Synthesis and Thermal Properties of Acrylonitrile/Butyl Acrylate/Fumaronitrile and Acrylonitrile/Ethyl Hexyl Acrylate/Fumaronitrile Terpolymers as a Potential Precursor for Carbon Fiber
Abstract
:1. Introduction
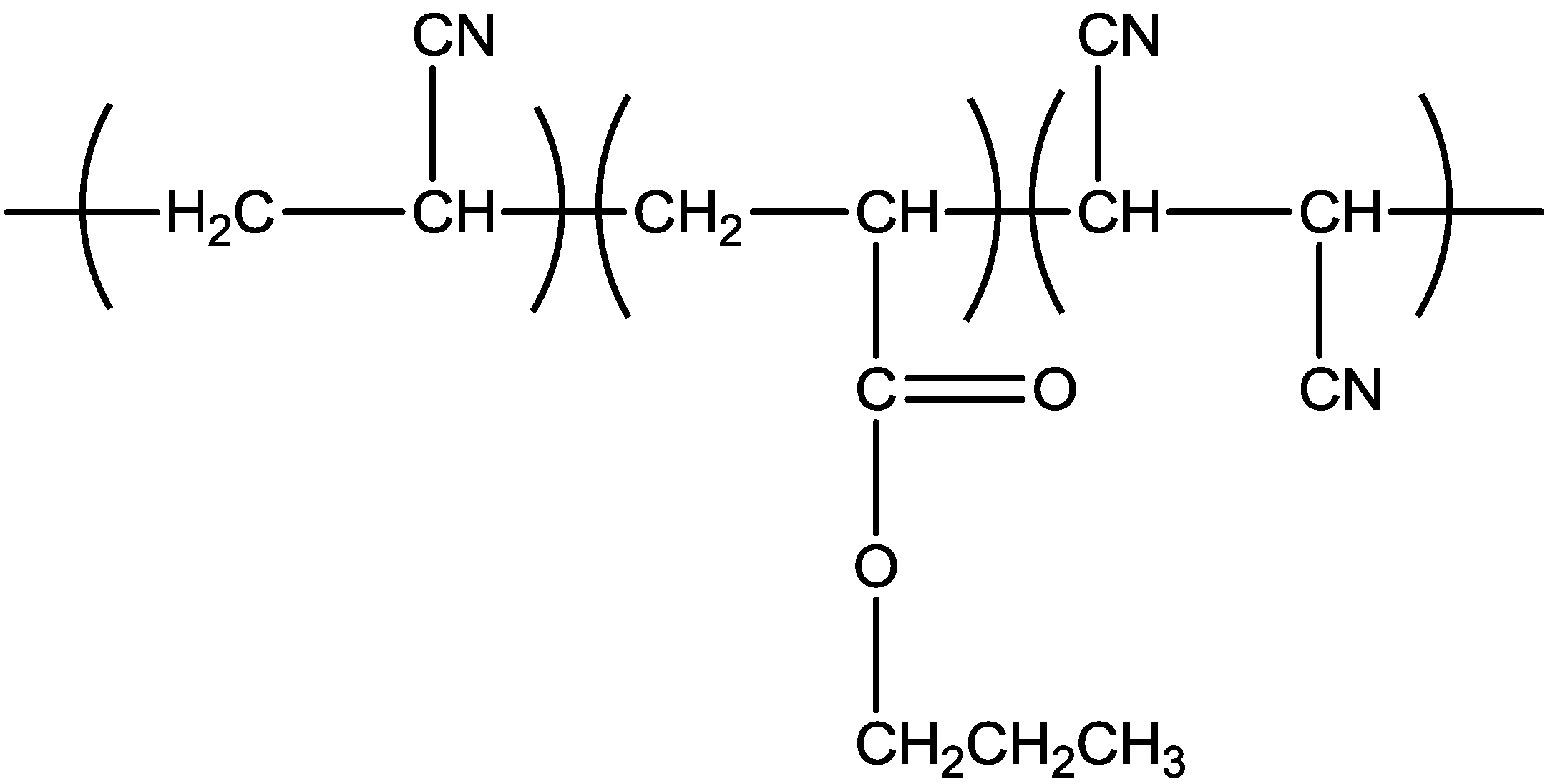
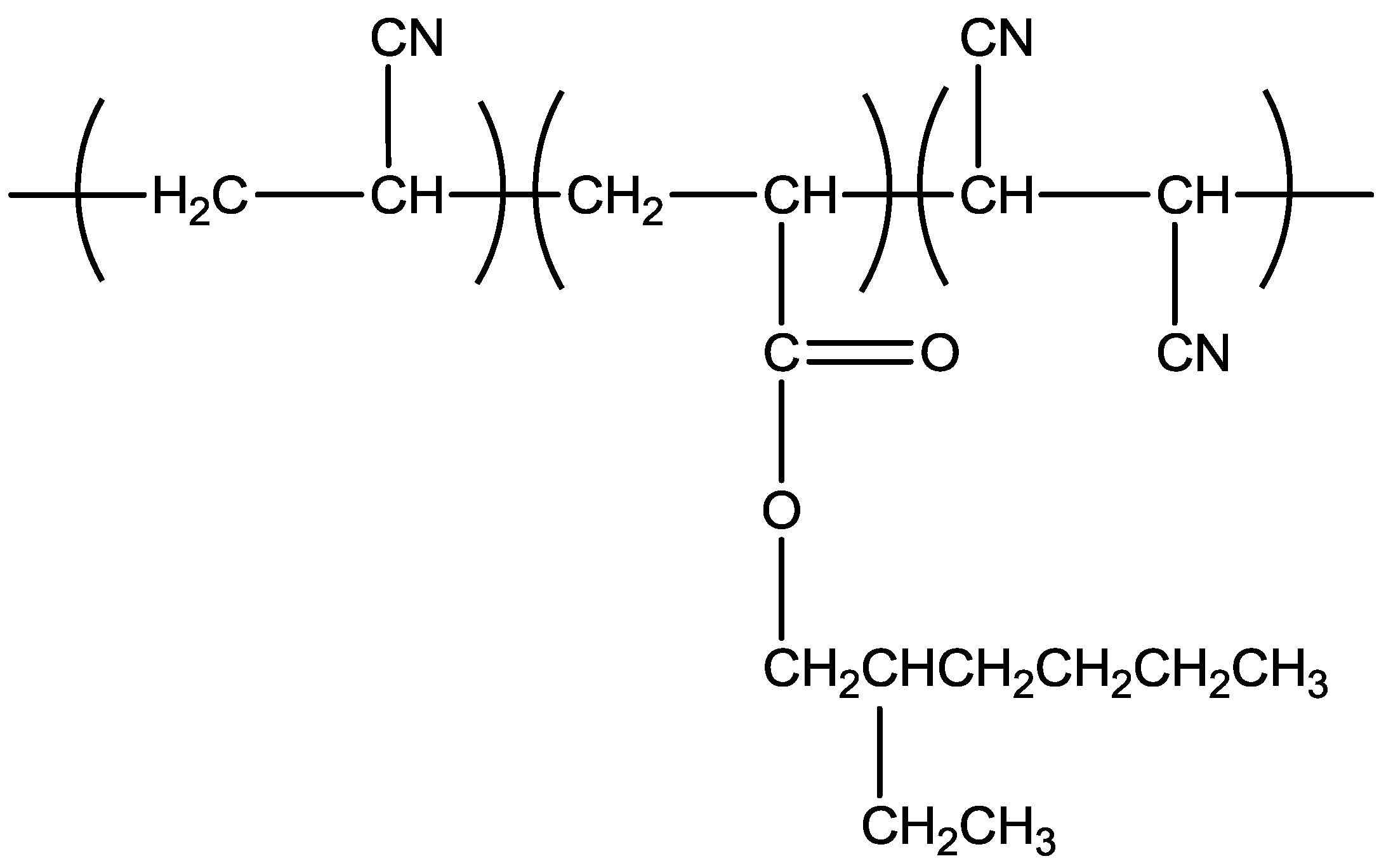
2. Results and Discussion
2.1. FTIR Spectroscopy
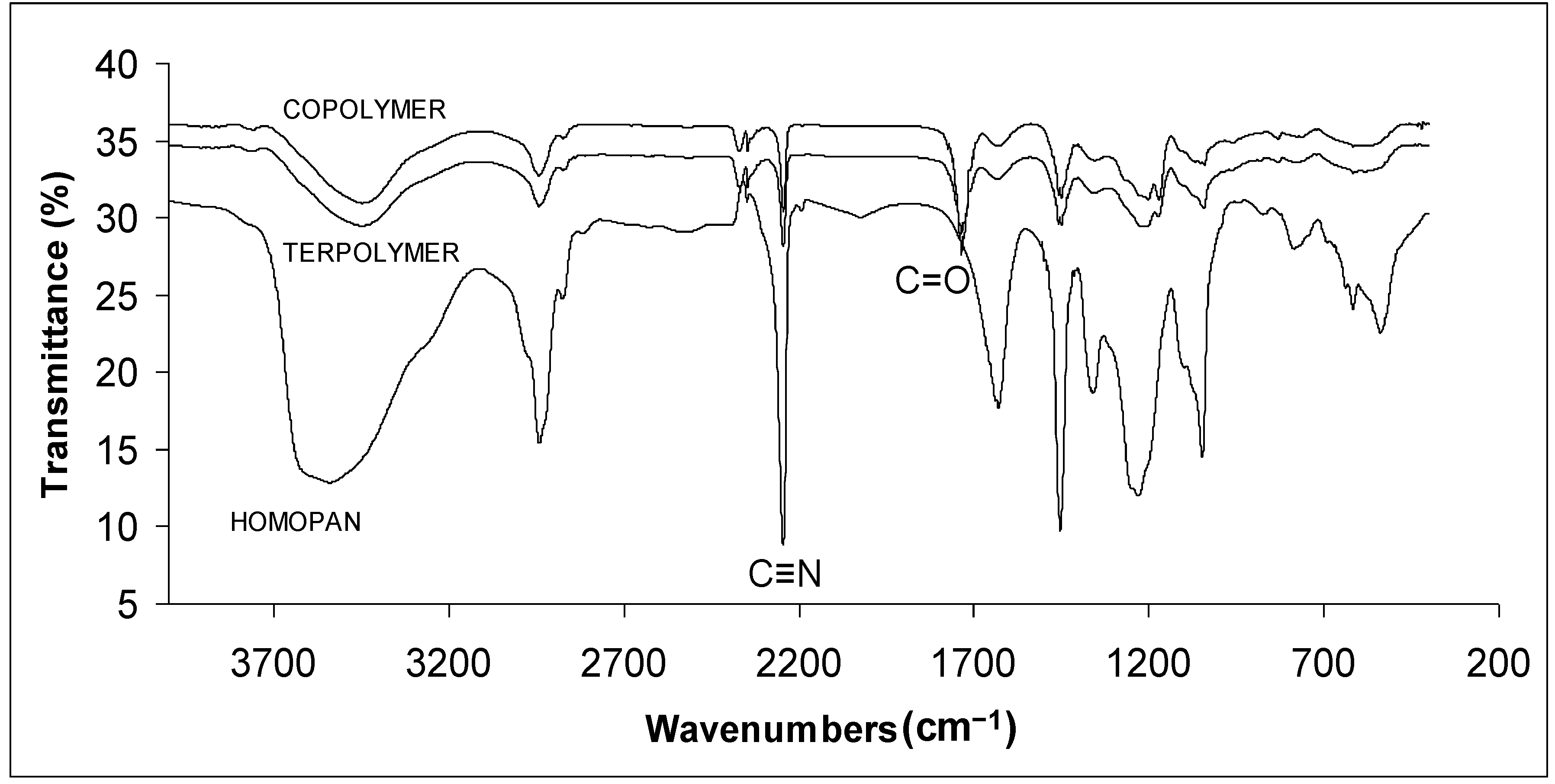
2.2. Conversions and Composition Analysis
| Monomers feed (mol%), M AN/comonomer/FN | Monomers conversion (%) | Monomers composition (mol%), m AN/comonomer/FN | Reacted monomer (%) m/M × 100 AN/comonomer/FN | Actual composition (mol%) AN/comonomer/FN |
|---|---|---|---|---|
| 100/0/0 | 85 | 85/0/0 | 85/0/0 | 100/0/0 |
| AN/BA/FN | ||||
| 95/5/0 | 77 | 71.35/4.50/0 | 75/90/0 | 94.1/5.9/0 |
| 90/10/0 | 75 | 64.06/8.90/0 | 71/89/0 | 87.8/12.2/0 |
| 90/2/8 | 73 | 64.58/1.78/6.24 | 72/89/78 | 88.9/2.5/8.6 |
| 90/4/6 | 67 | 57.29/3.48/4.74 | 64/87/79 | 87.5/5.3/7.2 |
| 90/6/4 | 68 | 58.33/5.10/3.08 | 65/85/77 | 87.7/7.7/4.6 |
| 90/8/2 | 61 | 49.48/6.96/1.51 | 55/87/76 | 85.4/12/2.6 |
| AN/EHA/FN | ||||
| 95/5/0 | 76 | 69.79/4.55/0 | 73/91/0 | 93.9/6.1/0 |
| 90/10/0 | 77 | 64.58/9.0/0 | 72/90/0 | 87.8/12.2/0 |
| 90/2/8 | 71 | 61.46/1.82/6.32 | 68/91/79 | 88.3/2.6/9.1 |
| 90/4/6 | 68 | 57.29/3.60/4.62 | 64/90/77 | 87.45/5.5/7.1 |
| 90/6/4 | 67 | 55.21/5.28/3.12 | 61/88/78 | 86.8/8.3/4.9 |
| 90/8/2 | 66 | 53.13/6.96/1.54 | 59/87/77 | 86.2/11.3/2.5 |
2.3. DSC Studies
2.3.1. Effect of Comonomer on Tg
| AN/comonomer/FN (mol%) | Glass transition, Tg (°C) |
|---|---|
| 100/0/0 | 210 ± 1 |
| AN/BA/FN | |
| 90/2/8 | 69 ± 1 |
| 90/4/6 | 67 ± 2 |
| 90/6/4 | 67 ± 2 |
| 90/8/2 | 63 ± 1 |
| 90/10/0 | 70 ± 1 |
| AN/EHA/FN | |
| 90/2/8 | 67 ± 1 |
| 90/4/6 | 65 ± 1 |
| 90/6/4 | 63 ± 1 |
| 90/8/2 | 60 ± 2 |
| 90/10/0 | 63 ± 1 |
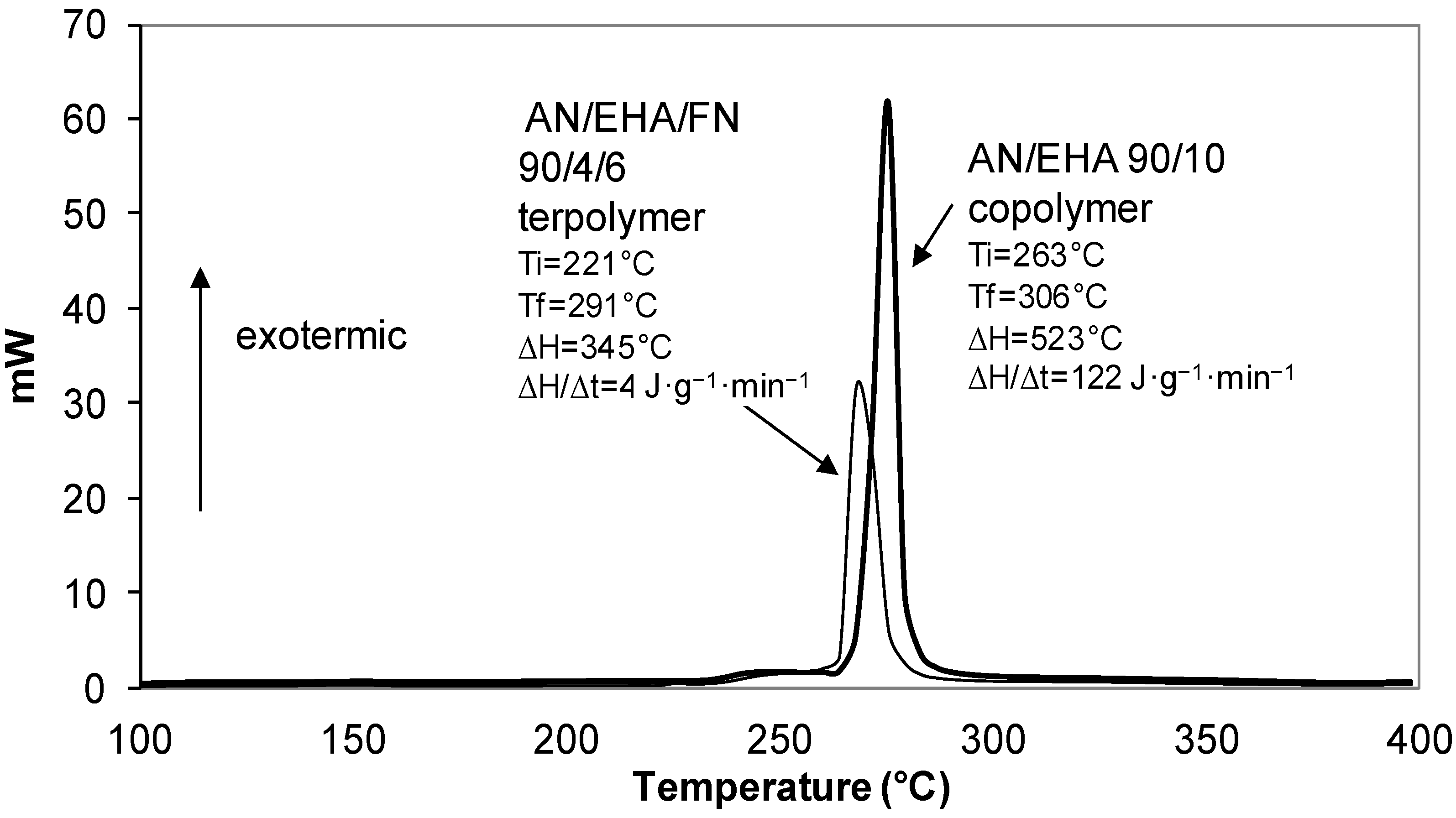
2.3.2. Effect of Comonomer and Termonomer on Stabilization Temperature
| AN/comonomer/FN (mol%) | Ti (°C) | Tf (°C) | ∆T (°C) | ∆H (J·g−1) | ∆H/∆t (J·g−1·min−1) |
|---|---|---|---|---|---|
| 100/0/0 | 246 | 345 | 99 | 758 | 77 |
| AN/BA/FN | |||||
| 90/10/0 | 264 | 321 | 57 | 590 | 104 |
| 90/2/8 | 228 | 309 | 81 | 371 | 46 |
| 90/4/6 | 233 | 317 | 84 | 392 | 47 |
| 90/6/4 | 241 | 331 | 90 | 437 | 49 |
| 90/8/2 | 260 | 353 | 93 | 489 | 53 |
| AN/EHA/FN | |||||
| 90/10/0 | 263 | 306 | 43 | 523 | 122 |
| 90/2/8 | 250 | 321 | 71 | 321 | 45 |
| 90/4/6 | 221 | 291 | 70 | 345 | 49 |
| 90/6/4 | 220 | 304 | 84 | 356 | 42 |
| 90/8/2 | 241 | 327 | 86 | 381 | 44 |
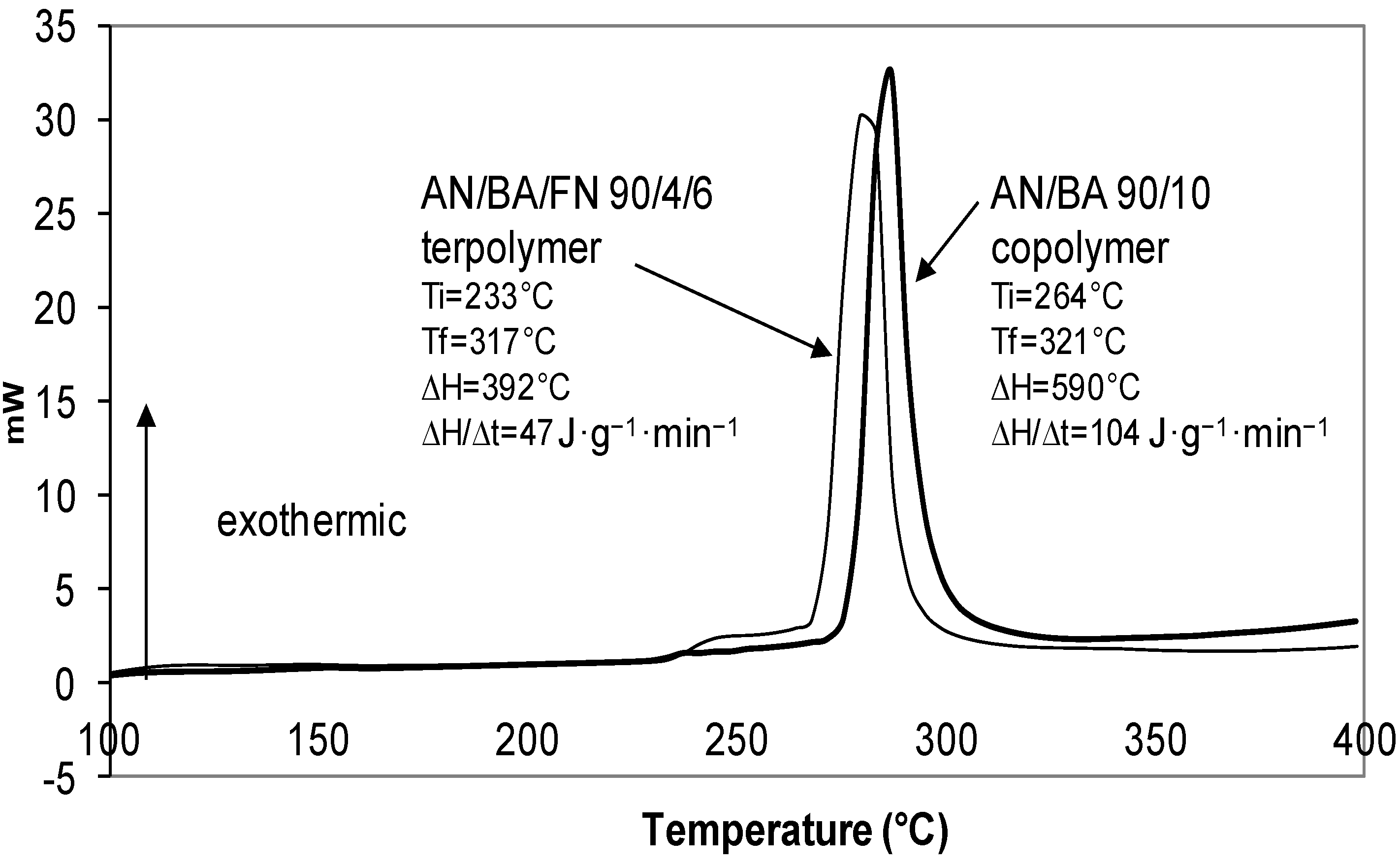
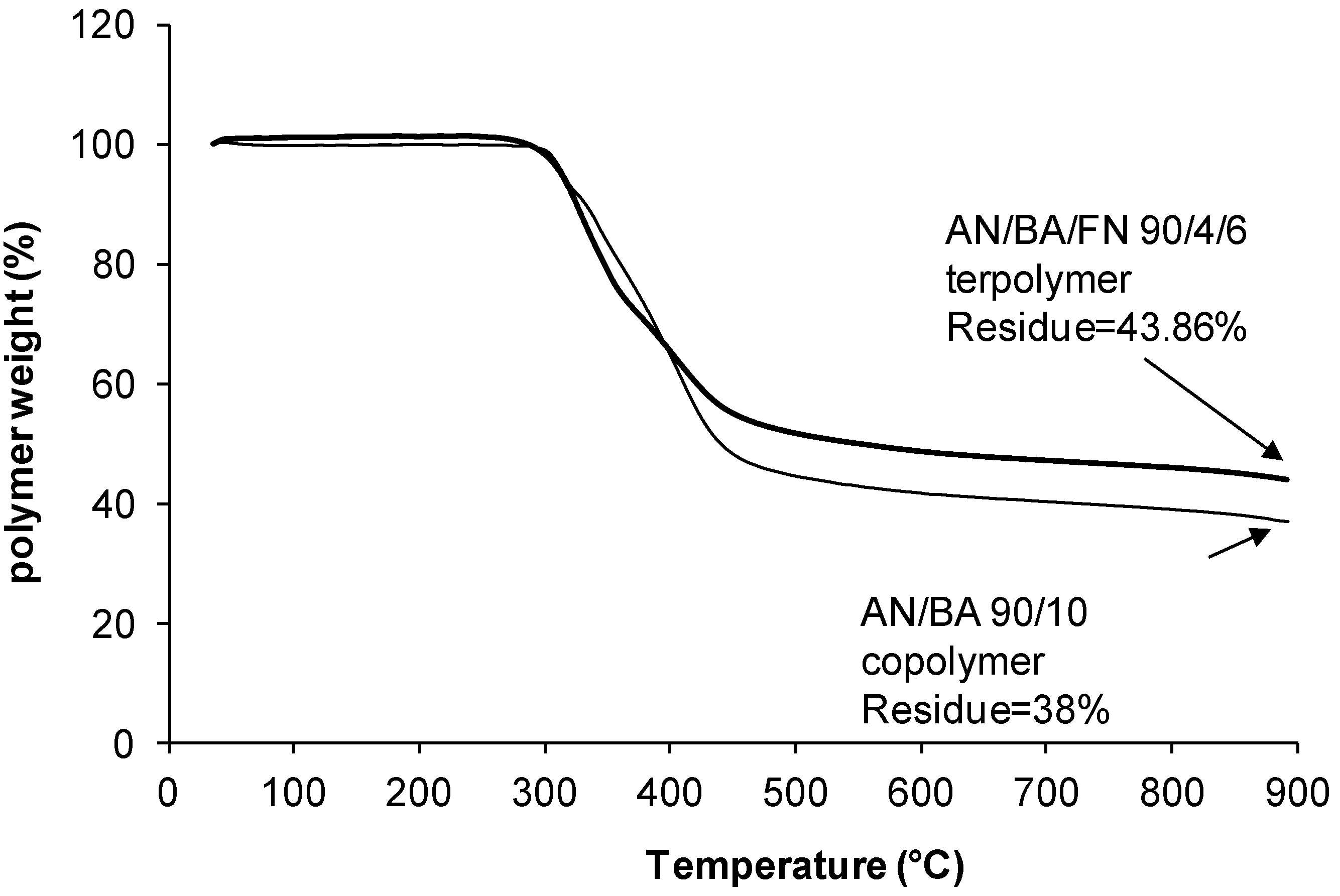
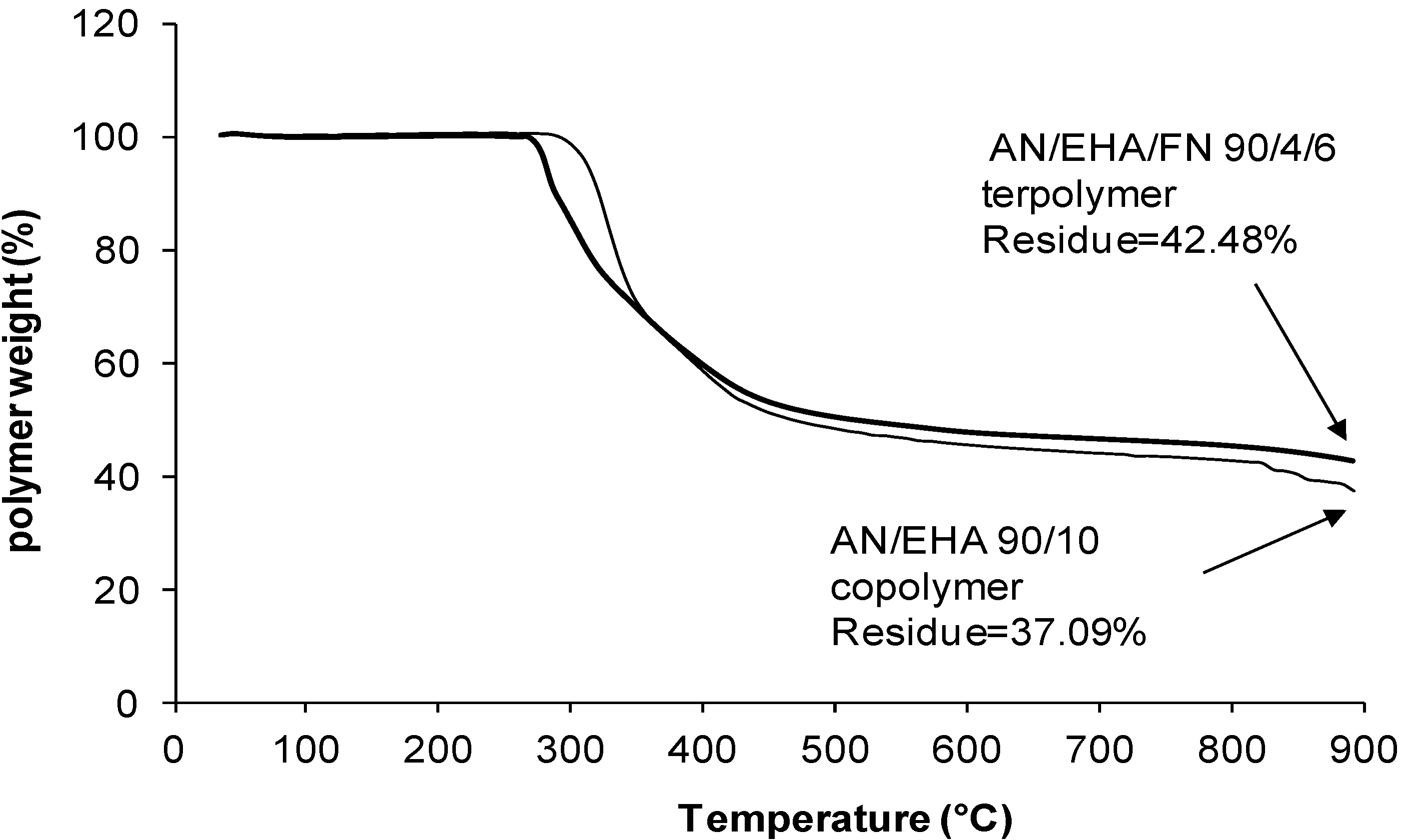
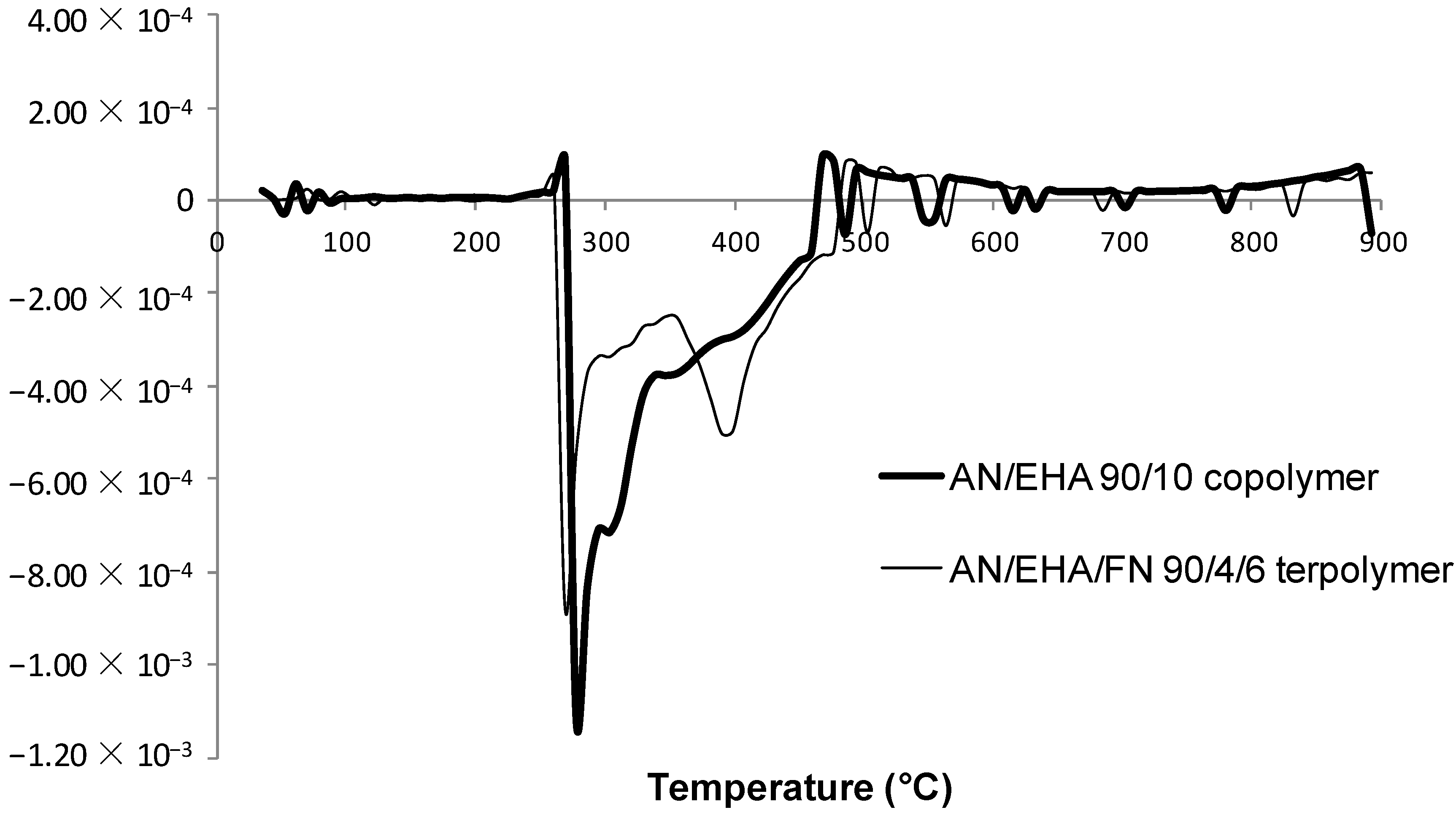
2.4. TGA Studies
| AN/comonomer/FN (mol%) | Weight loss (%) | Char yield (%) | |||
|---|---|---|---|---|---|
| Step 1 30–250 °C | Step 2 Part 1 250–350 °C | Step 2 Part 2 350–600 °C | Step 3 600–950 °C | ||
| 100/0/0 | 1.61 | 23.21 | 13.17 | 14.27 | 47.7 |
| AN/BA/FN | |||||
| 90/10/0 | 0.18 | 34.62 | 20.14 | 7.06 | 38.0 |
| 90/2/8 | 0.15 | 29.99 | 14.69 | 10.04 | 45.1 |
| 90/4/6 | 0.18 | 32.52 | 15.01 | 8.43 | 43.9 |
| 90/6/4 | 0.17 | 34.21 | 15.98 | 9.53 | 40.1 |
| 90/8/2 | 0.19 | 33.76 | 17.01 | 9.06 | 40.0 |
| AN/EHA/FN | |||||
| 90/10/0 | 0.45 | 35.80 | 15.0 | 11.67 | 37.1 |
| 90/2/8 | 1.67 | 30.18 | 14.14 | 10.12 | 43.9 |
| 90/4/6 | 0.24 | 33.21 | 15.73 | 8.34 | 42.5 |
| 90/6/4 | 0.78 | 35.01 | 16.23 | 7.79 | 40.2 |
| 90/8/2 | 1.12 | 38.65 | 14.29 | 6.84 | 39.1 |
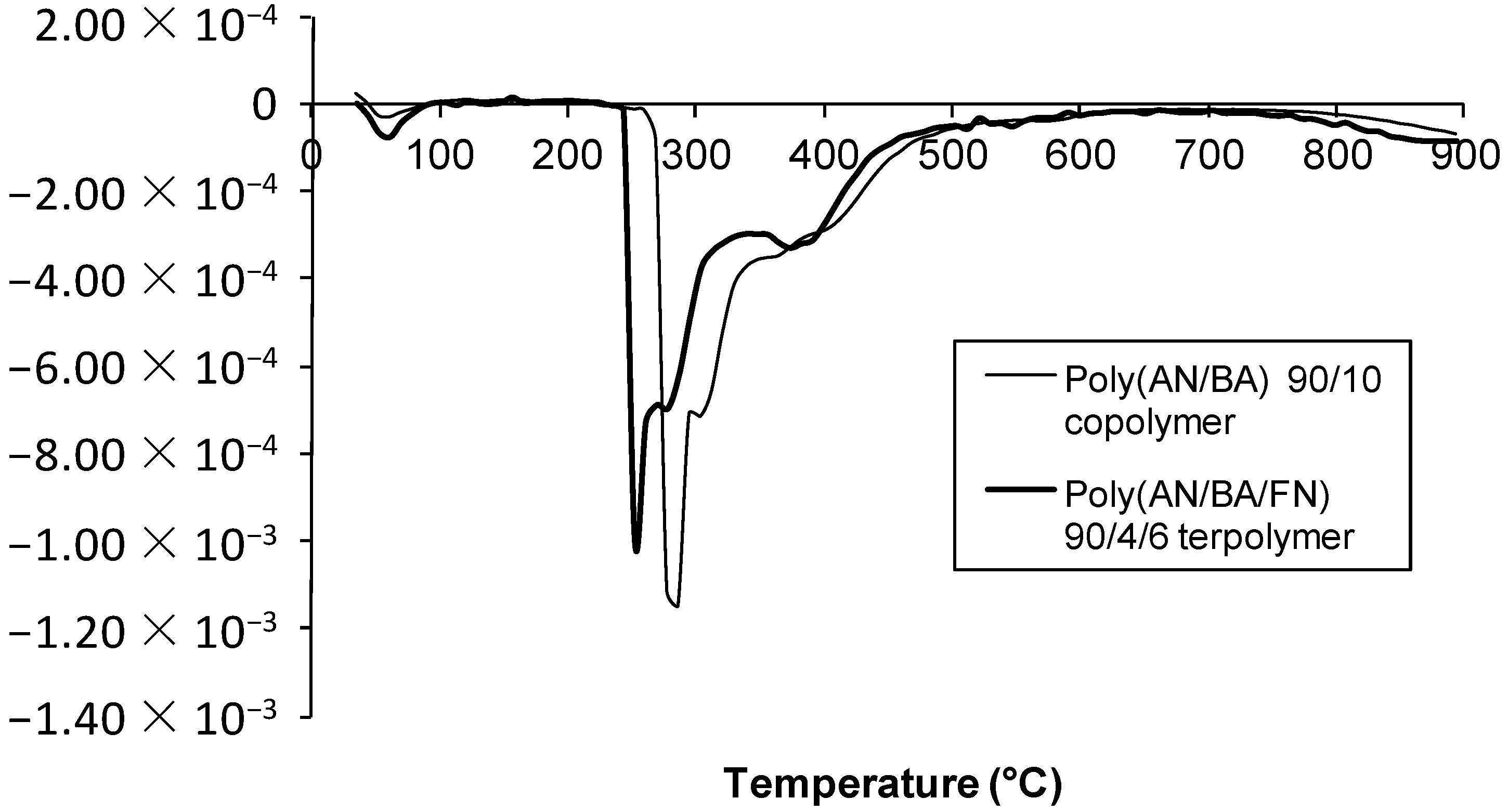
3. Experimental Section
3.1. Synthesis and Characterization
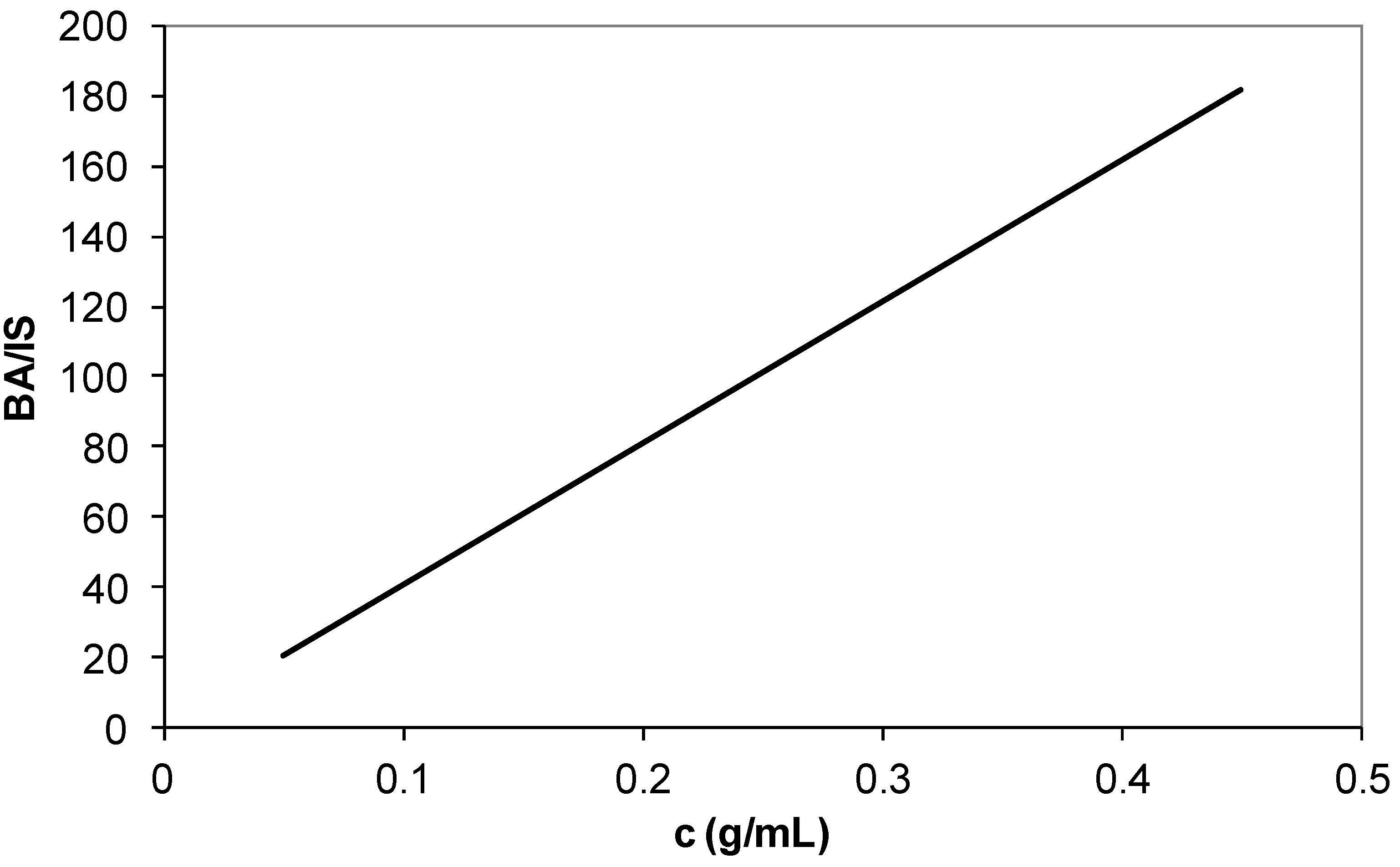
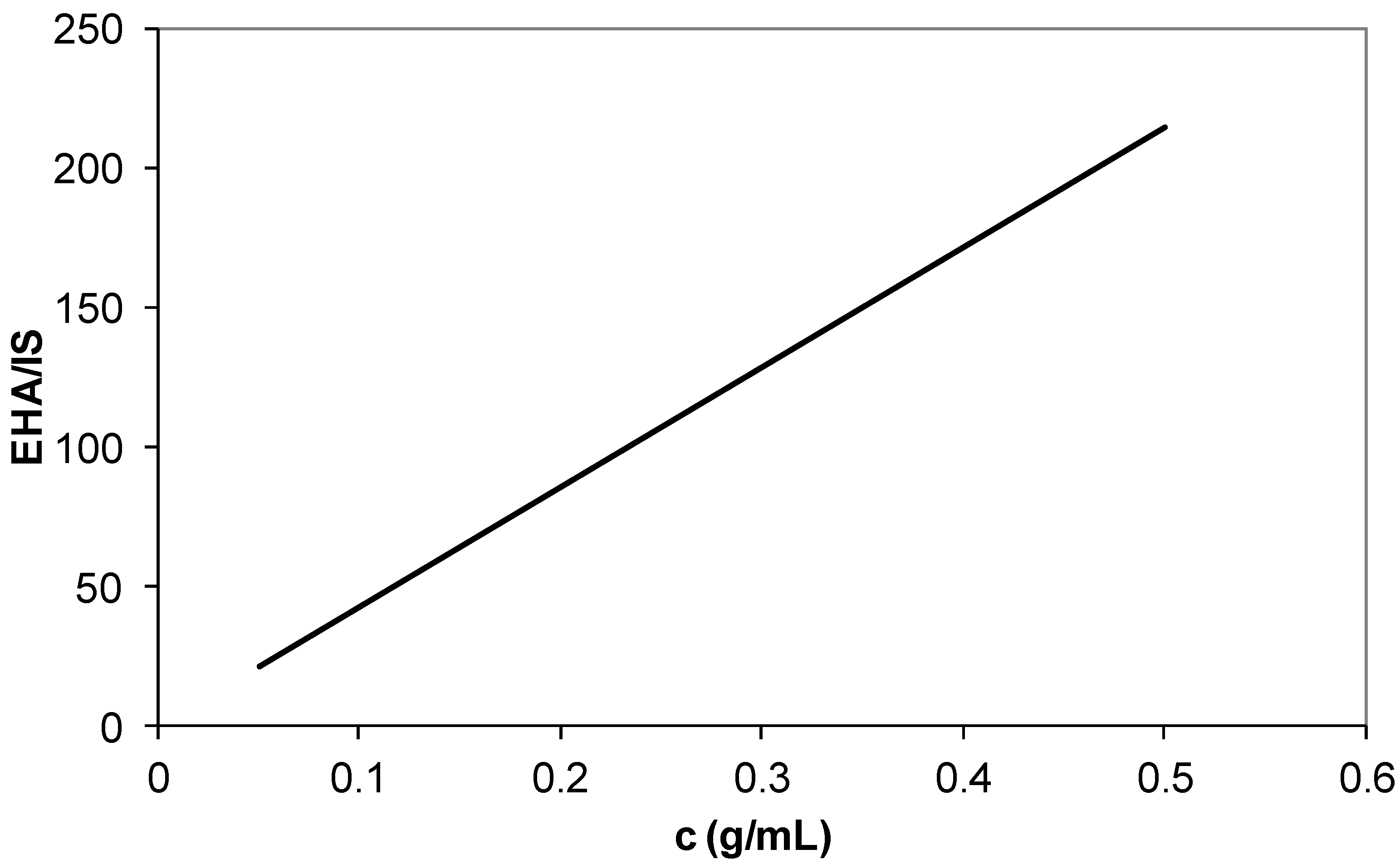
3.2. Composition Analysis of Polymers


3.3. Differential Scanning Calorimetry (DSC)
3.4. Thermogravimetric Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhu, J.; Wei, S.; Rutmana, D.; Haldolaarachchigec, N.; Young, D.P.; Guo, Z. Magnetic polyacrylonitrile-Fe@FeO nanocomposite fibers—Electrospinning, stabilization and carbonization. Polymer 2011, 52, 2947–2955. [Google Scholar] [CrossRef]
- Yusof, N.; Ismail, A.F. Post spinning and pyrolysis processes of polyacrylonitrile (PAN)-based carbon fiber and activated carbon fiber: A review. J. Anal. Appl. Pyrolysis 2012, 93, 1–13. [Google Scholar] [CrossRef]
- Sánchez-Soto, P.J.; Avilés, M.A.; Rio, J.C.D.; Ginés, J.M.; Pascual, J.; Pérez-Rodríguez, J.L. Thermal study of the effect of several solvents on polymerization of acrylonitrile and their subsequent pyrolysis. J. Anal. Appl. Pyrolysis 2001, 58, 155–172. [Google Scholar]
- Tsai, J.S.; Lin, C.H. Effect of comonomer composition on the properties of polyacrylonitrile precursor and resulting carbon fiber. J. Appl. Polym. Sci. 1991, 43, 679–685. [Google Scholar] [CrossRef]
- Nataraj, S.K.; Yang, K.S.; Aminabhavi, T.M. Polyacrylonitrile-based nanofibers—A state-of-the-art review. Progr. Polym. Sci. 2012, 37, 487–513. [Google Scholar] [CrossRef]
- Rahaman, M.S.A.; Ismail, A.F.; Mustafa, A. A review of heat treatment on polyacrylonitrile fiber. Polym. Degrad. Stab. 2007, 92, 1421–1432. [Google Scholar] [CrossRef]
- Aviles, M.A.; Ginés, J.M.; Rio, J.C.D.; Pascual, J.; Pérez-Rodríguez, J.L.; Sánchez-Soto, P.J. Thermal analysis of acrylonitrile polymerization and cyclization in the presence of N, N-dimethylformamide. J. Therm. Anal. Calorim. 2002, 67, 177–188. [Google Scholar] [CrossRef]
- Devasia, R.; Nair, R.C.P.; Ninan, K.N. Copolymerization of acrylonitrile with itaconic acid in dimethylformamide: Effect of triethylamine. Eur. Polym. J. 2003, 39, 537–544. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, J.; Wang, G. Evolution of structure and properties of PAN precursors during their conversion to carbon fibers. Carbon 2003, 41, 2805–2812. [Google Scholar] [CrossRef]
- Sedghi, A.; Farsani, R.E.; Shokuhfar, A. The effect of commercial polyacrylonitrile fibers characterizations on the produced carbon fibers properties. J. Mater. Process. Technol. 2008, 198, 60–67. [Google Scholar] [CrossRef]
- Bahrami, S.H.; Bajaj, P.; Sen, K. Thermal behavior of acrylonitrile carboxylic acid copolymers. J. Appl. Polym. Sci. 2003, 88, 685–698. [Google Scholar] [CrossRef]
- Deng, W.; Lobovsky, A.; Lacono, S.T.; Wu, T.; Tomar, N.; Budy, S.M.; Long, T.; Hoffman, W.P.; Smith, D.W. Poly(acrylonitrile-co-1-vinylimidazole): A new melt processable carbon fiber precursor. Polymer 2011, 52, 622–628. [Google Scholar] [CrossRef]
- Gupta, A.K.; Paliwal, D.K.; Bajaj, P. Effect of an acidic comonomer on thermooxidative stabilization of polyacrylonitrile. J. Appl. Polym. Sci. 1995, 58, 1161–1174. [Google Scholar] [CrossRef]
- Martin, S.C.; Liggat, J.J.; Snape, C.E. In situ NMR investigation into the thermal degradation and stabilisation of PAN. Polym. Degrad. Stab. 2001, 74, 407–412. [Google Scholar] [CrossRef]
- Min, B.G.; Son, T.W.; Jo, W.H.; Choi, S.G. Thermal stability of polyacrylonitrile in the melt formed by hydration. J. Appl. Polym. Sci. 1992, 46, 1793–1798. [Google Scholar] [CrossRef]
- Bajaj, P.; Sreekumar, T.V.; Sen, K. Effect of reaction medium on radical copolymerization of acrylonitrile with vinyl acids. J. Appl. Polym. Sci. 2001, 79, 1640–1652. [Google Scholar] [CrossRef]
- Tian, Y.; Hann, K.; Zhang, W.; Zhang, J.; Rong, H.; Wang, D.; Yan, B.; Liu, S.; Yun, M. Influence of residence time on the structure of polyacrylonitrile in ionic liquids during melt spinning process. Mater. Lett. 2013, 92, 119–121. [Google Scholar] [CrossRef]
- Bajaj, P.; Sreekumar, T.V.; Sen, K. Thermal behaviour of acrylonitrile copolymers having methacrylic and itaconic acid comonomers. Polymer 2001, 42, 1707–1718. [Google Scholar] [CrossRef]
- Naskar, A.K.; Walker, R.A.; Proulx, S.; Edie, D.D.; Ogale, A.A. UV assisted stabilization routes for carbon fiber precursors produced from melt-processible polyacrylonitrile terpolymer. Carbon 2005, 43, 1065–1072. [Google Scholar] [CrossRef]
- Ouyang, Q.; Cheng, L.; Wang, H.; Li, K. Mechanism and kinetics of the stabilization reactions of itaconic acid-modified polyacrylonitrile. Polym. Degrad. Stab. 2008, 93, 1415–1421. [Google Scholar] [CrossRef]
- Min, B.G.; Son, T.W.; Kim, B.C.; Lee, C.J.; Jo, W.H. Effect of solvent or hydrophilic polymer on the hydration melting behavior of polyacrylonitrile. J. Appl. Polym. Sci. 1994, 54, 457–462. [Google Scholar] [CrossRef]
- Rangarajan, P.; Bhanu, V.A.; Godshall, D.; Wilkes, G.L.; McGrath, J.E.; Baird, D.G. Dynamic oscillatory shear properties of potentially melt processable high acrylonitrile terpolymers. Polymer 2002, 43, 2699–2709. [Google Scholar] [CrossRef]
- Bhanu, V.A.; Rangarajan, P.; Wiles, K.; Bortner, M.; Sankarpandian, M.; Godshall, D.; Glass, T.E.; Banthia, A.K.; Yang, J.; Wilkes, G.; et al. Synthesis and characterization of acrylonitrile methyl acrylate statistical copolymers as melt processable carbon fiber precursors. Polymer 2002, 43, 4841–4850. [Google Scholar] [CrossRef]
- Godshall, D.; Rangarajan, P.; Baird, D.G.; Wilkes, G.L.; Bhanu, V.A.; McGrath, J.E. Incorporation of methyl acrylate in acrylonitrile based copolymers: Effects on melting behavior. Polymer 2003, 44, 4221–4228. [Google Scholar] [CrossRef]
- Bang, Y.H.; Lee, S.; Cho, H.H. Effect of methyl acrylate composition on the microstructure changes of high molecular weight polyacrylonitrile for heat treatment. J. Appl. Polym. Sci. 1998, 68, 2205–2213. [Google Scholar] [CrossRef]
- Liu, Y.; Chae, H.G.; Kumar, S. Gel-spun carbon nanotubes/polyacrylonitrile composite fibers. Part II: Stabilization reaction kinetics and effect of gas environment. Carbon 2011, 49, 4477–4486. [Google Scholar] [CrossRef]
- Bajaj, P.; Sen, K.; Bahrami, S.H. Solution polymerization of acrylonitrile with vinyl acids in dimethylformamide. J. Appl. Polym. Sci. 1996, 59, 1539–1550. [Google Scholar] [CrossRef]
- Jamil, S.N.A.M.; Daik, R.; Ahmad, I. Redox copolymerization of acrylonitrile with fumaronitrile as a precursor for carbon fibre. J. Polym. Res. 2007, 14, 379–385. [Google Scholar] [CrossRef]
- Bajaj, P.; Paliwal, D.K.; Gupta, A.K. Acrylonitrile–acrylic acids copolymers.I. Synthesis and characterization. J. Appl. Polym. Sci. 1993, 49, 823–833. [Google Scholar] [CrossRef]
- Jensen, J.O. Vibrational frequencies and structural determinations of fumaronitrile. J. Mol. Struct. THEOCHEM 2003, 631, 231–240. [Google Scholar] [CrossRef]
- Braun, D.; Hu, F. Free radical quaterpolymerization of acceptor-and donor-monomers. Polym. Bull. 2003, 49, 449–456. [Google Scholar] [CrossRef]
- Sarac, A.S. Redox polymerization. Progr. Polym. Sci. 1999, 24, 1149–1204. [Google Scholar] [CrossRef]
- Zuo, Y.; Lan, T.; Dai, L.; Lin, G.; Pan, R. Group-transfer copolymerization. Polymer 1996, 37, 875–877. [Google Scholar] [CrossRef]
- Wan, L.S.; Xu, Z.K.; Huang, X.J.; Wang, Z.G.; Wang, J.L. Copolymerization of acrylonitrile with N-vinyl-2-pyrrolidone to improve the hemocompatibility of polyacrylonitrile. Polymer 2005, 46, 7715–7723. [Google Scholar] [CrossRef]
- Mukundan, T.; Bhanu, V.A.; Wiles, K.B.; Johnson, H.; Bortner, M.; Baird, D.G.; Naskar, A.K.; Ogale, A.A.; Edie, D.D.; McGrath, J.E. A photocrosslinkable melt processible acrylonitrile terpolymer as carbon fiber precursor. Polymer 2006, 47, 4163–4171. [Google Scholar] [CrossRef]
- Gupta, A.K.; Paliwal, D.K.; Bajaj, P. Effect of the nature and mole fraction of acidic comonomer on the stabilization of polyacrylonitrile. J. Appl. Polym. Sci. 1996, 59, 1819–1825. [Google Scholar] [CrossRef]
- Rintoul, I.; Wandrey, C. Polymerization of ionic monomers in polar solvents: Kinetics and mechanism of the free radical copolymerization of acrylamide/acrylic acid. Polymer 2005, 46, 4525–4532. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jamil, S.N.A.M.; Daik, R.; Ahmad, I. Synthesis and Thermal Properties of Acrylonitrile/Butyl Acrylate/Fumaronitrile and Acrylonitrile/Ethyl Hexyl Acrylate/Fumaronitrile Terpolymers as a Potential Precursor for Carbon Fiber. Materials 2014, 7, 6207-6223. https://doi.org/10.3390/ma7096207
Jamil SNAM, Daik R, Ahmad I. Synthesis and Thermal Properties of Acrylonitrile/Butyl Acrylate/Fumaronitrile and Acrylonitrile/Ethyl Hexyl Acrylate/Fumaronitrile Terpolymers as a Potential Precursor for Carbon Fiber. Materials. 2014; 7(9):6207-6223. https://doi.org/10.3390/ma7096207
Chicago/Turabian StyleJamil, Siti Nurul Ain Md, Rusli Daik, and Ishak Ahmad. 2014. "Synthesis and Thermal Properties of Acrylonitrile/Butyl Acrylate/Fumaronitrile and Acrylonitrile/Ethyl Hexyl Acrylate/Fumaronitrile Terpolymers as a Potential Precursor for Carbon Fiber" Materials 7, no. 9: 6207-6223. https://doi.org/10.3390/ma7096207
APA StyleJamil, S. N. A. M., Daik, R., & Ahmad, I. (2014). Synthesis and Thermal Properties of Acrylonitrile/Butyl Acrylate/Fumaronitrile and Acrylonitrile/Ethyl Hexyl Acrylate/Fumaronitrile Terpolymers as a Potential Precursor for Carbon Fiber. Materials, 7(9), 6207-6223. https://doi.org/10.3390/ma7096207




