Effect of Annealing Temperature on the Mechanical and Corrosion Behavior of a Newly Developed Novel Lean Duplex Stainless Steel
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
| UNS No. | C | Si | Mn | P | S | Cr | Ni | Mo | Cu | N |
|---|---|---|---|---|---|---|---|---|---|---|
| S32750 | ≤0.03 | ≤0.8 | ≤1.2 | ≤0.035 | ≤0.02 | 24.0/26.0 | 6.0/8.0 | 3.0/5.0 | ≤0.5 | 0.24/0.32 |
| S32101 | <0.04 | <1.0 | 4.0/6.0 | <0.04 | <0.03 | 21.0/22.0 | 1.35/1.70 | 0.10/0.80 | 0.10/0.80 | 0.20/0.25 |
| New DSS | 0.03 | 0.32 | 3.45 | 0.01 | 0.004 | 20.53 | 2.08 | 0.31 | 0.34 | 0.17 |
2.2. Uniaxial Tensile Test
2.3. Electrochemical Measurement
2.4. Optical Metallographic Microscopy and SEM-EDS Analysis
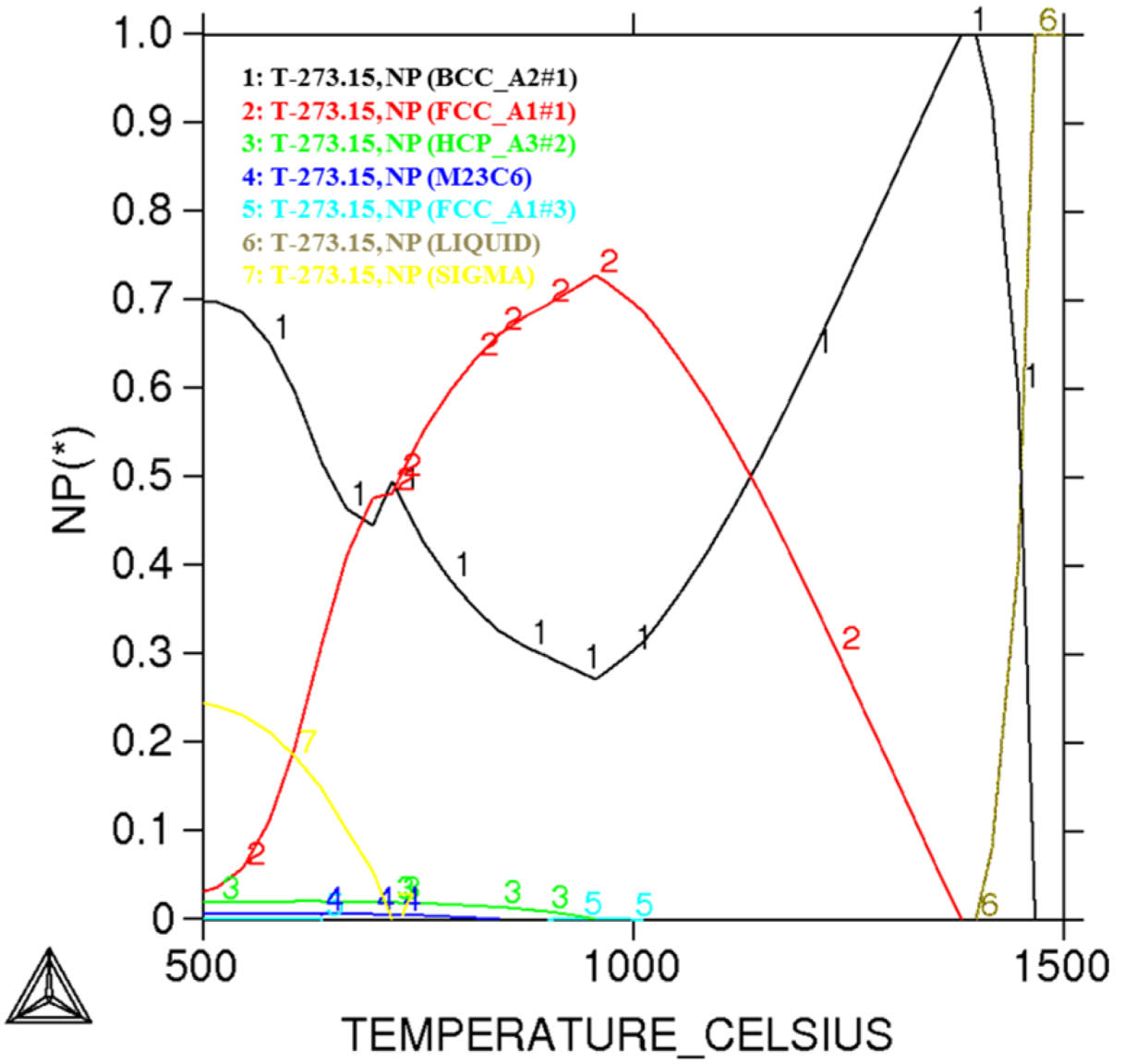
| Annealing temperature | Phase | Volume fraction (%) | Cr (%) | Ni (%) | Mo (%) | N (%) | PREN16 | PREN30 |
|---|---|---|---|---|---|---|---|---|
| 1000 °C | Ferrite | 52.1 | 21.59 | 1.51 | 0.40 | 0.05 | 23.710 | 24.410 |
| Austenite | 47.9 | 19.37 | 2.70 | 0.21 | 0.30 | 24.863 | 29.063 | |
| 1020 °C | Ferrite | 57.9 | 21.45 | 1.68 | 0.32 | 0.05 | 23.306 | 24.006 |
| Austenite | 42.1 | 19.27 | 2.62 | 0.29 | 0.34 | 25.667 | 30.427 | |
| 1050 °C | Ferrite | 61.4 | 21.24 | 1.72 | 0.33 | 0.05 | 23.129 | 23.829 |
| Austenite | 38.6 | 19.40 | 2.66 | 0.28 | 0.36 | 26.084 | 31.124 | |
| 1080 °C | Ferrite | 62.9 | 21.25 | 1.52 | 0.32 | 0.05 | 23.106 | 23.806 |
| Austenite | 37.1 | 19.31 | 3.03 | 0.30 | 0.37 | 26.220 | 31.400 | |
| 1110 °C | Ferrite | 66.2 | 21.15 | 1.73 | 0.35 | 0.05 | 23.105 | 23.805 |
| Austenite | 33.8 | 19.32 | 2.77 | 0.22 | 0.41 | 26.606 | 32.346 | |
| 1150 °C | Ferrite | 69.1 | 21.05 | 1.85 | 0.32 | 0.05 | 22.906 | 23.606 |
| Austenite | 30.9 | 19.37 | 2.60 | 0.29 | 0.44 | 27.367 | 33.527 |
2.5. Magnetic Force Microscopy (MFM) and Scanning Kelvin Probe Force Microscopy (SKPFM) Measurements
3. Results and Discussion
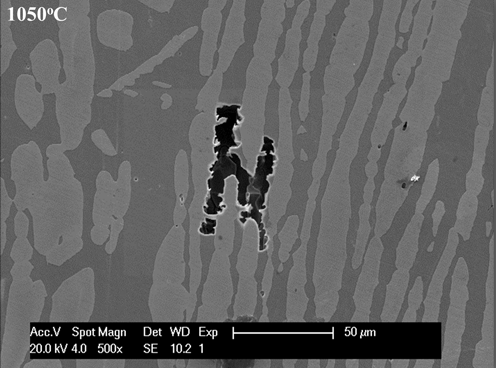
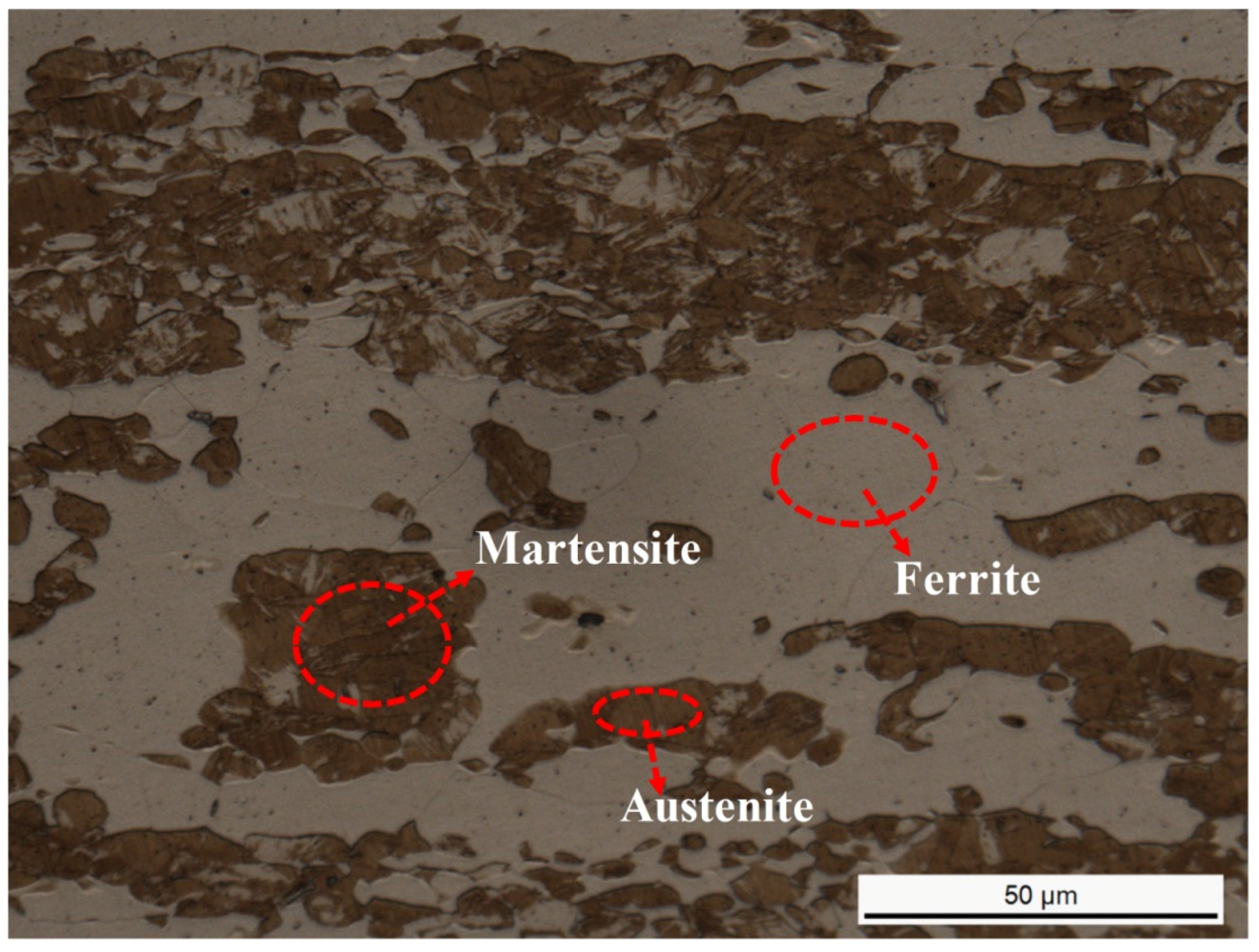
3.1. Microstructures and Elements Distribution

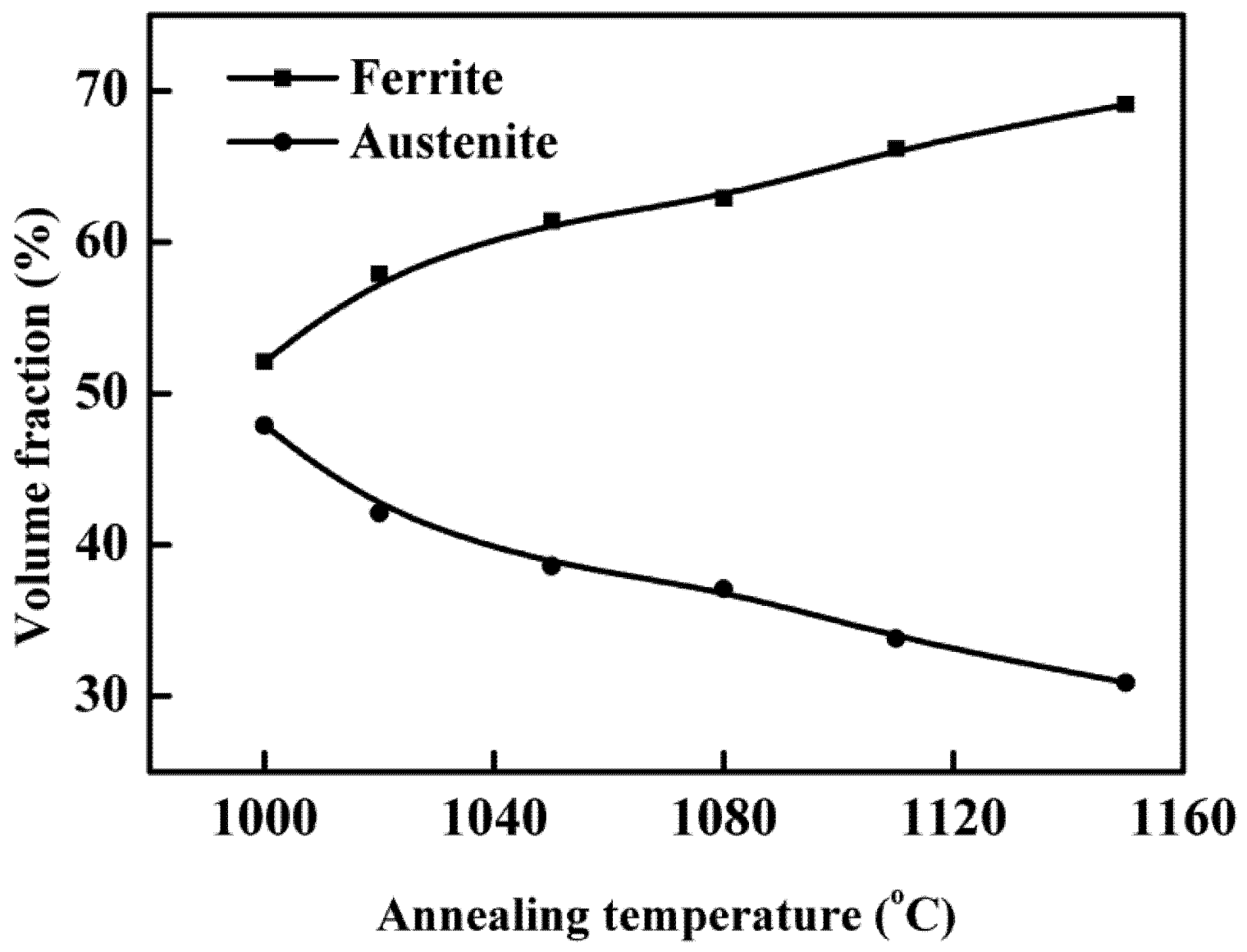
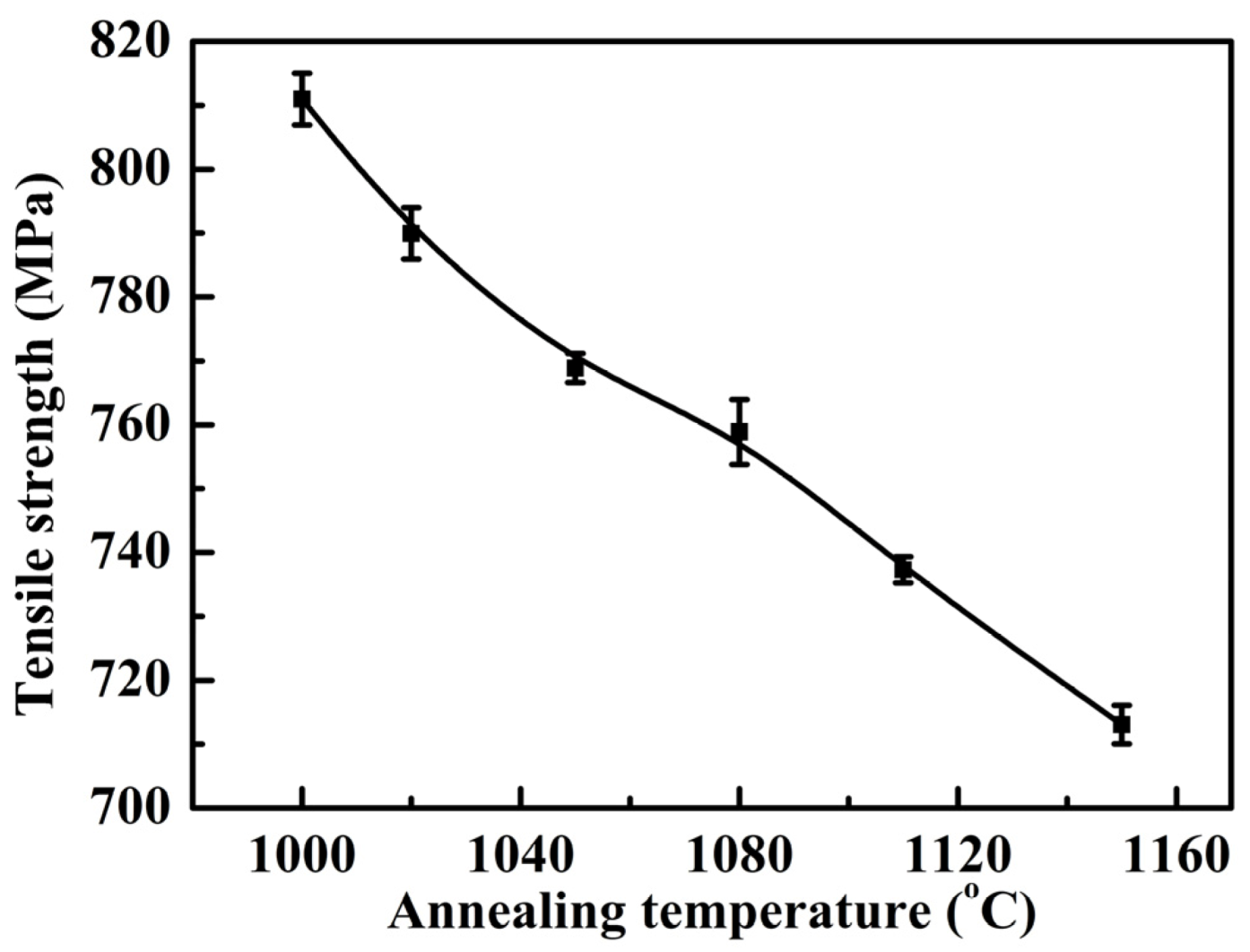
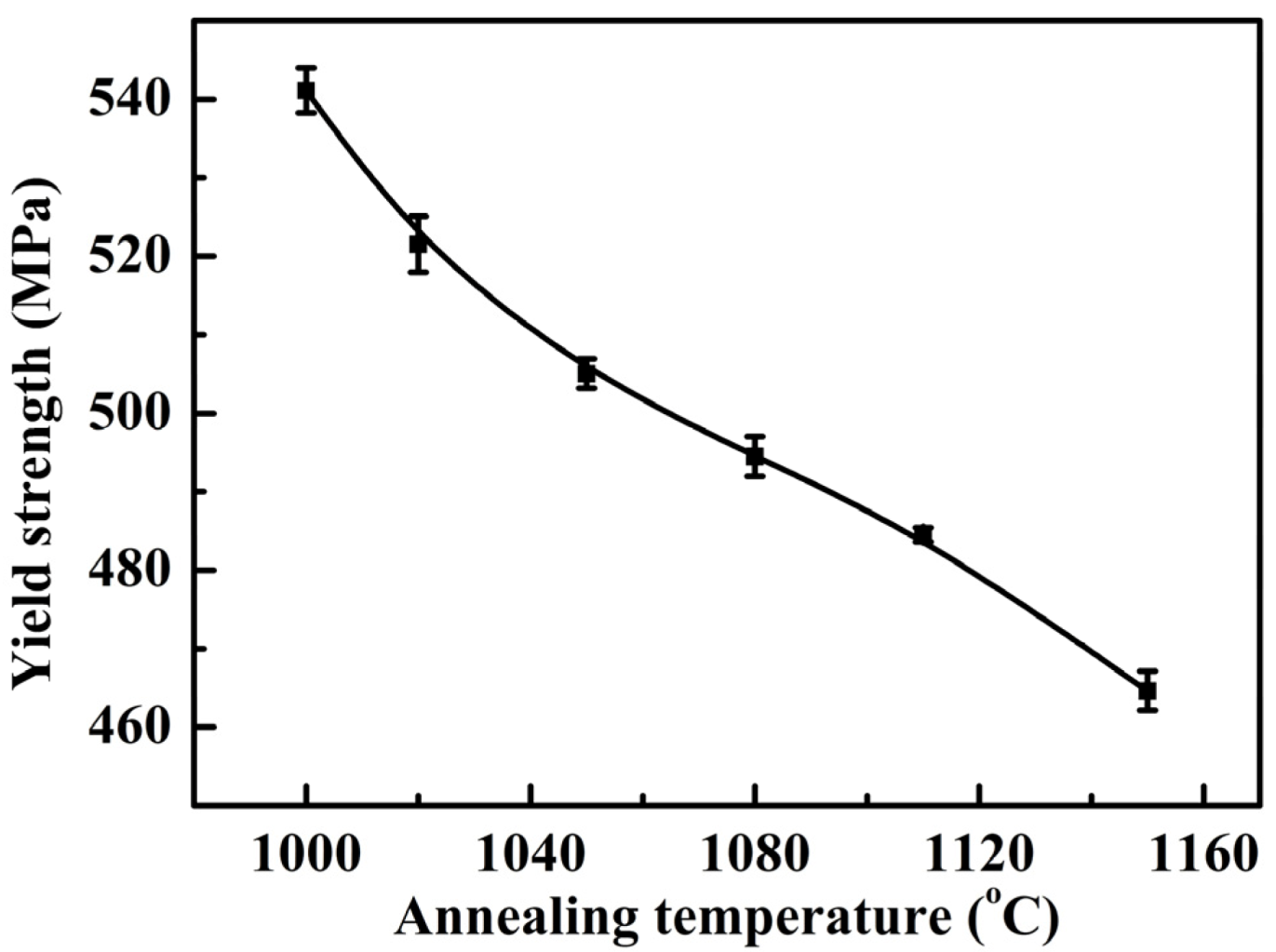
3.2. Mechanical Properties

| Alloy | Ultimate tensile strength, MPa | 0.2% proof stress (min) MPa | Elongation (min) A5, % |
|---|---|---|---|
| UNS S32750 [1] | 800–1000 | 550 | 25 |
| UNS S32101 [8] | 650–700 | 450 | 30 |
| Newly Developed DSS | 737–811 | 465 | 47.3 |
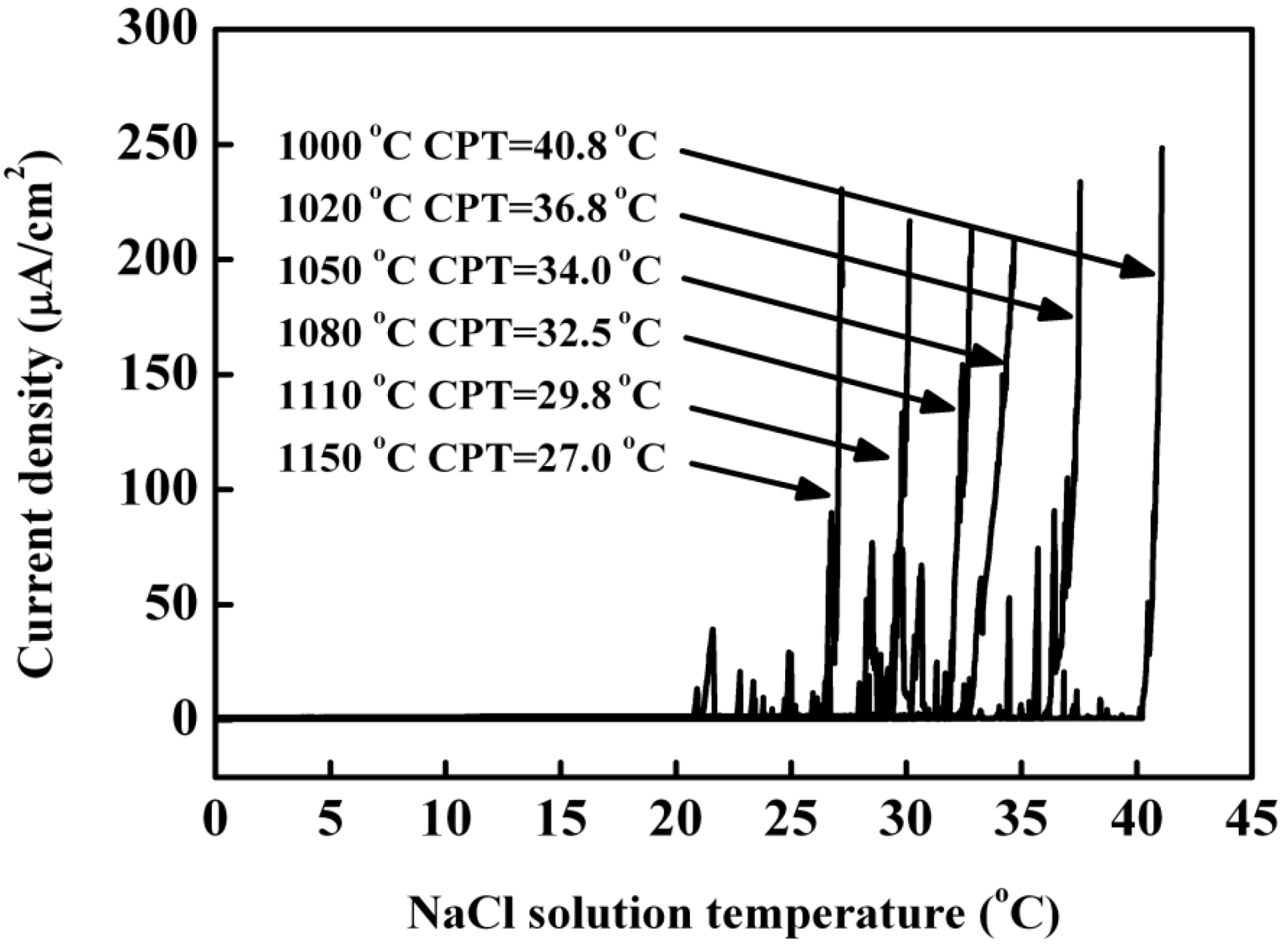
3.3. Pitting Corrosion Behavior
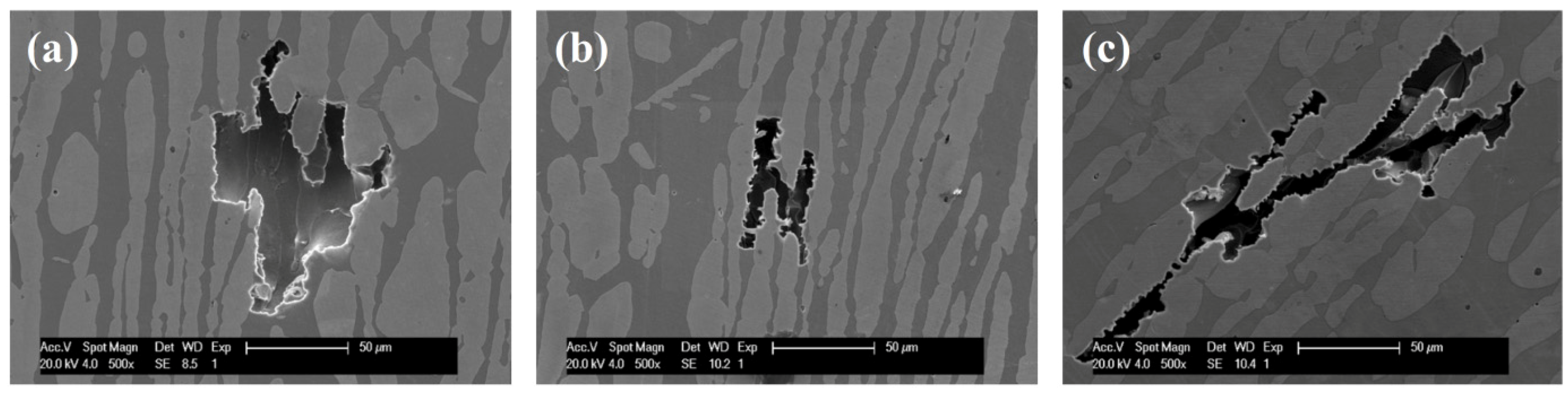

3.4. MFM and SKPFM Results

| Annealing temperature (°C) | Average Volta potential differenceFerrite-Austenite (mV) |
|---|---|
| 1000 | 37.5 |
| 1050 | 50 |
| 1150 | 75 |
4. Conclusions
- (1)
- The volume fraction of austenite decreased continuously and the ferrite increased with the annealing temperature increasing from 1000 to 1150 °C.
- (2)
- The tensile strength and yield strength decreased with the increase of annealing temperature, which could be explained by the grain size and solid solution strengthening effects of alloying elements.
- (3)
- The elongation at break reached the maximum of 52.7% after annealing at 1050 °C due to martensite transformation associated with TRIPeffect.
- (4)
- The critical pitting temperature decreased with increasing of annealing temperature. The localized pitting attack preferentially occurred at ferrite phase. The pitting corrosion behaviorin chloride solutions could be explained by redistribution of main alloying elements, such as chromium, molybdenum and nitrogen in two phases, resulting in the change of PREN16 of ferrite and austenite phases.
- (5)
- Ferrite had a lower Volta potential than austenite phase. The Volta potential difference between ferrite and austenite increased as the increase of annealing temperature. The Volta potential difference between ferrite and austenite was in good conformity with the difference between PRENFerrite and PRENAustenite.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nilsson, J.O. Super duplex stainless-steels. Mater. Sci. Technol. 1992, 8, 685–700. [Google Scholar] [CrossRef]
- Chen, T.H.; Yang, J.R. Effects of solution treatment and continuous cooling on sigma-phase precipitation in a 2205 duplex stainless steel. Mater. Sci. Eng. A 2001, 311, 28–41. [Google Scholar] [CrossRef]
- He, H.; Zhang, T.; Zhao, C.Z.; Hou, K.; Meng, G.Z.; Shao, Y.W.; Wang, F.H. Effect of alternating voltage passivation on the corrosion resistance of duplex stainless steel. J. Appl. Electrochem. 2009, 39, 737–745. [Google Scholar] [CrossRef]
- Lee, K.M.; Cho, H.S.; Choi, D.C. Effect of isothermal treatment of SAF 2205 duplex stainless steel on migration of delta/gamma interface boundary and growth of austenite. J. Alloys Compd. 1999, 285, 156–161. [Google Scholar]
- Nilsson, J.O.; Wilson, A. Influence of isothermal phase-transformations on toughness and pitting corrosion of super duplex stainless-steel SAF-2507. Mater. Sci. Technol. 1993, 9, 545–554. [Google Scholar] [CrossRef]
- Sato, Y.S.; Nelson, T.W.; Sterling, C.J.; Steel, R.J.; Pettersson, C.O. Microstructure and mechanical properties of friction stir welded SAF 2507 super duplex stainless steel. Mater. Sci. Eng. A 2005, 397, 376–384. [Google Scholar] [CrossRef]
- Weber, L.; Uggowitzer, P.J. Partitioning of chromium and molybdenum in super duplex stainless steels with respect to nitrogen and nickel content. Mater. Sci. Eng. A 1998, 242, 222–229. [Google Scholar] [CrossRef]
- Alfonsson, E. Lean Duplex—The First Decade of Service Experience. In Proceedings of the 8th Duplex Stainless Steels Conference, Beaune, France, 13–15 October 2010.
- Zhang, W.; Jiang, L.Z.; Hu, J.C.; Song, H.M. Effect of ageing on precipitation and impact energy of 2101 economical duplex stainless steel. Mater. Charact. 2009, 60, 50–55. [Google Scholar] [CrossRef]
- Zhang, L.H.; Zhang, W.; Jiang, Y.M.; Deng, B.; Sun, D.M.; Li, J. Influence of annealing treatment on the corrosion resistance of lean duplex stainless steel 2101. Electrochim. Acta 2009, 54, 5387–5392. [Google Scholar] [CrossRef]
- Sieurin, H.; Sandstrom, R.; Westin, E.M. Fracture toughness of the lean duplex stainless steel LDX 2101. Metall. Mater. Trans. A 2006, 37A, 2975–2981. [Google Scholar] [CrossRef]
- Zhang, L.H.; Jiang, Y.M.; Deng, B.; Zhang, W.; Xu, J.L.; Li, J. Effect of aging on the corrosion resistance of 2101 lean duplex stainless steel. Mater. Charact. 2009, 60, 1522–1528. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, J.H. Effect of annealing temperature on transformation induced plasticity effect of a lean duplex stainless steel. Mater. Charact. 2013, 79, 37–42. [Google Scholar] [CrossRef]
- Herrera, C.; Ponge, D.; Raabe, D. Design of a novel Mn-based 1 GPa duplex stainless TRIP steel with 60% ductility by a reduction of austenite stability. Acta Mater. 2011, 59, 4653–4664. [Google Scholar] [CrossRef]
- Choi, J.Y.; Ji, J.H.; Hwang, S.W.; Park, K.T. Strain induced martensitic transformation of Fe-20Cr-5Mn-0.2Ni duplex stainless steel during cold rolling: Effects of nitrogen addition. Mater. Sci. Eng. A 2011, 528, 6012–6019. [Google Scholar] [CrossRef]
- Pohl, M.; Storz, O.; Glogowski, T. Effect of intermetallic precipitations on the properties of duplex stainless steel. Mater. Charact. 2007, 58, 65–71. [Google Scholar] [CrossRef]
- Deng, B.; Wang, Z.Y.; Jiang, Y.M.; Sun, T.; Xu, J.L.; Li, J. Effect of thermal cycles on the corrosion and mechanical properties of UNS S31803 duplex stainless steel. Corros. Sci. 2009, 51, 2969–2975. [Google Scholar] [CrossRef]
- British Standards (BS) European Norm (EN) International Organization for Standardization (ISO) 6892-1:2009, “Metallic materials Tensile testing; Part 1: Method of test at ambient temperature”. European Committee for Standardization: Brüssel, 2009.
- American Society for Testing and Materials (ASTM) Standard G150-99. In Standard Test Method for Electrochemical Critical Pitting Temperature Testing of Stainless Steels, Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2010.
- Tan, H.; Jiang, Y.M.; Deng, B.; Sun, T.; Xu, J.L.; Li, J. Effect of annealing temperature on the pitting corrosion resistance of super duplex stainless steel UNS S32750. Mater. Charact. 2009, 60, 1049–1054. [Google Scholar] [CrossRef]
- GarfiasMesias, L.F.; Sykes, J.M.; Tuck, C.D.S. The effect of phase compositions on the pitting corrosion of 25 Cr duplex stainless steel in chloride solutions. Corros. Sci. 1996, 38, 1319–1330. [Google Scholar] [CrossRef]
- Nonnenmacher, M.; Oboyle, M.P.; Wickramasinghe, H.K. Kelvin probe force microscopy. Appl. Phys. Lett. 1991, 58, 2921–2923. [Google Scholar] [CrossRef]
- Rohwerder, M.; Turcu, F. High-resolution Kelvin probe microscopy in corrosion science: Scanning Kelvin probe force microscopy (SKPFM) versus classical scanning Kelvin probe (SKP). Electrochim. Acta 2007, 53, 290–299. [Google Scholar] [CrossRef]
- Yousefieh, M.; Shamanian, M.; Saatchi, A. Influence of step annealing temperature on the microstructure and pitting corrosion resistance of SDSS UNS S32760 welds. J. Mater. Eng. Perform. 2011, 20, 1678–1683. [Google Scholar] [CrossRef]
- Offerman, S.E.; van Dijk, N.H.; Sietsma, J.; Grigull, S.; Lauridsen, E.M.; Margulies, L.; Poulsen, H.F.; Rekveldt, M.T.; van der Zwaag, S. Grain nucleation and growth during phase transformations. Science 2002, 298, 1003–1005. [Google Scholar] [CrossRef]
- Badji, R.; Bacroix, B.; Bouabdallah, M. Texture, microstructure and anisotropic properties in annealed 2205 duplex stainless steel welds. Mater. Charact. 2011, 62, 833–843. [Google Scholar] [CrossRef]
- Zhang, X.D.; Hansen, N.; Gao, Y.K.; Huang, X.X. Hall-Petch and dislocation strengthening in graded nanostructured steel. Acta Mater. 2012, 60, 5933–5943. [Google Scholar] [CrossRef]
- Merello, R.; Botana, F.J.; Botella, J.; Matres, M.V.; Marcos, M. Influence of chemical composition on the pitting corrosion resistance of non-standard low-Ni high-Mn-N duplex stainless steels. Corros. Sci. 2003, 45, 909–921. [Google Scholar] [CrossRef]
- Bae, S.H.; Lee, H.W. Effect of Mo contents on corrosion behaviors of welded duplex stainless steel. Met. Mater. Int. 2013, 19, 563–569. [Google Scholar] [CrossRef]
- Munoz, A.I.; Anton, J.G.; Nuevalos, S.L.; Guinon, J.L.; Herranz, V.N. Corrosion studies of austenitic and duplex stainless steels in aqueous lithium bromide solution at different temperatures. Corros. Sci. 2004, 46, 2955–2974. [Google Scholar] [CrossRef]
- Zhong, C.; Liu, F.; Wu, Y.T.; Le, J.J.; Liu, L.; He, M.F.; Zhu, J.C.; Hu, W.B. Protective diffusion coatings on magnesium alloys: A review of recent developments. J. Alloys Compd. 2012, 520, 11–21. [Google Scholar] [CrossRef]
- Zhong, C.; He, M.F.; Liu, L.; Wu, Y.T.; Chen, Y.J.; Deng, Y.D.; Shen, B.; Hu, W.B. Lower temperature fabrication of continuous intermetallic coatings on AZ91D magnesium alloy in molten salts. J. Alloys Compd. 2010, 504, 377–381. [Google Scholar] [CrossRef]
- Le, J.J.; Liu, L.; Liu, F.; Deng, Y.D.; Zhong, C.; Hu, W.B. Interdiffusion kinetics of the intermetallic coatings on AZ91D magnesium alloy formed in molten salts at lower temperatures. J. Alloys Compd. 2014, 610, 173–179. [Google Scholar] [CrossRef]
- Mori, G.; Bauernfeind, D. Pitting and crevice corrosion of superaustenitic stainless steels. Mater. Corros. 2004, 55, 164–173. [Google Scholar] [CrossRef]
- Sathirachinda, N.; Pettersson, R.; Pan, J.S. Depletion effects at phase boundaries in 2205 duplex stainless steel characterized with SKPFM and TEM/EDS. Corros. Sci. 2009, 51, 1850–1860. [Google Scholar] [CrossRef]
- Guo, L.Q.; Li, M.; Shi, X.L.; Yan, Y.; Li, X.Y.; Qiao, L.J. Effect of annealing temperature on the corrosion behavior of duplex stainless steel studied by in situ techniques. Corros. Sci. 2011, 53, 3733–3741. [Google Scholar] [CrossRef]
- Sathirachinda, N.; Gubner, R.; Pan, J.; Kivisakk, U. Characterization of phases in duplex stainless steel by magnetic force microscopy/scanning kelvin probe force microscopy. Electrochem. Solid State Lett. 2008, 11, C41–C45. [Google Scholar] [CrossRef]
- Stratmann, M. The investigation of the corrosion propereties of metals, covered with adsobed electrolyte layers—A new experimental technique. Corros. Sci. 1987, 27, 869–872. [Google Scholar] [CrossRef]
- Stratmann, M.; Streckel, H. On the atmospheric corrosion of metals which are covered with thin electrolyte layers.1. Verification of the experimental technique. Corros. Sci. 1990, 30, 681–696. [Google Scholar] [CrossRef]
- Yee, S.G.; Oriani, R.A.; Stratmann, M. Application of a kelvin microprobe to the corrosion of metals in humid atmospheres. J. Electrochem. Soc. 1991, 138, 55–61. [Google Scholar] [CrossRef]
- Schmutz, P.; Frankel, G.S. Characterization of AA2024-T3 by scanning Kelvin probe force microscopy. J. Electrochem. Soc. 1998, 145, 2285–2295. [Google Scholar] [CrossRef]
- Guillaumin, V.; Schmutz, P.; Frankel, G.S. Characterization of corrosion interfaces by the scanning Kelvin probe force microscopy technique. J. Electrochem. Soc. 2001, 148, B163–B173. [Google Scholar] [CrossRef]
- Femenia, M.; Canalias, C.; Pan, J.; Leygraf, C. Scanning Kelvin probe force microscopy and magnetic force microscopy for characterization of duplex stainless steels. J. Electrochem. Soc. 2003, 150, B274–B281. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Guo, Y.; Hu, J.; Li, J.; Jiang, L.; Liu, T.; Wu, Y. Effect of Annealing Temperature on the Mechanical and Corrosion Behavior of a Newly Developed Novel Lean Duplex Stainless Steel. Materials 2014, 7, 6604-6619. https://doi.org/10.3390/ma7096604
Guo Y, Hu J, Li J, Jiang L, Liu T, Wu Y. Effect of Annealing Temperature on the Mechanical and Corrosion Behavior of a Newly Developed Novel Lean Duplex Stainless Steel. Materials. 2014; 7(9):6604-6619. https://doi.org/10.3390/ma7096604
Chicago/Turabian StyleGuo, Yanjun, Jincheng Hu, Jin Li, Laizhu Jiang, Tianwei Liu, and Yanping Wu. 2014. "Effect of Annealing Temperature on the Mechanical and Corrosion Behavior of a Newly Developed Novel Lean Duplex Stainless Steel" Materials 7, no. 9: 6604-6619. https://doi.org/10.3390/ma7096604