Mechanisms of in Vivo Degradation and Resorption of Calcium Phosphate Based Biomaterials
Abstract
:1. Introduction
| Compound Name | Chemical Formula | Symbol | Mineral | Ca/P Ionic Ratio | Density (g/cm3) | Solubility at 25 °C (mg/L) |
|---|---|---|---|---|---|---|
| Monocalcium phosphate monohydrate | Ca(H2PO4)2·H2O | MCPM | - | 0.5 | 2.23 | ~18,000 |
| Monocalcium phosphate anhydrous | Ca(H2PO4)2 | MCPA | - | 0.5 | 2.58 | ~17,000 |
| Dicalcium phosphate dehydrate | CaHPO4·2H2O | DCPD | Brushite | 1.0 | 2.27 | ~88 |
| Dicalcium phosphate anhydrous | CaHPO4 | DCPA | Monetite | 1.0 | 2.92 | ~48 |
| Octacalcium phosphate | Ca8(HPO4)2(PO4)4·5H2O | OCP | - | 1.33 | 2.61 | ~8.1 |
| α-Tricalcium phosphate | α-Ca3(PO4)2 | α-TCP | - | 1.5 | 2.86 | ~2.5 |
| β-Tricalcium phosphate | β-Ca3(PO4)2 | Β-TCP | - | 1.5 | 3.07 | ~0.5 |
| Amorphous calcium phosphate | Ca3(PO4)2·nH2O n = 3–4.5; 15%–20% H,O | ACP | - | 1.5 | 3.01 | 25.6–32.8 |
| Precipitated hydroxyapatite | Ca10−x(HPO4)x(PO4)6−x(OH)2−x | PHA | - | 1.33–1.67 | 3.16 | Not available |
| Calcium-deficient hydroxyapatite | Ca10−x(HPO4)x(PO4)6−x(OH)2−x (0 < x < 1) | CDHA | - | 1.5–1.67 | 3.16 | ~9.4 |
| Hydroxyapatite | Ca10(PO4)6(OH)2 | HA | Hydroxyapatite | 1.67 | 3.16 | ~0.3 |
| Oxyapatite | Ca10(PO4)6O | OXA | - | 1.67 | 3.20 | Not available |
| Fluorapatite | Ca10(PO4)6F2 | FA | - | 1.67 | 3.18 | ~0.2 |
| Tetracalcium phosphate | Ca2(PO4)2O | TTCP | Hilgenstockite | 2.0 | 3.05 | ~0.7 |
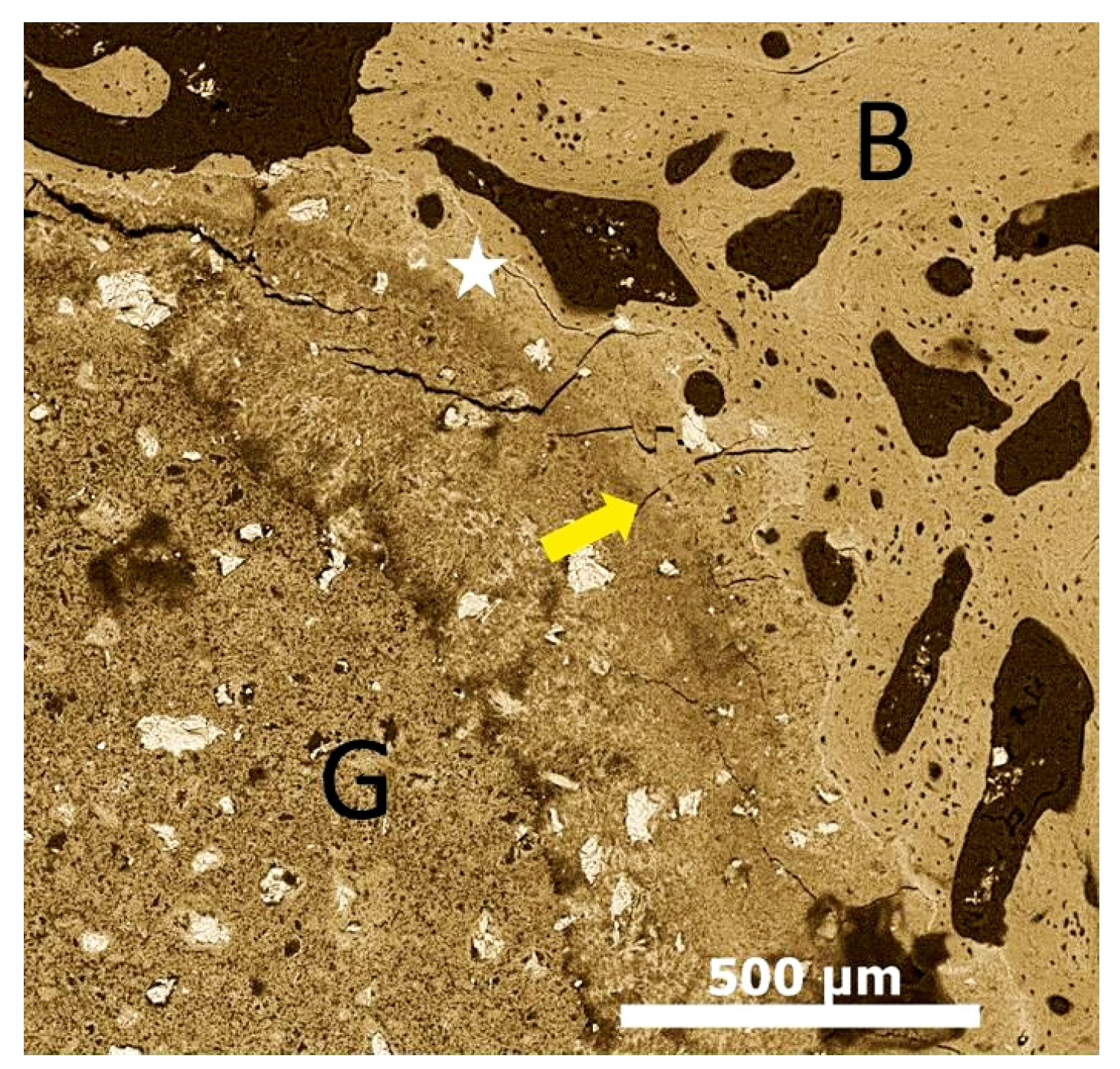
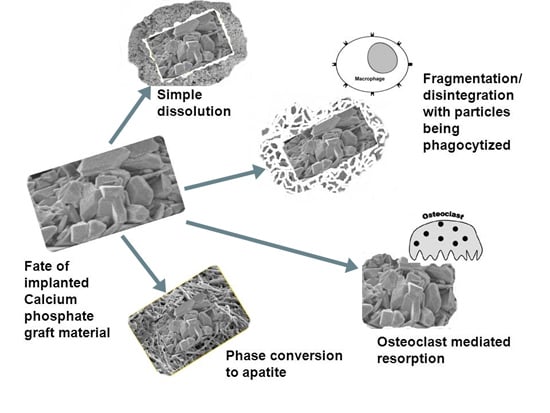
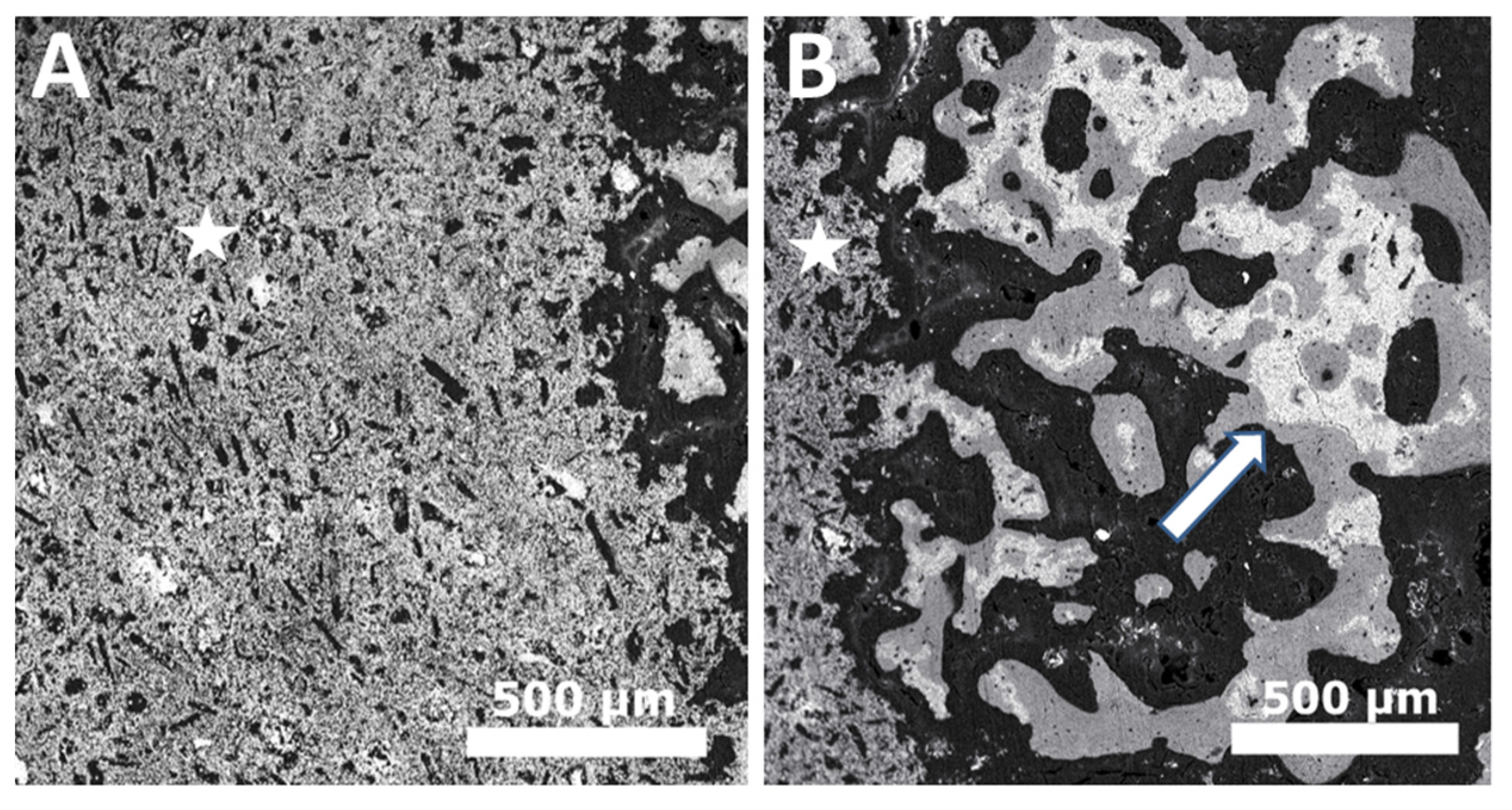
2. In Vivo Degradation and Resorption of Calcium Phosphates
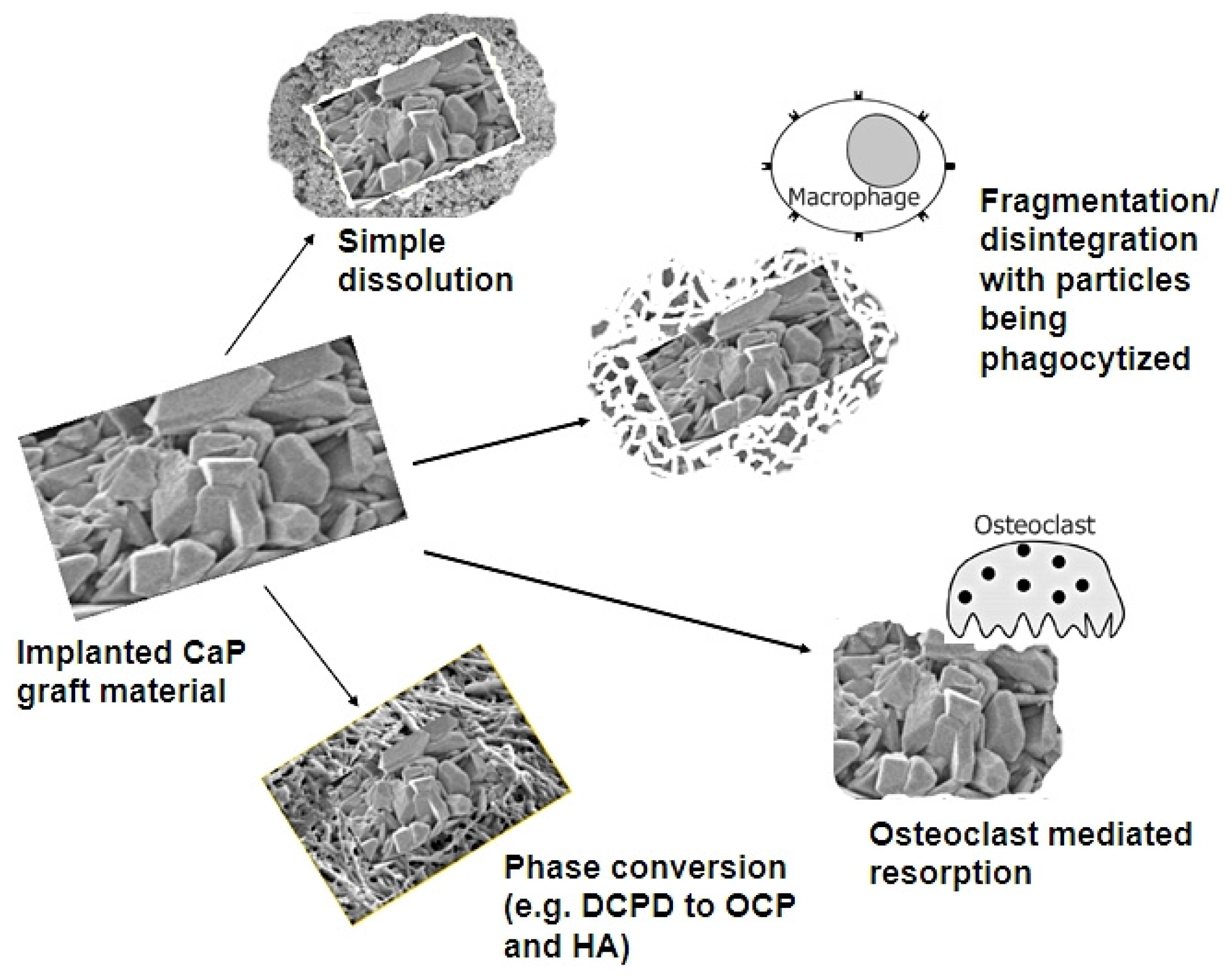

3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bohner, M. Calcium orthophosphates in medicine: From ceramics to calcium phosphate cements. Injury 2000, 31, D37–D47. [Google Scholar] [CrossRef]
- Tamimi, F.; Sheikh, Z.; Barralet, J. Dicalcium phosphate cements: Brushite and monetite. Acta Biomater. 2012, 8, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Legeros, R.Z. Biodegradation and bioresorption of calcium phosphate ceramics. Clin. Mater. 1993, 14, 65–88. [Google Scholar] [CrossRef]
- Sheikh, Z.; Sima, C.; Glogauer, M. Bone replacement materials and techniques used for achieving vertical alveolar bone augmentation. Materials 2015, 8, 2953–2993. [Google Scholar] [CrossRef]
- Barney, V.C.; Levin, M.P.; Adams, D.F. Bioceramic implants in surgical periodontal defects: A comparison study. J. Periodontol. 1986, 57, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Z.; Zhao, J.; Zhang, X.; Sun, X.; Xia, L.; Chang, Q.; Ye, D.; Jiang, X. Vertical alveolar ridge augmentation with beta-tricalcium phosphate and autologous osteoblasts in canine mandible. Biomaterials 2009, 30, 2489–2498. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Tamimi, F.; Alkhraisat, M.H.; Prados-Frutos, J.C.; Rastikerdar, E.; Gbureck, U.; Barralet, J.E.; Lopez-Cabarcos, E. Vertical bone augmentation with 3d-synthetic monetite blocks in the rabbit calvaria. J. Clin. Periodontol. 2011, 38, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Marinno, F.T.; Torres, J.; Tresguerres, I.; Jerez, L.B.; Cabarcos, E.L. Vertical bone augmentation with granulated brushite cement set in glycolic acid. J. Biomed. Mater. Res. A 2007, 81A, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Glogauer, M. Successful ridge augmentation: The challenge of periodontal tissue engineering. EC Dent. Sci. 2015, 2, 216–218. [Google Scholar]
- Vallet-Regí, M. Ceramics for medical applications. J. Chem. Soc. Dalton Trans. 2001, 97–108. [Google Scholar] [CrossRef]
- Fabbri, M.; Celotti, G.; Ravaglioli, A. Granulates based on calcium phosphate with controlled morphology and porosity for medical applications: Physico-chemical parameters and production technique. Biomaterials 1994, 15, 474–477. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.; Gil, F.; Ginebra, M.; Driessens, F.; Planell, J.; Best, S. Production and characterization of new calcium phosphate bone cements in the cahpov 4–α-ca 3 (po 4) 2 system: pH, workability and setting times. J. Mater. Sci. Mater. Med. 1999, 10, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphates in nature, biology and medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef] [PubMed]
- Combes, C.; Rey, C. Amorphous calcium phosphates: Synthesis, properties and uses in biomaterials. Acta Biomater. 2010, 6, 3362–3378. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.; Yaszemski, M.J.; Mikos, A.G.; Laurencin, C.T. Tissue engineering of bone: Material and matrix considerations. J. Bone Jt. Surg. 2008, 90, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Chen, Q.; Blaker, J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Schantz, J.T.; Lam, C.X.F.; Tan, K.C.; Lim, T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regen. Med. 2007, 1, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Crea, A.; Deli, G.; Littarru, C.; Lajolo, C.; Orgeas, G.V.; Tatakis, D.N. Intrabony defects, open-flap debridement, and decortication: A randomized clinical trial. J. Periodontol. 2014, 85, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R. Bone transplants and implants. In Fundamental and Clinical Bone Physiology; Urist, M.R., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1980; pp. 331–368. [Google Scholar]
- Goldberg, V.M. Selection of bone grafts for revision total hip arthroplasty. Clin. Orthop. Relat. Res. 2000, 68–76. [Google Scholar] [CrossRef]
- Cornell, C.N. Osteoconductive materials and their role as substitutes for autogenous bone grafts. Orthop. Clin. N. Am. 1999, 30, 591–598. [Google Scholar] [CrossRef]
- Radin, S.; Ducheyne, P. Effect of bioactive ceramic composition and structure on in vitro behavior. III. Porous versus dense ceramics. J. Biomed. Mater. Res. 1994, 28, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Gallur, A.; Flautre, B.; Anselme, K.; Descamps, M.; Thierry, B.; Hardouin, P. Comparative study of tissue reactions to calcium phosphate ceramics among cancellous, cortical, and medullar bone sites in rabbits. J. Biomed. Mater. Res. 1998, 42, 357–367. [Google Scholar] [CrossRef]
- Daculsi, G.; LeGeros, R.; Heughebaert, M.; Barbieux, I. Formation of carbonate-apatite crystals after implantation of calcium phosphate ceramics. Calcif. Tissue Int. 1990, 46, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Descamps, M.; Dejou, J.; Koubi, G.; Hardouin, P.; Lemaitre, J.; Proust, J.P. The biodegradation mechanism of calcium phosphate biomaterials in bone. J. Biomed. Mater. Res. 2002, 63, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Rae, T. The macrophage response to implant materials-with special reference to those used in orthopedics. CRC Crit. Rev. Biocompat. 1986, 2, 97–126. [Google Scholar]
- Velard, F.; Braux, J.; Amedee, J.; Laquerriere, P. Inflammatory cell response to calcium phosphate biomaterial particles: An overview. Acta Biomater. 2013, 9, 4956–4963. [Google Scholar] [CrossRef] [PubMed]
- Hallab, N.J.; Jacobs, J.J. Biologic effects of implant debris. Bull. NYU Hosp. Jt. Dis. 2009, 67, 182. [Google Scholar] [PubMed]
- Hannink, G.; Arts, J.C. Bioresorbability, porosity and mechanical strength of bone substitutes: What is optimal for bone regeneration? Injury 2011, 42, S22–S25. [Google Scholar] [CrossRef] [PubMed]
- Heymann, D.; Pradal, G.; Benahmed, M. Cellular mechanisms of calcium phosphate ceramic degradation. Histol. Histopathol. 1999, 14, 871–877. [Google Scholar] [PubMed]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, foreign body giant cells and their response to implantable biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef]
- Xia, Z.; Triffitt, J.T. A review on macrophage responses to biomaterials. Biomed. Mater. 2006, 1, R1. [Google Scholar] [CrossRef] [PubMed]
- Ohlin, A.; Johnell, O.; Lerner, U.H. The pathogenesis of loosening of total hip arthroplasties: The production of factors by periprosthetic tissues that stimulate in vitro bone resorption. Clin. Orthop. Relat. Res. 1990, 253, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Li, Y.; de Bruijn, J.; de Groot, K.; Zhang, X. Tissue responses of calcium phosphate cement: A study in dogs. Biomaterials 2000, 21, 1283–1290. [Google Scholar] [CrossRef]
- Ooms, E.; Wolke, J.; Van Der Waerden, J.; Jansen, J. Trabecular bone response to injectable calcium phosphate (ca-p) cement. J. Biomed. Mater. Res. 2002, 61, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Theiss, F.; Apelt, D.; Brand, B.A.; Kutter, A.; Zlinszky, K.; Bohner, M.; Matter, S.; Frei, C.; Auer, J.A.; von Rechenberg, B. Biocompatibility and resorption of a brushite calcium phosphate cement. Biomaterials 2005, 26, 4383–4394. [Google Scholar] [CrossRef] [PubMed]
- Heymann, D.; Guicheux, J.; Gouin, F.; Passuti, N.; Daculsi, G. Cytokines, growth factors and osteoclasts. Cytokine 1998, 10, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Baslé, M.F.; Chappard, D.; Grizon, F.; Filmon, R.; Delecrin, J.; Daculsi, G.; Rebel, A. Osteoclastic resorption of ca-p biomaterials implanted in rabbit bone. Calcif. Tissue Int. 1993, 53, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Leeuwenburgh, S.C.; Jo, J.; Wang, H.; Yamamoto, M.; Jansen, J.A.; Tabata, Y. Mineralization, biodegradation, and drug release behavior of gelatin/apatite composite microspheres for bone regeneration. Biomacromolecules 2010, 11, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, M.; Orly, I.; Menanteau, J. The influence of calcium phosphate biomaterials on human bone cell activities. An in vitro approach. J. Biomed. Mater. Res. 1990, 24, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Orly, I.; Gregoire, M.; Menanteau, J.; Dard, M. Effects of synthetic calcium phosphates on the 3h-thymidine incorporation and alkaline phosphatase activity of human fibroblasts in culture. J. Biomed. Mater. Res. 1989, 23, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, M.; Orly, I.; Kerebel, L.; Kerebel, B. In vitro effects of calcium phosphate biomaterials on fibroblastic cell behavior. Biol. Cell 1987, 59, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Lacey, D.; Timms, E.; Tan, H.-L.; Kelley, M.; Dunstan, C.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Roodman, G.D. Role of cytokines in the regulation of bone resorption. Calcif. Tissue Int. 1993, 53, S94–S98. [Google Scholar] [CrossRef] [PubMed]
- Benahmed, M.D.; Heymann, D.; Berreur, M.; Cottrel, M.; Godard, A.; Daculsi, G.; Pradal, G. Ultrastructural study of degradation of calcium phosphate ceramic by human monocytes and modulation of this activity by hilda/lif cytokine. J. Histochem. Cytochem. 1996, 44, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, M.; Andreesen, R.; Krause, S.W.; Szabo, A.; Ritz, E.; Reichel, H. 1, 25-dihydroxyvitamin d3 production and vitamin d3 receptor expression are developmentally regulated during differentiation of human monocytes into macrophages. Blood 1993, 82, 1300–1307. [Google Scholar] [PubMed]
- Blifeld, C.; Prehn, J.L.; Jordan, S.C. Stimulus-specific 1, 25 (oh) 2d3 modulation of tnf and il-1-beta gene expression in human peripheral blood mononuclear cells and monocytoid cell lines. Transplantation 1991, 51, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Benahmed, M.; Blottiere, H.; Praloran, V.; Daculsi, G. Monocyte activity in the presence of calcium phosphate activated by 1, 25 (oh) 2 vd3 and interferon-γ. Biomaterials 1994, 15, 25–30. [Google Scholar] [CrossRef]
- Laquerriere, P.; Grandjean-Laquerriere, A.; Jallot, E.; Balossier, G.; Frayssinet, P.; Guenounou, M. Importance of hydroxyapatite particles characteristics on cytokines production by human monocytes in vitro. Biomaterials 2003, 24, 2739–2747. [Google Scholar] [CrossRef]
- Kimakhe, S.; Heymann, D.; Guicheux, J.; Pilet, P.; Giumelli, B.; Daculsi, G. Polymyxin b inhibits biphasic calcium phosphate degradation induced by lipopolysaccharide-activated human monocytes/macrophages. J. Biomed. Mater. Res. 1998, 40, 336–340. [Google Scholar] [CrossRef]
- Benahmed, M.; Heymann, D.; Pilet, P.; Bienvenu, J.; Daculsi, G. Lps increases biomaterial degradation by human monocytes in vitro. J. Biomed. Mater. Res. 1997, 34, 115–119. [Google Scholar] [CrossRef]
- Kim, Y.-W.; Kim, J.-J.; Kim, Y.H.; Rho, J.-Y. Effects of organic matrix proteins on the interfacial structure at the bone–biocompatible nacre interface in vitro. Biomaterials 2002, 23, 2089–2096. [Google Scholar] [CrossRef]
- Constantz, B.R.; Barr, B.M.; Ison, I.C.; Fulmer, M.T.; Baker, J.; McKinney, L.A.; Goodman, S.B.; Gunasekaren, S.; Delaney, D.C.; Ross, J.; et al. Histological, chemical, and crystallographic analysis of four calcium phosphate cements in different rabbit osseous sites. J. Biomed. Mater. Res. 1998, 43, 451–461. [Google Scholar] [CrossRef]
- Apelt, D.; Theiss, F.; El-Warrak, A.O.; Zlinszky, K.; Bettschart-Wolfisberger, R.; Bohner, M.; Matter, S.; Auer, J.A.; von Rechenberg, B. In vivo behavior of three different injectable hydraulic calcium phosphate cements. Biomaterials 2004, 25, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Gisep, A.; Wieling, R.; Bohner, M.; Matter, S.; Schneider, E.; Rahn, B. Resorption patterns of calcium-phosphate cements in bone. J. Biomed. Mater. Res. A 2003, 66, 532–540. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Apatites in biological systems. Prog. Cryst. Growth Charact. Mater. 1981, 4, 1–45. [Google Scholar] [CrossRef]
- Ben-Nissan, B. Advances in Calcium Phosphate Biomaterials; Springer: Berlin, Germany, 2014. [Google Scholar]
- Driessens, F.C.; van Dijk, J.W.; Borggreven, J.M. Biological calcium phosphates and their role in the physiology of bone and dental tissues i. Composition and solubility of calcium phosphates. Calcif. Tissue Res. 1978, 26, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K. Calcium phosphate cement. In Advances in Calcium Phosphate Biomaterials; Springer: Berlin, Germany, 2014; pp. 199–227. [Google Scholar]
- Ginebra, M.-P.; Canal, C.; Espanol, M.; Pastorino, D.; Montufar, E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012, 64, 1090–1110. [Google Scholar] [CrossRef] [PubMed]
- Grossardt, C.; Ewald, A.; Grover, L.M.; Barralet, J.E.; Gbureck, U. Passive and active in vitro resorption of calcium and magnesium phosphate cements by osteoclastic cells. Tissue Eng. A 2010, 16, 3687–3695. [Google Scholar] [CrossRef] [PubMed]
- Frayssinet, P.; Gineste, L.; Conte, P.; Fages, J.; Rouquet, N. Short-term implantation effects of a dcpd-based calcium phosphate cement. Biomaterials 1998, 19, 971–977. [Google Scholar] [CrossRef]
- Kuemmerle, J.M.; Oberle, A.; Oechslin, C.; Bohner, M.; Frei, C.; Boecken, I.; von Rechenberg, B. Assessment of the suitability of a new brushite calcium phosphate cement for cranioplasty—An experimental study in sheep. J. Cranio-Maxillofac. Surg. 2005, 33, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Alkhraisat, M.H.; Marino, F.T.; Retama, J.R.; Jerez, L.B.; Lopez-Cabarcos, E. Beta-tricalcium phosphate release from brushite cement surface. J. Biomed. Mater. Res. A 2008, 84A, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, F.; Le Nihouannen, D.; Eimar, H.; Sheikh, Z.; Komarova, S.; Barralet, J. The effect of autoclaving on the physical and biological properties of dicalcium phosphate dihydrate bioceramics: Brushite vs. Monetite. Acta Biomater. 2012, 8, 3161–3169. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.T.; Torres, J.; Hamdan, M.; Rodriguez, C.R.; Cabarcos, E.L. Advantages of using glycolic acid as a retardant in a brushite forming cement. J. Biomed. Mater. Res. B 2007, 83B, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Ma, P.-W.; Zhang, L.-L.; Yin, Y.-J.; Yao, K.-D.; Zhang, F.-J.; Zhang, Y.-D.; Li, X.-L.; Nie, W. Β-tcp/mcpm-based premixed calcium phosphate cements. Acta Biomater. 2009, 5, 3165–3177. [Google Scholar] [CrossRef] [PubMed]
- Grover, L.M.; Knowles, J.C.; Fleming, G.J.P.; Barralet, J.E. In vitro ageing of brushite calcium phosphate cement. Biomaterials 2003, 24, 4133–4141. [Google Scholar] [CrossRef]
- Pioletti, D.P.; Takei, H.; Lin, T.; van Landuyt, P.; Ma, Q.J.; Kwon, S.Y.; Sung, K.L.P. The effects of calcium phosphate cement particles on osteoblast functions. Biomaterials 2000, 21, 1103–1114. [Google Scholar] [CrossRef]
- Boanini, E.; Gazzano, M.; Bigi, A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Hyuga, H.; Kondo, N.; Kita, H.; Sasaki, M.; Mitamura, M.; Hashimoto, K.; Toda, Y. Substitution model of monovalent (Li, Na, and K), divalent (Mg), and trivalent (Al) metal ions for β-tricalcium phosphate. J. Am. Ceram. Soc. 2006, 89, 688–690. [Google Scholar] [CrossRef]
- Bohner, M.; Galea, L.; Doebelin, N. Calcium phosphate bone graft substitutes: Failures and hopes. J. Eur. Ceram. Soc. 2012, 32, 2663–2671. [Google Scholar] [CrossRef]
- Yang, L.; Perez-Amodio, S.; Barrère-de Groot, F.Y.; Everts, V.; van Blitterswijk, C.A.; Habibovic, P. The effects of inorganic additives to calcium phosphate on in vitro behavior of osteoblasts and osteoclasts. Biomaterials 2010, 31, 2976–2989. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Bernard, S.; Xue, W.; Bose, S. Calcium phosphate-based resorbable ceramics: Influence of mgo, zno, and sio2 dopants. J. Am. Ceram. Soc. 2006, 89, 2675–2688. [Google Scholar] [CrossRef]
- Banerjee, S.S.; Tarafder, S.; Davies, N.M.; Bandyopadhyay, A.; Bose, S. Understanding the influence of mgo and sro binary doping on the mechanical and biological properties of β-tcp ceramics. Acta Biomater. 2010, 6, 4167–4174. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Braux, J.; Velard, F.; Guillaume, C.; Bouthors, S.; Jallot, E.; Nedelec, J.-M.; Laurent-Maquin, D.; Laquerrière, P. A new insight into the dissociating effect of strontium on bone resorption and formation. Acta Biomater. 2011, 7, 2593–2603. [Google Scholar] [CrossRef] [PubMed]
- Buache, E.; Velard, F.; Bauden, E.; Guillaume, C.; Jallot, E.; Nedelec, J.-M.; Laurent-Maquin, D.; Laquerriere, P. Effect of strontium-substituted biphasic calcium phosphate on inflammatory mediators production by human monocytes. Acta Biomater. 2012, 8, 3113–3119. [Google Scholar] [CrossRef] [PubMed]
- Renaudin, G.; Laquerriere, P.; Filinchuk, Y.; Jallot, E.; Nedelec, J.-M. Structural characterization of sol-gel derived sr-substituted calcium phosphates with anti-osteoporotic and anti-inflammatory properties. J. Mater. Chem. 2008, 18, 3593–3600. [Google Scholar] [CrossRef]
- Botelho, C.; Brooks, R.; Spence, G.; McFarlane, I.; Lopes, M.; Best, S.; Santos, J.; Rushton, N.; Bonfield, W. Differentiation of mononuclear precursors into osteoclasts on the surface of si-substituted hydroxyapatite. J. Biomed. Mater. Res. A 2006, 78, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Ito, A.; Kojima, H.; Sakane, M.; Miyakawa, S.; Uemura, T.; LeGeros, R.Z. Inhibitory effect of Zn2+ in zinc-containing β-tricalcium phosphate on resorbing activity of mature osteoclasts. J. Biomed. Mater. Res. A 2008, 84, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Patntirapong, S.; Habibovic, P.; Hauschka, P.V. Effects of soluble cobalt and cobalt incorporated into calcium phosphate layers on osteoclast differentiation and activation. Biomaterials 2009, 30, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Bose, S. Osteoclastogenesis and osteoclastic resorption of tricalcium phosphate: Effect of strontium and magnesium doping. J. Biomed. Mater. Res. A 2012, 100, 2450–2461. [Google Scholar] [CrossRef] [PubMed]
- Flautre, B.; Lemaitre, J.; Maynou, C.; van Landuyt, P.; Hardouin, P. Influence of polymeric additives on the biological properties of brushite cements: An experimental study in rabbit. J. Biomed. Mater. Res. A 2003, 66A, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Li, B. Effects of collagen on the properties of ttcp/mcpm bone cement. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2010, 27, 328–331. [Google Scholar] [PubMed]
- Alkhraisat, M.H.; Rueda, C.; Jerez, L.B.; Marino, F.T.; Torres, J.; Gbureck, U.; Cabarcos, E.L. Effect of silica gel on the cohesion, properties and biological performance of brushite cement. Acta Biomater. 2010, 6, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Huan, Z.G.; Chang, J. Novel bioactive composite bone cements based on the beta-tricalcium phosphate-monocalcium phosphate monohydrate composite cement system. Acta Biomater. 2009, 5, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; van Landuyt, P.; Merkle, H.P.; Lemaitre, J. Composition effects on the pH of a hydraulic calcium phosphate cement. J. Mater. Sci. Mater. Med. 1997, 8, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Theiss, F.; Apelt, D.; Hirsiger, W.; Houriet, R.; Rizzoli, G.; Gnos, E.; Frei, C.; Auer, J.A.; von Rechenberg, B. Compositional changes of a dicalcium phosphate dihydrate cement after implantation in sheep. Biomaterials 2003, 24, 3463–3474. [Google Scholar] [CrossRef]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable materials for bone repair and tissue engineering applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef]
- Sheikh, Z.; Zhang, Y.L.; Grover, L.; Merle, G.E.; Tamimi, F.; Barralet, J. In vitro degradation and in vivo resorption of dicalcium phosphate cement based grafts. Acta Biomater. 2015, 26, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Geffers, M.; Christel, T.; Barralet, J.E.; Gbureck, U. Chelate setting of alkali ion substituted calcium phosphates. Ceram. Int. 2015, 41, 10010–10017. [Google Scholar] [CrossRef]
- Gbureck, U.; Hozel, T.; Klammert, U.; Wurzler, K.; Muller, F.A.; Barralet, J.E. Resorbable dicalcium phosphate bone substitutes prepared by 3D powder printing. Adv. Funct. Mater. 2007, 17, 3940–3945. [Google Scholar] [CrossRef]
- Tamimi, F.; Torres, J.; Gbureck, U.; Lopez-Cabarcos, E.; Bassett, D.C.; Alkhraisat, M.H.; Barralet, J.E. Craniofacial vertical bone augmentation: A comparison between 3d printed monolithic monetite blocks and autologous onlay grafts in the rabbit. Biomaterials 2009, 30, 6318–6326. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheikh, Z.; Abdallah, M.-N.; Hanafi, A.A.; Misbahuddin, S.; Rashid, H.; Glogauer, M. Mechanisms of in Vivo Degradation and Resorption of Calcium Phosphate Based Biomaterials. Materials 2015, 8, 7913-7925. https://doi.org/10.3390/ma8115430
Sheikh Z, Abdallah M-N, Hanafi AA, Misbahuddin S, Rashid H, Glogauer M. Mechanisms of in Vivo Degradation and Resorption of Calcium Phosphate Based Biomaterials. Materials. 2015; 8(11):7913-7925. https://doi.org/10.3390/ma8115430
Chicago/Turabian StyleSheikh, Zeeshan, Mohamed-Nur Abdallah, Ahmed Abdalla Hanafi, Syed Misbahuddin, Haroon Rashid, and Michael Glogauer. 2015. "Mechanisms of in Vivo Degradation and Resorption of Calcium Phosphate Based Biomaterials" Materials 8, no. 11: 7913-7925. https://doi.org/10.3390/ma8115430
APA StyleSheikh, Z., Abdallah, M.-N., Hanafi, A. A., Misbahuddin, S., Rashid, H., & Glogauer, M. (2015). Mechanisms of in Vivo Degradation and Resorption of Calcium Phosphate Based Biomaterials. Materials, 8(11), 7913-7925. https://doi.org/10.3390/ma8115430