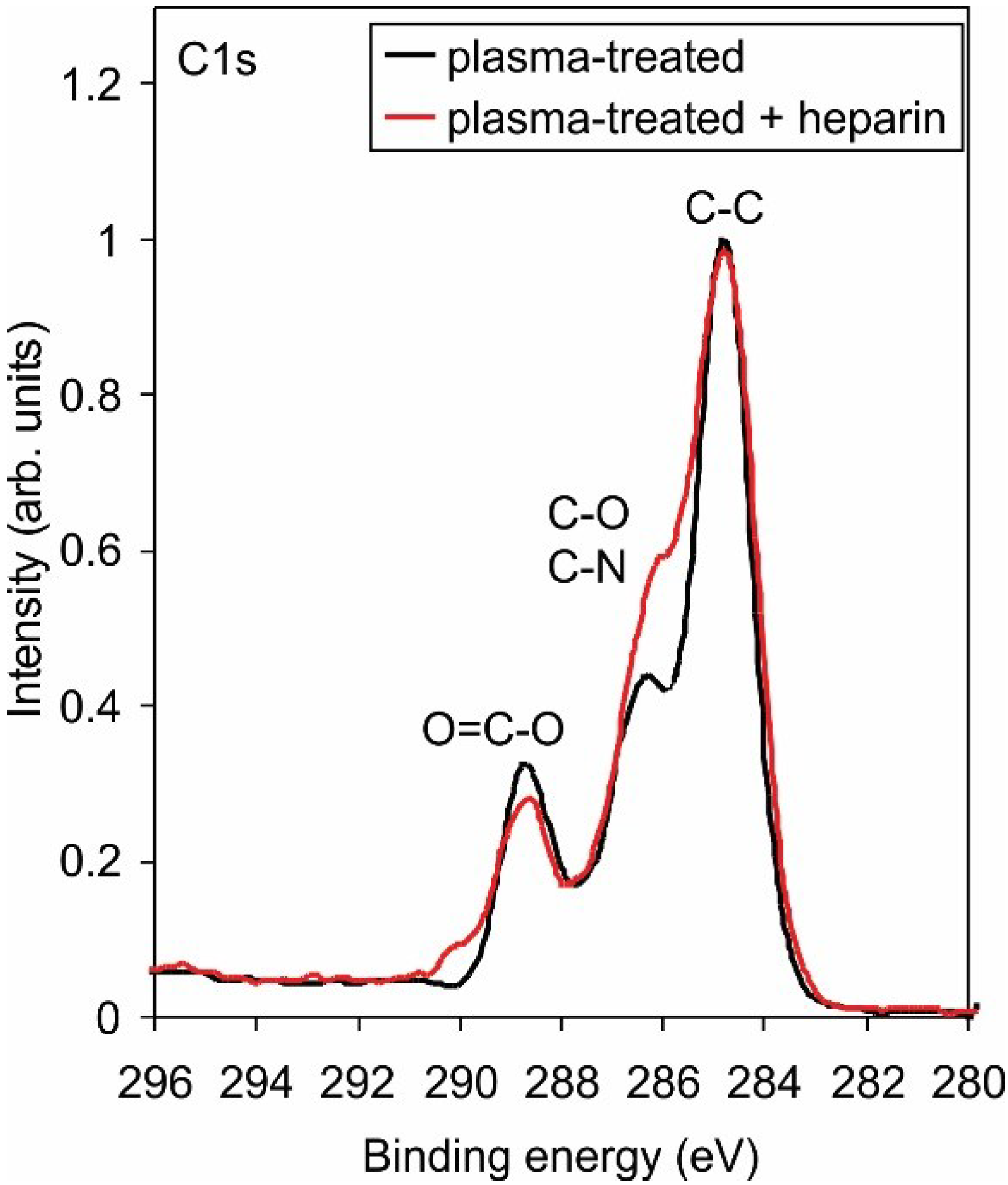
2.3. Biological Response

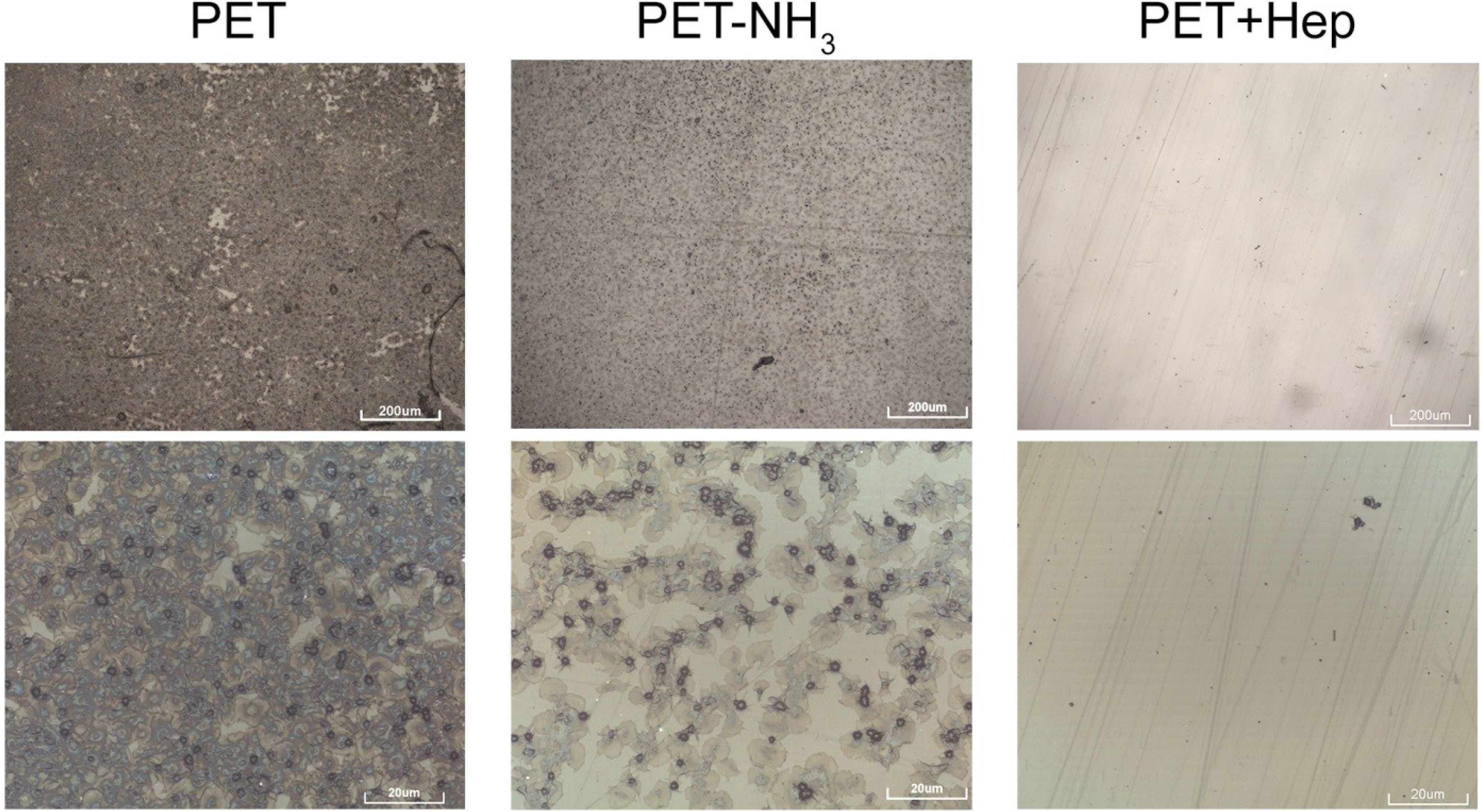
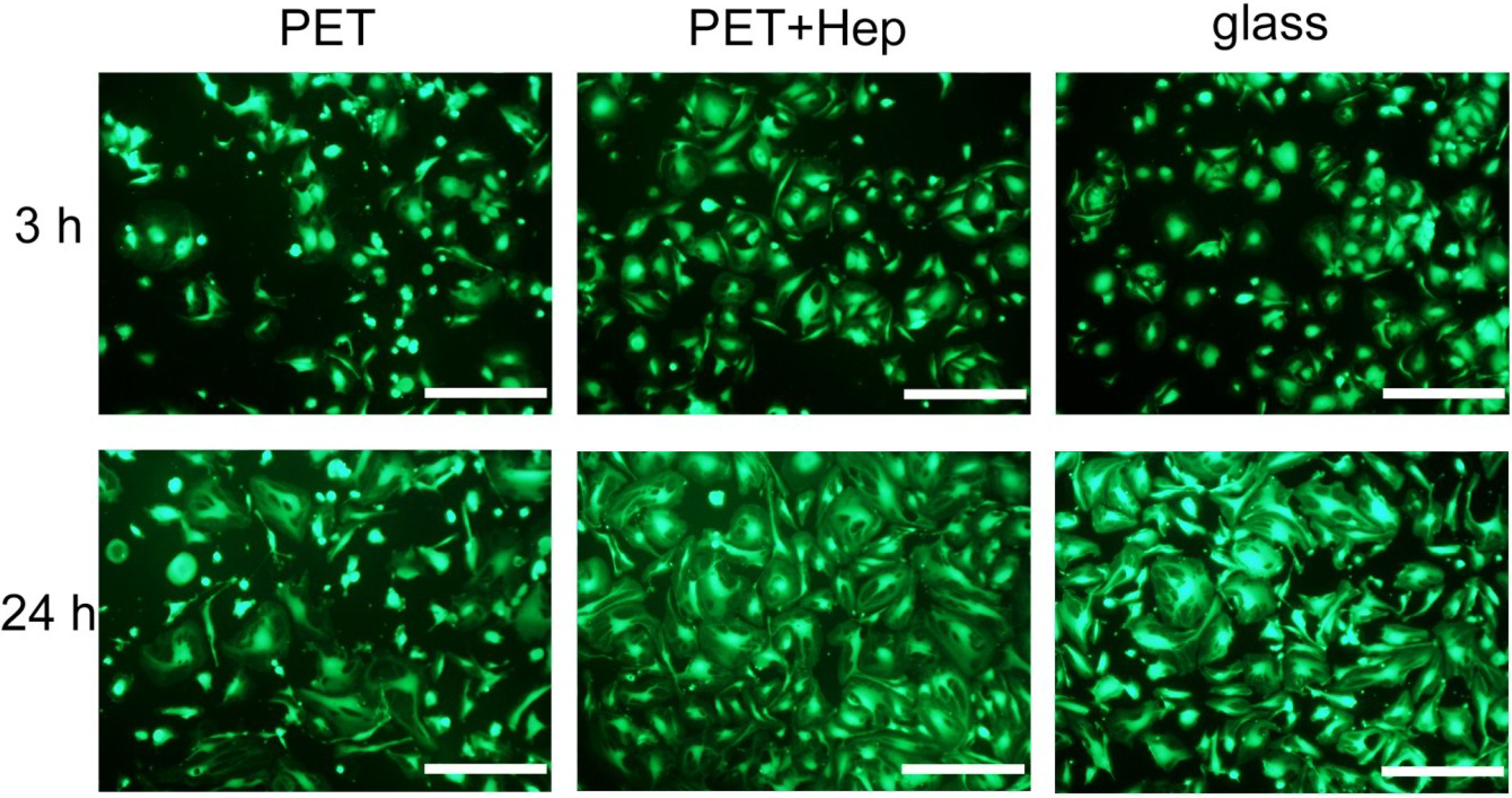

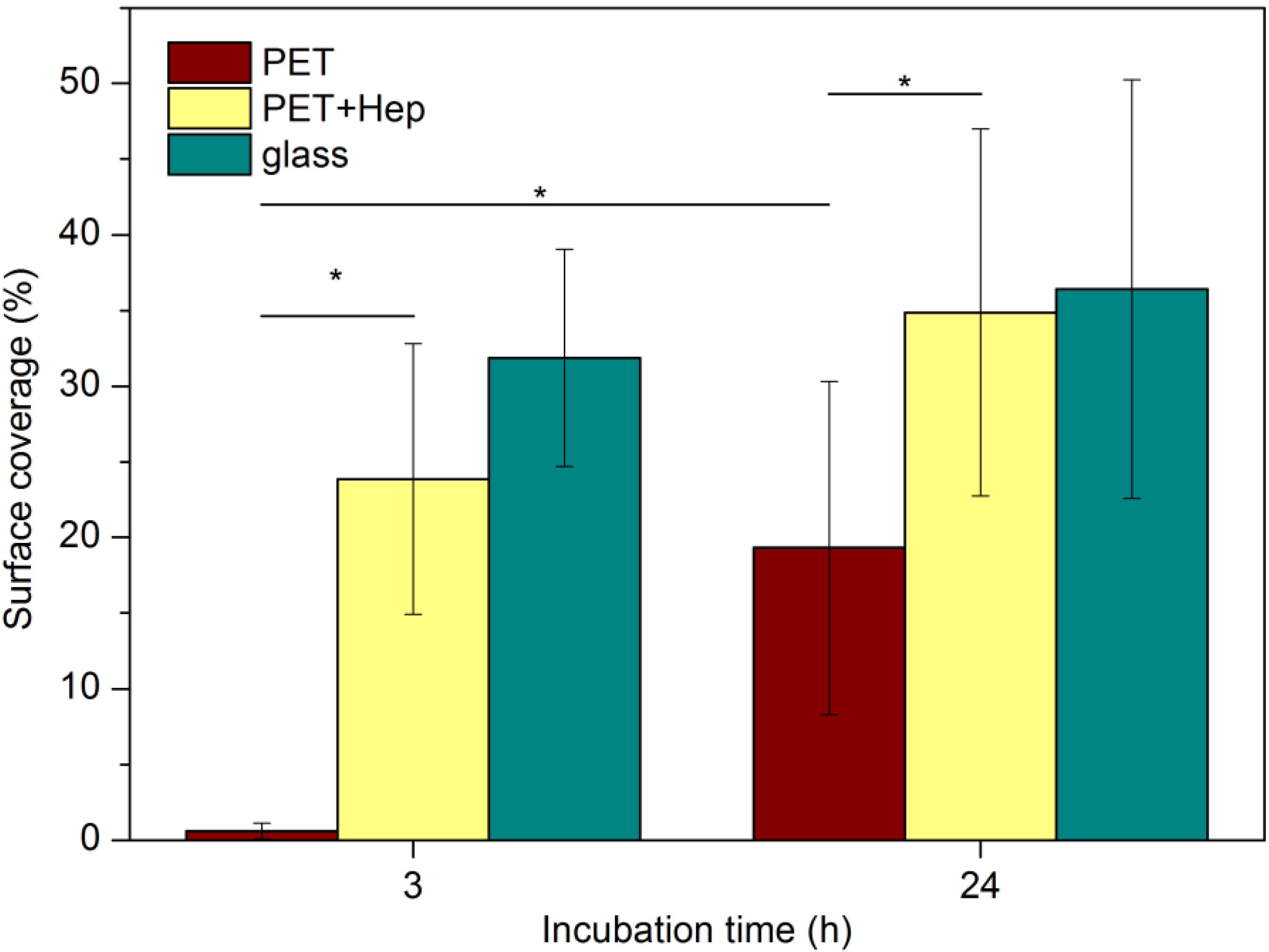
3. Experimental Section
3.1. Plasma Treatment
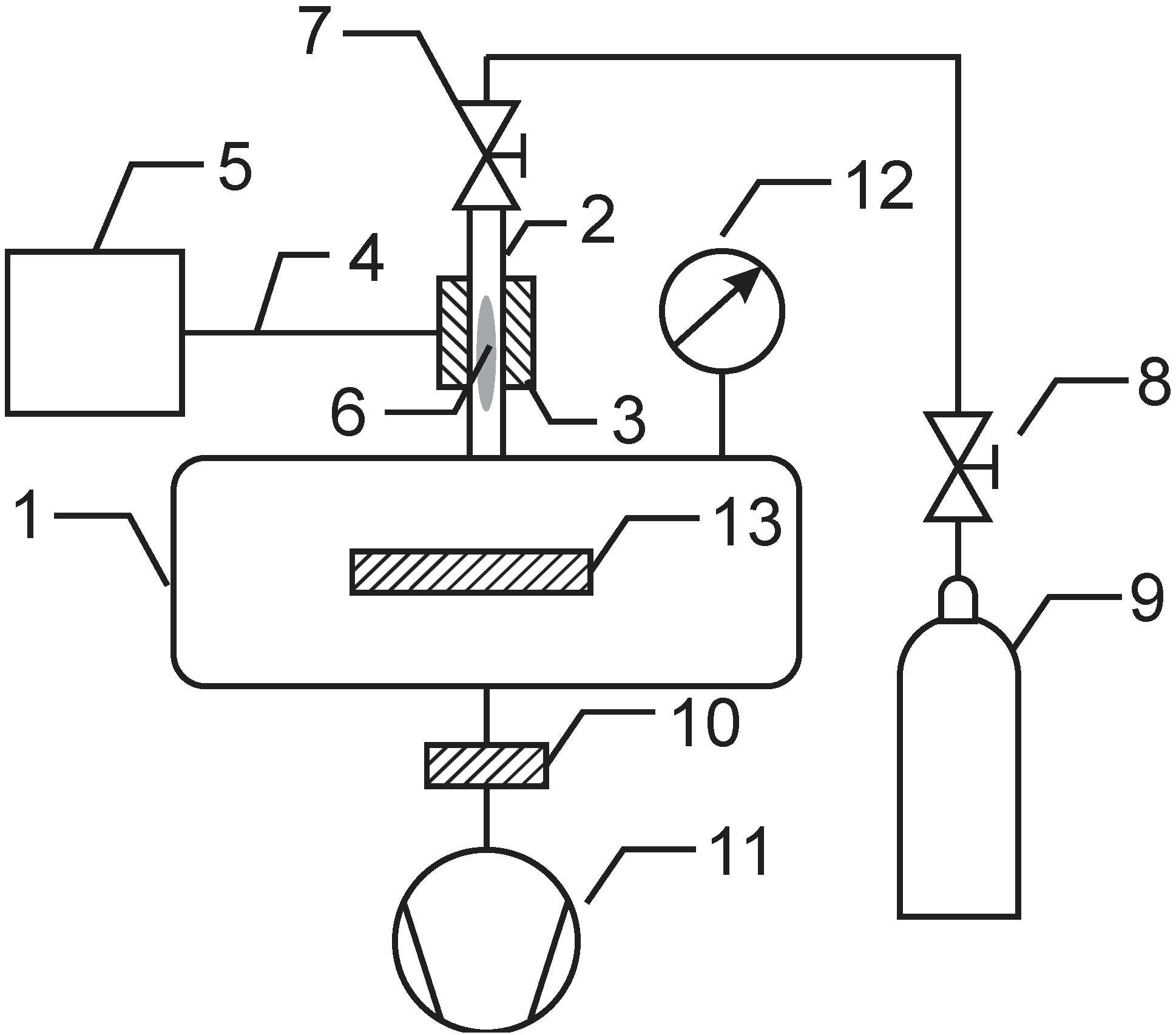
3.2. Surface Characterization
3.3. Heparin Immobilisation
3.4. Blood Incubation and Endothelisation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Modic, M.; Junkar, I.; Stana-Kleinschek, K.; Kostanjšek, R.; Mozetič, M. Morphology transformations of platelets on plasma activated surfaces. Plasma Process. Polym. 2014, 11, 596–605. [Google Scholar] [CrossRef]
- Indest, T.; Laine, J.; Johansson, L.-S.; Stana-Kleinschek, K.; Strnad, S.; Dworczak, R.; Ribitsch, V. Adsorption of fucoidan and chitosan sulfate on chitosan modified PET films monitored by QCM-D. Biomacromolecules 2009, 10, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Doliska, A.; Strnad, S.; Stana-Kleinschek, K. Heparin adsorption onto model poly(ethylene terephtalate) (PET) surfaces monitored by QCM-D. Mater. Technol. 2012, 46, 81–85. [Google Scholar]
- Doliška, A.; Strnad, S.; Stana, J.; Martinelli, E.; Ribitsch, V.; Stana-Kleinschek, K. In vitro haemocompatibility evaluation of PET surfaces using the quartz crystal microbalance technique. J. Biomater. Sci. Polym. Ed. 2012, 23, 697–714. [Google Scholar] [CrossRef] [PubMed]
- Mushunje, A.; Zhou, A.; Carrell, R.W.; Huntington, J.A. Heparin-induced substrate behavior of antithrombin cambridge II. Blood 2003, 102, 4028–4034. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Johnson, D.J.; Esmon, C.T.; Huntington, J.A. Structure of the antithrombin-thrombin-heparin ternary complex reveals the antithrombotic mechanism of heparin. Nat. Struct. Mol. Biol. 2004, 11, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Bech, L.; Meylheuc, T.; Lepoittevin, B.; Roger, P. Chemical surface modification of poly(ethylene terephthalate) fibers by aminolysis and grafting of carbohydrates. J. Polym. Sci. Polym. Chem. 2007, 45, 2172–2183. [Google Scholar] [CrossRef]
- Murugesan, S.; Xie, J.; Linhardt, R.J. Immobilization of heparin: Approaches and applications. Curr. Top. Med. Chem. 2008, 8, 80–100. [Google Scholar] [CrossRef] [PubMed]
- Gore, S.; Andersson, J.; Biran, R.; Underwood, C.; Riesenfeld, J. Heparin surfaces: Impact of immobilization chemistry on hemocompatibility and protein adsorption. J. Biomed. Mater. Res. B 2014, 102, 1817–1824. [Google Scholar] [CrossRef]
- Recek, N.; Jaganjac, M.; Kolar, M.; Milkovic, L.; Mozetic, M.; Stana-Kleinschek, K.; Vesel, A. Protein adsorption on various plasma-treated polyethylene terephthalate substrates. Molecules 2013, 18, 12441–12463. [Google Scholar] [CrossRef] [PubMed]
- Karbassi, E.; Asadinezhad, A.; Lehocky, M.; Humpolicek, P.; Vesel, A.; Novak, I.; Saha, P. Antibacterial performance of alginic acid coating on polyethylene film. Int. J. Mol. Sci. 2014, 15, 14684–14696. [Google Scholar] [CrossRef] [PubMed]
- Asadinezhad, A.; Novak, I.; Lehocky, M.; Bilek, F.; Vesel, A.; Junkar, I.; Saha, P.; Popelka, A. Polysaccharides coatings on medical-grade PVC: A probe into surface characteristics and the extent of bacterial adhesion. Molecules 2010, 15, 1007–1027. [Google Scholar] [CrossRef] [PubMed]
- Popelka, A.; Novak, I.; Lehocky, M.; Junkar, I.; Mozetic, M.; Kleinova, A.; Janigova, I.; Slouf, M.; Bilek, F.; Chodak, I. A new route for chitosan immobilization onto polyethylene surface. Carbohydr. Polym. 2012, 90, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Junkar, I.; Cvelbar, U.; Lehocky, M. Plasma treatment of biomedical materials. Mater. Tehnol. 2011, 45, 221–226. [Google Scholar]
- Vesel, A.; Junkar, I.; Cvelbar, U.; Kovac, J.; Mozetic, M. Surface modification of polyester by oxygen- and nitrogen-plasma treatment. Surf. Interface Anal. 2008, 40, 1444–1453. [Google Scholar] [CrossRef]
- Lopez-Garcia, J.; Lehocky, M.; Humpolicek, P.; Novak, I. On the correlation of surface charge and energy in non-thermal plasma-treated polyethylene. Surf. Interface Anal. 2014, 46, 625–629. [Google Scholar] [CrossRef]
- Belmonte, T.; Pintassilgo, C.D.; Czerwiec, T.; Henrion, G.; Hody, V.; Thiebaut, J.M.; Loureiro, J. Oxygen plasma surface interaction in treatments of polyolefines. Surf. Coat. Technol. 2005, 200, 26–30. [Google Scholar] [CrossRef]
- Kang, I.K.; Kwon, O.H.; Lee, Y.M.; Sung, Y.K. Preparation and surface characterization of functional group-grafted and heparin-immobilized polyurethanes by plasma glow discharge. Biomaterials 1996, 17, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Seo, E.J.; Kang, I.K. Synthesis and characterization of heparinized polyurethanes using plasma glow discharge. Biomaterials 1999, 20, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Pandiyaraj, K.N.; Selvarajan, V.; Rhee, Y.H.; Kim, H.W.; Shah, S.I. Glow discharge plasma-induced immobilization of heparin and insulin on polyethylene terephthalate film surfaces enhances anti-thrombogenic properties. Mater. Sci. Eng. C 2009, 29, 796–805. [Google Scholar] [CrossRef]
- Favia, P.; Palumbo, F.; d’Agostino, R.; Lamponi, S.; Magnani, A.; Barbucci, R. Immobilization of heparin and highly-sulphated hyaluronic acid onto plasma-treated polyethylene. Plasmas Polym. 1998, 3, 77–96. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kang, I.K.; Huh, M.W.; Yoon, S.C. Surface characterization and in vitro blood compatibility of poly(ethylene terephthalate) immobilized with insulin and/or heparin using plasma glow discharge. Biomaterials 2000, 21, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Chandy, T.; Das, G.S.; Wilson, R.F.; Rao, G.H.R. Use of plasma glow for surface-engineering biomolecules to enhance bloodcompatibility of dacron and PTFE vascular prosthesis. Biomaterials 2000, 21, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Ren, L.; Feng, W.; Zhai, Z.; Wang, Y. Surface characterization of polyethylene terephthalate films treated by ammonia low-temperature plasma. Appl. Surf. Sci. 2012, 258, 7207–7212. [Google Scholar] [CrossRef]
- Casimiro, J.; Lepoittevin, B.; Boisse-Laporte, C.; Barthés-Labrousse, M.-G.; Jegou, P.; Brisset, F.; Roger, P. Introduction of primary amino groups on poly(ethylene terephthalate) surfaces by ammonia and a mix of nitrogen and hydrogen plasma. Plasma Chem. Plasma Process. 2011, 32, 305–323. [Google Scholar] [CrossRef]
- Öteyaka, M.Ö.; Chevallier, P.; Turgeon, S.; Robitaille, L.; Laroche, G. Low pressure radio frequency ammonia plasma surface modification on poly(ethylene terephthalate) films and fibers: Effect of the polymer forming process. Plasma Chem. Plasma Process. 2011, 32, 17–33. [Google Scholar] [CrossRef]
- Tan, Q.G.; Ji, J.; Zhao, F.; Fan, D.Z.; Sun, F.Y.; Shen, J.C. Fabrication of thromboresistant multilayer thin film on plasma treated poly (vinyl chloride) surface. J. Mater. Sci. Mater. Med. 2005, 16, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Steffen, H.J.; Schmidt, J.; Gonzalez-Elipe, A. Biocompatible surfaces by immobilization of heparin on diamond-like carbon films deposited on various substrates. Surf. Interface Anal. 2000, 29, 386–391. [Google Scholar] [CrossRef]
- Valeur, E.; Bradley, M. Amide bond formation: Beyond the myth of coupling reagents. Chem. Soc. Rev. 2009, 38, 606–631. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, E.; Jimenez-Redondo, M.; Tanarro, I.; Herrero, V.J. Neutral and ion chemistry in low pressure DC plasmas of H2/N2 mixtures: Routes for the efficient production of NH3 and NH4+. Phys. Chem. Chem. Phys. 2011, 13, 19561–19572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevallier, P.; Castonguay, M.; Turgeon, S.; Dubrulle, N.; Mantovani, D.; McBreen, P.H.; Wittmann, J.C.; Laroche, G. Ammonia RF–plasma on PTFE surfaces: Chemical characterization of the species created on the surface by vapor–phase chemical derivatization. J. Phys. Chem. B 2001, 105, 12490–12497. [Google Scholar] [CrossRef]
- Lopez-Santos, C.; Yubero, F.; Cotrino, J.; Gonzalez-Elipe, A.R. Surface functionalization, oxygen depth profiles, and wetting behavior of pet treated with different nitrogen plasmas. ACS Appl. Mater. Interfaces 2010, 2, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Stana, J.; Stropnik, D.; Strnad, S.; Indest, T.; Jevšek, M.; Košir, G. Introducing and optimization of the in vitro method for determining the hemocompatibility of modified poly(ethylene)terephthalate surfaces. Acta Medico-Biotech. 2009, 2, 61–67. [Google Scholar]
- Goodman, S.L. Sheep, pig, and human platelet–material interactions with model cardiovascular biomaterials. J. Biomed. Mater. Res. 1999, 45, 240–250. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolar, M.; Mozetič, M.; Stana-Kleinschek, K.; Fröhlich, M.; Turk, B.; Vesel, A. Covalent Binding of Heparin to Functionalized PET Materials for Improved Haemocompatibility. Materials 2015, 8, 1526-1544. https://doi.org/10.3390/ma8041526
Kolar M, Mozetič M, Stana-Kleinschek K, Fröhlich M, Turk B, Vesel A. Covalent Binding of Heparin to Functionalized PET Materials for Improved Haemocompatibility. Materials. 2015; 8(4):1526-1544. https://doi.org/10.3390/ma8041526
Chicago/Turabian StyleKolar, Metod, Miran Mozetič, Karin Stana-Kleinschek, Mirjam Fröhlich, Boris Turk, and Alenka Vesel. 2015. "Covalent Binding of Heparin to Functionalized PET Materials for Improved Haemocompatibility" Materials 8, no. 4: 1526-1544. https://doi.org/10.3390/ma8041526
APA StyleKolar, M., Mozetič, M., Stana-Kleinschek, K., Fröhlich, M., Turk, B., & Vesel, A. (2015). Covalent Binding of Heparin to Functionalized PET Materials for Improved Haemocompatibility. Materials, 8(4), 1526-1544. https://doi.org/10.3390/ma8041526








